Published online Dec 24, 2017. doi: 10.5500/wjt.v7.i6.349
Peer-review started: August 14, 2017
First decision: September 4, 2017
Revised: September 18, 2017
Accepted: November 1, 2017
Article in press: November 2, 2017
Published online: December 24, 2017
Processing time: 131 Days and 0.6 Hours
To identify risk factors associated with the formation of biliary strictures post liver transplantation over a period of 10-year in Queensland.
Data on liver donors and recipients in Queensland between 2005 and 2014 was obtained from an electronic patient data system. In addition, intra-operative and post-operative characteristics were collected and a logistical regression analysis was performed to evaluate their association with the development of biliary strictures.
Of 296 liver transplants performed, 285 (96.3%) were from brain dead donors. Biliary strictures developed in 45 (15.2%) recipients. Anastomotic stricture formation (n = 25, 48.1%) was the commonest complication, with 14 (58.3%) of these occurred within 6-mo of transplant. A percutaneous approach or endoscopic retrograde cholangiography was used to treat 17 (37.8%) patients with biliary strictures. Biliary reconstruction was initially or ultimately required in 22 (48.9%) patients. In recipients developing biliary strictures, bilirubin was significantly increased within the first post-operative week (Day 7 total bilirubin 74 μmol/L vs 49 μmol/L, P = 0.012). In both univariate and multivariate regression analysis, Day 7 total bilirubin > 55 μmol/L was associated with the development of biliary stricture formation. In addition, hepatic artery thrombosis and primary sclerosing cholangitis were identified as independent risk factors.
In addition to known risk factors, bilirubin levels in the early post-operative period could be used as a clinical indicator for biliary stricture formation.
Core tip: Biliary stricture formation post liver transplantation is a frequent cause for patient morbidity and mortality and is referred to as the Achilles’ Heel of transplant. Strictures can be anastomotic or non-anastomotic depending on their number and anatomical location. Early stricture identification is key to providing successful treatment options. Known risk factors for biliary stricture formation include surgical technique, bile leak, hepatic artery thrombosis, primary sclerosing cholangitis, donation after circulatory death donors and increased cold ischemic time. This study identifies risk factors and clinical indicators for the development of biliary strictures post liver transplantation. It also discusses the importance of bilirubin and its potential role when implementing surveillance tools for biliary stricture formation post-transplant.
- Citation: Forrest EA, Reiling J, Lipka G, Fawcett J. Risk factors and clinical indicators for the development of biliary strictures post liver transplant: Significance of bilirubin. World J Transplant 2017; 7(6): 349-358
- URL: https://www.wjgnet.com/2220-3230/full/v7/i6/349.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i6.349
Orthotopic liver transplantation is currently the gold-standard treatment for patients with end-stage liver disease[1,2]. Post-operative biliary complications, in particular biliary stricture formation, are a frequent cause for patient morbidity and mortality and is often referred to as the Achilles’ heel of liver transplantation. Hospital re-admissions and clinical interventions used to treat biliary complications post-transplant are also a significant cost to health systems[3]. Despite advances in treatment techniques for biliary strictures post liver transplant, including non-surgical methods, formation is still observed in approximately 5%-32% of recipients[2,4]. Biliary tract complications post liver transplant include anastomotic strictures (AS), non-anastomotic strictures (NAS), bile leaks, stone formation, sludge and sphincter of Oddi dysfunction. It is important to note however, that biliary complications are often sub-clinical and studies have showed approximately 19% of the total number are clinically relevant[4].
Usually manifesting 5-8 mo post-transplant, AS occur when there is a narrowing of the anastomosis between the donor and the recipient bile ducts[4,5]. With a reported incidence of 4%-9%, the development of AS are thought to be associated with biliary ischemia, provoking a localized fibrotic response[5]. In symptomatic patients, treatment options include balloon dilatation or stenting using endoscopic retrograde cholangiography (ERCP) or placement of a biliary drain using percutaneous trans-hepatic cholangiography (PTC). In 16%-32% of patients, surgical interventions including re-operation of the biliary anastomosis or re-transplant are used[6,7]. Published literature indicates that risk factors for AS formation are mainly due to suboptimal surgical technique or the presence of a bile leak in the post-operative period[8].
In contrast, NAS are a narrowing of the biliary duct system at any site outside of the biliary tree and proximal to the biliary anastomosis, this can be both extra-hepatic and intra-hepatic. The pathophysiology remains largely unknown, however, fibrosis following injury to the biliary epithelium is the proposed pathological process for the development of NAS. Macroangiopathy and microangiopathy are two proposed etiologies of NAS. Those NAS occurring within the first year of transplant are thought to be associated with hepatic artery thrombosis (HAT), those that occur without HAT are often referred to as ischemic type biliary lesions. The incidence of NAS is varied, with 1%-20% incidence reported in the literature[9]. NAS are complex due to their location, often occurring in multiples and are longer in length. Due to complicated management issues, morbidity and mortality related to NAS is higher compared with AS[10]. Known risk factors for NAS formation include hepatic artery thrombosis, chronic ductopenic rejection, ABO incompatibility, primary sclerosing cholangitis (primary pathology), donation after circulatory death donors (DCD), prolonged use of vasopressors, older age of donor, preservation injury and prolonged cold and warm ischemia times[5,11]. Endoscopic treatment methods, including ERCP with balloon dilatation and stenting, are also used to treat NAS, however, patients often require multiple treatments with a reported 50%-75% success rate[5,12]. Secondary graft loss is common with up to 50% of patients experiencing graft loss and either requiring re-transplant or succumb to their illness whilst waiting for a life-saving re-transplant[13-15].
The aim of this study was to identify risk factors and clinical indicators associated with the formation of biliary strictures post orthotopic liver transplantation in the state of Queensland, Australia over a 10-year period. In addition to this, the study aimed to investigate potential post-transplant surveillance methods that could be used to identify patients at risk of biliary stricture formation.
We retrospectively analyzed all adult liver transplant recipients in the state of Queensland, Australia between 1st January 2005 and 31st December 2014 with studied follow up until 30th June 2015. Transplants analyzed consisted of varied graft types, including whole liver, right split and heart-liver-lung (HLL). HLL graft types were excluded from this study as these transplants were performed and followed up at a different transplant center within the state of Queensland (n = 5). Of the transplants studied, 25 were repeat liver transplants. De-identified donor and recipient data was collected from internal hospital records. The study protocol was approved by the Human Research Ethics Committee for the state of Queensland, Australia (HREC/13/QPAH/382) as well as the University of Queensland Ethics Committee (2015001248). In addition, approval was obtained from the Queensland Government to access confidential patient information, held by Queensland Health, for the Purpose of Research under the provision of section 280 of the Public Health Act 2005.
To prevent coagulopathy, the organ retrieval process routinely involved a 25000 IU flush of Heparin into the donor. The dopamine antagonist Chlorpromazine was used in addition to Heparin as per the discretion of the retrieval surgeon. Rapid cooling of organs was achieved by the instigation of a 2 L cold saline flush, followed by University of Wisconsin cold storage solution (UW Solution) through aortic and portal vein cannulas. Organs were transported in static cold storage prior to transplantation.
Orthotopic transplants at our center was exclusively performed using the piggyback technique. Right split grafts were transplanted using either the piggyback or venovenous bypass technique if indicated. A 1 L saline flush of the liver was infused during the inferior vena cava (IVC) anastomosis. Prior to reperfusion, 500 mL of blood was vented from the IVC. Hepatic artery anastomosis was performed after reperfusion had occurred. Right split liver transplant was performed using the piggy back. Venous-arterial extension grafts were used when required for both whole and right split liver transplantation. The biliary anastomosis was performed using one of two methods, Roux-en-Y hepaticojejunostomy or end-to-end choledochocholedochostomy. Use of each method was based on consideration of the patient’s past medical history and surgeon preference. Early in our cohort, T-tubes were routinely inserted to drain bile in transplant recipients, however, these were later replaced by silicon stents, used at the surgeons’ discretion. The macrolide calcineurin inhibitor, Tacrolimus, was used in conjunction with oral corticosteroids and Azathioprine post-transplant. Tacrolimus dose was titrated based on blood levels, a therapeutic level of > 8 but < 10 μg/L was considered optimal. Patients were initially followed up daily in an outpatient clinic post hospital inpatient discharge, following this twice weekly if three week’s post-transplant, weekly if two months post-transplant, monthly if three months post-transplant and finally with third monthly blood tests if 12-mo post-transplant.
Donor and recipient demographic data associated with the formation of biliary strictures was collected for this study. For recipients, this included age, gender, body mass index, reason for transplant, previous transplant and follow-up period. Donor demographic data included age, gender, body mass index, cause of death, donor type and cause of death. In addition to these parameters, intraoperative data was collected, including cold ischemic time (CIT), warm ischemic time (WIT), hepatic artery warm ischemic time, time of portal vein anastomosis, type of biliary anastomosis performed and the use of T-drains. Post-operative Day 0 to Day 7 liver functions tests, including total bilirubin were also collected.
Biliary stricture formation and the time frame that this occurred post-transplant were classified into the following categories; anastomotic stricture, ischemic type biliary stricture (ITBS) and recurrence of primary sclerosing cholangitis. For continuity of diagnosis, biliary stricture identification was made by an experienced transplant surgeon (JF) in our center using patient records and visualization of radiological imaging and reports. A stricture was defined as a narrowing of the bile duct with dilatation of the proximal biliary duct. No strict diameter cut-offs were used to define the structure. Routine post-operative magnetic resonance cholangiopancreatography (MRCP) was not performed at our center. Instead, imaging is guided by patient symptomatology. For the purposes of this study, a biliary stricture was considered a true stricture only in those requiring intervention. The requirement for post-operative ERCP and percutaneous drainage was recorded. In addition to this, we examined the number of patients who required re-transplantation and those who ultimately died as a result of biliary complications. The incidence of hepatic artery thrombosis was also established.
All continuous variables are expressed as median (interquartile range) and all categorical variables as frequency (percentage)[16]. Multivariable logistical regression analysis was performed to determine the risk factors for biliary strictures following transplantation[17]. All factors with a P-value of 0.1 or less in univariable regression were included in the model. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using IMB SPSS Statistics for Macintosh, Version 23.0 (IBM Corp. IMB SPSS statistics, Armonk, NY). No external statistical review was obtained.
Demographic data on donors and recipients are shown in Table 1. Between 1st January 2005 and 31st December 2014, a total of 296 patients underwent liver transplantation (Table 1). The average age of liver donors that developed biliary complications was higher than those that did not (48 and 42 years, P = 0.10). Most donors either had a normal BMI or were overweight, 137 (46.3%) and 117 (39.5%) respectively. Of the 11 DCD donors in the cohort, the majority did not develop biliary strictures (n = 10). Stroke was the leading cause of death in both groups, but was more substantial in the biliary stricture group (64.4% vs 49.8%, P = 0.28).
| Characteristics | Overall (n = 296) | Biliary strictures (n = 45) | Nil biliary strictures (n = 251) | P value |
| Donor characteristics | ||||
| Age (yr) | 42 (28-54) | 48 (40-57) | 42 (27-54) | NS |
| Gender (male) | 165 (55.7) | 25 (55.6) | 140 (55.8) | NS |
| BMI (kg/m2) | NS | |||
| < 18.5 | 5 (1.7) | 0 (0.0) | 5 (2.0) | |
| 18.5-24.9 | 137 (46.3) | 18 (40.0) | 119 (47.4) | |
| 25-29.9 | 117 (39.5) | 20 (44.4) | 97 (38.6) | |
| > 30 | 37 (12.5) | 7 (15.5) | 30 (12.0) | |
| Donor type | NS | |||
| Donation after brain death | 285 (96.3) | 44 (97.8) | 241 (96.0) | |
| Donation after circulatory death | 11 (3.7) | 1 (2.2) | 10 (4.0) | |
| Cause of death | NS | |||
| Stroke | 154 (52.0) | 29 (64.4) | 125 (49.8) | |
| Hypoxia | 43 (14.5) | 6 (13.3) | 37 (14.7) | |
| Accident | 37 (12.5) | 3 (6.7) | 34 (13.5) | |
| Other | 62 (20.9) | 7 (15.6) | 55 (21.9) | |
| Recipient characteristics | ||||
| Age at transplant (yr) | 52 (45-57) | 53 (40-58) | 52 (45-57) | NS |
| Gender (male) | 207 (69.9) | 31 (68.8) | 176 (70.1) | NS |
| BMI (kg/m2) (n = 267) | 267 | 44 | 223 | NS |
| Underweight (≤ 18.5) | 5 (1.9) | 0 (0.0) | 5 (2.0) | |
| Normal weight (18.5-24.9) | 94 (35.2) | 17 (38.6) | 77 (30.7) | |
| Overweight (25-29.9) | 93 (34.8) | 15 (34.1) | 78 (31.1) | |
| Obese ≥ 30 | 75 (28.1) | 12 (27.3) | 63 (25.1) | |
| Reason for transplant | NS | |||
| Viral hepatitis (B, C) ± hepatocellular carcinoma | 124 (41.9) | 19 (42.2) | 105 (41.8) | |
| Hepatocellular carcinoma without hepatitis | 22 (7.4) | 1 (2.2) | 21 (8.4) | |
| Alcohol | 33 (11.1) | 6 (13.3) | 27 (10.8) | |
| Biliary1 | 29 (9.8) | 8 (17.8) | 21 (8.4) | |
| Non-alcoholic steatohepatitis | 14 (4.7) | 3 (6.7) | 11 (4.4) | |
| Acute/fulminant liver failure | 12 (4.1) | 2 (4.4) | 10 (4.0) | |
| Complications first transplant | 21 (7.1) | 3 (6.7) | 18 (7.2) | |
| Other | 41 (13.8) | 3 (6.7 ) | 38 (15.1) | |
| Previous transplant | 25 (8.5) | 5 (11.1) | 20 (8.0) | NS |
Liver transplant recipients had a median age of 52 years, the majority were male. Overweight or obese patients accounted for 93 (34.8%) and 75 (28.1%) of the recipient population respectively. The indication for transplantation did not differ between the two groups, with viral hepatitis B, C with or without hepatocellular carcinoma accounting for approximately 40% of transplants. Recipients demographic data overall did not differ when comparing those with or without biliary complications. Of the parameters analyzed for this study, 98.5% of the data was available.
Table 2 presents the data on the transplant procedure characteristics, comparing those with and without biliary complications. Overall, no significant difference were found between the two groups.
| Characteristic | Total (n = 296) | Biliary strictures (n = 52) | Nil biliary strictures (n = 244) | P value |
| Cold ischemic time (min) | 415 (308-520) | 414 (319-530) | 415 (307-520) | NS |
| Warm ischemic time (min) | 27 (23-32) | 28 (23-33) | 27 (23-32) | NS |
| Time until hepatic artery anastomosis (min) | 74 (61-88) | 70 (60-86) | 74 (61-89) | NS |
| Time between portal vein and hepatic artery anastomosis (min) | 47 (35-60) | 41 (36-57) | 48 (35-60) | NS |
| Anastomosis used | 246 | NS | ||
| Duct to duct | 213 (72.0) | 29 (64.4) | 184 (73.3) | |
| Roux-en-Y | 78 (26.4) | 16 (35.6) | 62 (24.7) | |
| T-drain used (n = 231) | 23 (7.8) | 3 (6.7) | 19 (8.0) | NS |
A total of 45 (15.2%) recipients developed biliary strictures throughout the study period. One patient developed two complications. Anastomotic stricture formation was the commonest complication with 15 (33.3%) of these occurring within 6-mo of transplantation (Table 3). Anastomotic stricture formation was the leading cause for intervention with ERCP/PTC and reoperation of the biliary anastomosis (17, 58.6% and 15, 68.2% respectively) (Table 3). The development of ITBS accounted for 22.2% of all biliary stricture formation. ITBS were the primary indication with five (41.7%) patients undergoing an additional transplant within the first six-months of initial graft placement. Some patients were represented more than once as they underwent two interventions for biliary stricture formation. Serum Tacrolimus levels on post-operative Days 1-7 were found not to be significantly associated with the development of biliary strictures.
| Complication | Total frequency (n = 45)1 | Requiring intervention (ERCP/PTC) (n = 29)1 | Reoperation of biliary anastomosis (n = 22)1 | Retransplant (n = 12)1 |
| Anastomotic stricture | 25 (55.6) | 17 (58.6) | 15 (68.2) | 2 (16.7) |
| < 6 mo | 15 (33.3) | 10 (34.4) | 8 (36.4) | 1 (8.3) |
| > 6 mo | 10 (22.2) | 7 (24.1) | 7 (31.8) | 1 (8.3) |
| Right split graft | 3 (6.7) | 1 (3.4) | 2 (9.1) | 1 (8.3) |
| Ischemic type biliary stricture2 | 10 (22.2) | 7 (24.1) | 6 (27.3) | 6 (50.0) |
| < 6 mo | 8 (17.8) | 5 (17.2) | 5 (22.7) | 5 (41.7) |
| > 6 mo | 2 (4.4) | 2 (6.9) | 1 (4.5) | 1 (8.3) |
| DCD donor | 1 (2.2) | 1 (3.4) | 1 (4.5) | 1 (8.3) |
| Right split graft | 3 (6.7) | 2 (6.9) | 1 (4.5) | 1 (8.3) |
| PSC recurrence | 8 (17.8) | 3 (10.3) | 2 (9.1) | 1 (8.3) |
| Ischemic cholangiopathy due to HAT | 3 (6.7) | 3 (10.3) | 0 (0.0) | 3 (25.0) |
| Total patients1 | 46 (100.0) | 0 (100.0)3 | 23 (100.0) | 12 (100.0) |
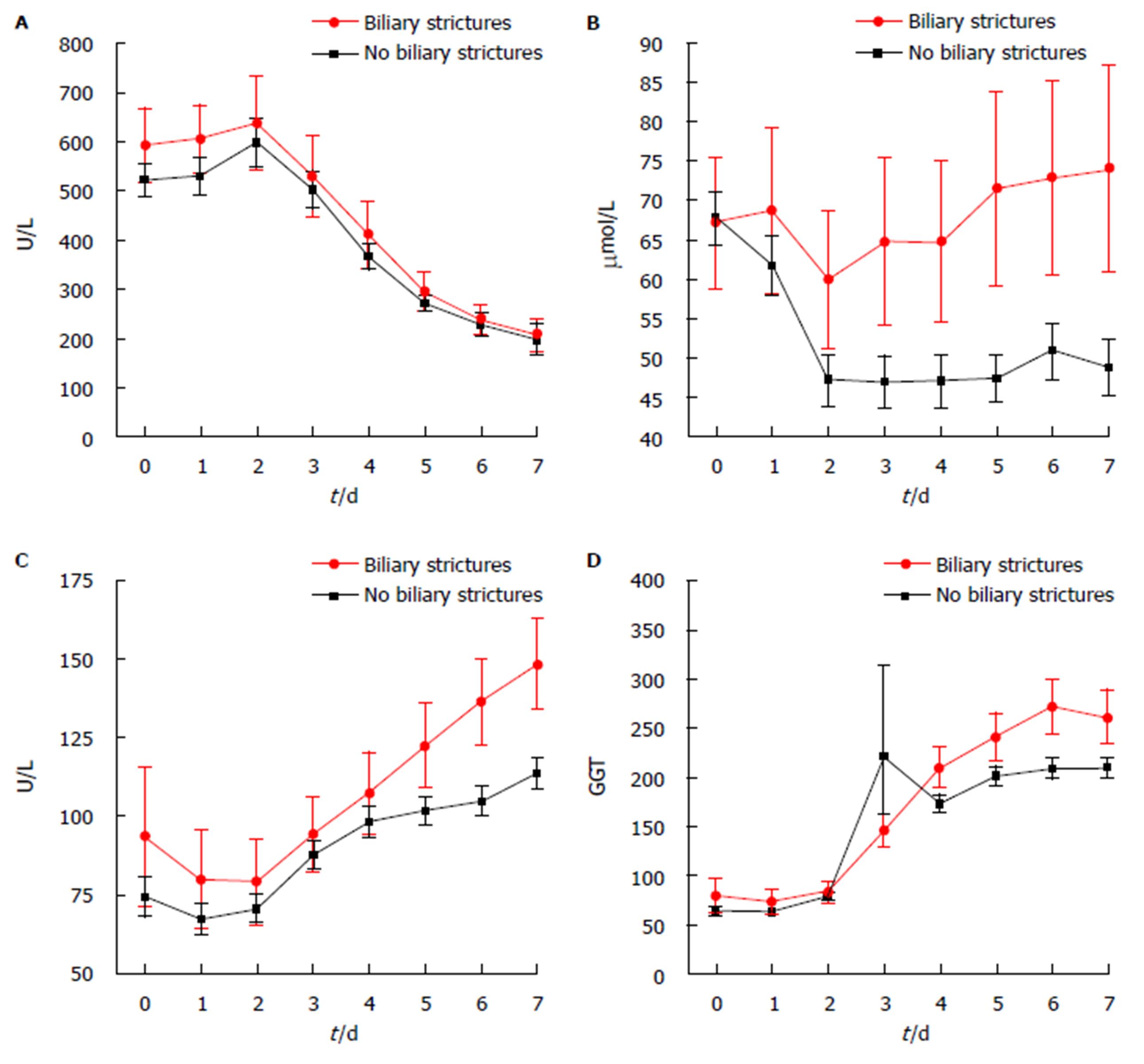
In recipients developing biliary strictures, total bilirubin was significantly increased within the first post-operative week (Day 7 total bilirubin 74 μmol/L vs 49 μmol/L, P = 0.012) (Figure 1). In both univariate and multivariate regression analysis, Day 7 total bilirubin > 55 μmol/L was associated with the development of biliary stricture formation (Table 4) with an odds ratio of 2.54 (1.22-5.29), P = 0.013. In addition, hepatic artery thrombosis and primary sclerosing cholangitis were identified as independent risk factors for biliary stricture formation (OR = 25.23, P ≤ 0.001 and OR = 3.10, P = 0.028, respectively). The sensitivity and specificity for Day 7 total bilirubin > 55 μmol/L was 38.6% and 77.6%, respectively.
| Characteristic | Univariate regression | Multivariate regression | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Biliary strictures (n = 45)1 | ||||
| Donor age > 55 yr | 1.54 (0.80-3.00) | NS | ||
| Cause of death - stroke | 1.88 (1.00-3.52) | 0.049 | 1.49 (0.72-3.08) | NS |
| Donation after circulatory death | 0.55 (0.07-4.39) | NS | ||
| Split vs whole graft | 1.09 (0.43-2.79) | NS | ||
| Primary sclerosing cholangitis as primary indication for transplant | 2.40 (0.82-7.07) | 0.11 | 3.10 (1.13-8.51) | 0.028 |
| Hepatic artery thrombosis | 22.93 (4.59-114.52) | < 0.001 | 25.23 (4.73-143.64) | < 0.001 |
| ≥ 2 prior transplants | 1.44 (0.51-4.07) | NS | ||
| Day 7 total bilirubin > 55 μmol/L | 2.19 (1.11-4.30) | 0.024 | 2.54 (1.22-5.29) | 0.013 |
In this study, we analyzed the risk factors associated with biliary stricture formation post liver transplant in an Australian cohort. Total bilirubin in the direct post-operative period was found to be associated with the development of biliary strictures, especially at post-operative Day 7.
The development of post-operative biliary strictures is still regarded as the Achilles’ heel of liver transplantation, causing significant patient morbidity and mortality. This study supports this statement, as it demonstrated an overall incidence of biliary stricture formation post deceased liver donor transplantation in an Australian center at 15%. This is comparable to other reported literature globally, with a 5%-15% incidence reported in deceased donors and a 28%-32% incidence reported in living liver donors[18]. These comparable incidence rates of biliary stricture formation are of interest, as unlike other overseas hospitals, our center does not routinely screen for biliary stricture formation with modalities such as MRCP. Instead, regular ultrasounds are used in the immediate inpatient setting. After this period, our center only images patients that are symptomatic or in those in which a stricture is suspected.
It is difficult to predict the formation of biliary strictures in adult patients post liver transplant. MRCP is a non-invasive technique that enables detailed visualization of the biliary tree, however is a limited and expensive resource[19]. Through analyzing post-operative recipient blood work, total bilirubin was found to be elevated in those that developed biliary strictures, in particular, a total bilirubin level > 55 μmol/L on post-operative Day 7. These findings suggest that total bilirubin levels could be used as an inexpensive tool to identify those patients more at risk of biliary stricture formation and these patients could potentially benefit from a surveillance MRCP.
It is important to note that bilirubin has previously been recognized as a significant marker in the identification of liver graft dysfunction and graft survival. This is demonstrated in the currently accepted definition of early allograft dysfunction which includes one or more of the following variables: (1) total bilirubin ≥ 171 μmol/L on post-operative day 7; (2) INR ≥ 1.6 on post-operative Day 7; and (3) an aminotransferase level (ALT or AST) ≥ 2000 IU/mL within the first seven post-operative days[20]. To support this, a study conducted by Wagener et al[21] concluded that elevated total bilirubin levels on post-operative Day 0-7 significantly correlated with graft dysfunction within the first 90 day post-operatively. This study went on further to report post-operative Days 1-2 bilirubin > 112 μmol/L should warn clinicians of potential EAD[21]. On the other hand, Olthoff et al[20] contradicts this statement suggesting that elevated total bilirubin levels on post-operative Days 1-3 should be excluded when predicting EAD as these values may reflect the pre-transplant status and not graft functionality.
The underlying mechanism of the association between elevated total bilirubin levels at Day 7 post-transplantation and biliary stricture formation remains to be determined. Previous studies have identified a more toxic bile composition in recipients developing non-anastomotic biliary strictures[22,23]. Furthermore, prolonged graft ischaemia was found to cause an unparalleled impairment of bile acid transporter expression in cholangiocytes leading to prolonged biliary transit time of bile acids inducing apoptosis[24,25]. Although not formally assessed in this study, increased total bilirubin levels at Day 7 post transplantation might be the results of impaired bile transporter function post transplantation and associated increased in bile toxicity resulting in stricture formation.
Currently, the literature reports biliary stricture formation presents as a later complication, between 5-8 mo post-transplant[18]. This is dependent on the type of stricture that has formed, with non-anastomotic stricture presenting between 3.3-5.9 mo[26,27]. Our study demonstrated AS formation as the most common complication (55.6%), with 33.3% of these forming within the first six-months post-transplant. Therefore, the proposed surveillance MRCP should be completed within the first three to six-months post-transplant.
Risk factors for biliary stricture formation have been well documented in the literature and are multifactorial including factors related to the recipient, donor and operative characteristics[5,11,28]. In line with previous findings, the results of our study identified stroke as donor cause of death, hepatic artery thrombosis (OR = 22.93) and Day 7 total bilirubin > 55 μmol/L as significant risk factors for biliary stricture formation on univariate regression. Upon multivariate analysis, PSC as primary indication for transplantation, HAT and Day 7 total bilirubin > 55 μmol/L were all significant risk factors. Specific donor characteristics, such as increased donor age (> 55 years) and DCD donor type were not found not to be a significant risk factor in the formation of biliary strictures in this study which could be due to our small cohort size and relative underrepresentation of DCD donor grafts.
In our cohort, we had a low percentage of ITBS compared to previous reports in the literature[29]. It was found that radiologically, it is difficult to distinguish between the development of PSC recurrence and ITBS formation[29]. For the purposes of this study, the investigator classified these questionable lesions as PSC recurrence, this in turn could have underestimated the presence of ITBS on our data set. Another point to note is that as DCD donation is infrequently used in our transplant center and therefore we were unable to assess this as a risk factor for stricture formation. Previously, the risk of biliary complications and ischemic cholangiopathy has been found to be significantly increased in DCD donors by Foley et al[19]. As immunological factors have been associated with biliary stricture formation, post-operative Days 1-7 serum Tacrolimus levels were measured by found not to be significantly associated with the development of biliary strictures.
Currently, there is no clear consensus as to which anastomotic reconstruction technique (duct-to-duct vs Roux-en-Y) of the biliary system is superior regarding biliary stricture formation. It is important to note that the surgical technique used if often dependent on the indication for transplant (e.g., PSC with previous diseased bile duct) or split liver graft and is usually weighed up against the need to restore original anatomy[11]. In saying this, a running suture, without a bile tube has been proven to be of benefit in preventing early biliary complications[30]. Although a greater percentage of patients that underwent Roux-en-Y anastomosis developed biliary complications compared to those in the end to end anastomosis group, Roux-en-Y was not found to be a significant risk factor for biliary stricture formation. Our study demonstrated a higher incidence of biliary stricture formation in the Roux-en-Y technique, with this being the less common method used at our center (26.4%). T-tube use was not associated with biliary stricture formation.
In addition, recipient WIT has been identified as a risk factor for non-anastomotic biliary stricture formation[31]. It has previously been found that there is a 2.64 (P ≤ 0.01) relative risk of developing non-anastomotic biliary strictures with every hour increase of WIT[14]. Our study did not identify CIT or WIT as a risk factor for biliary stricture formation, with the average CIT and WIT being comparable. Again, the smaller cohort represented in our study may have accounted for this finding.
Our study was limited by the fact that it consisted of a relatively small cohort and because data was collected retrospectively collected and some cases were not documented appropriately or contained missing data. Overall 98.5% of parameters included in the total dataset were available for analysis. Furthermore, due to the limited number of patients in our cohort that received right split grafts (n = 37, 12.5%), we were unable to draw comparisons between whole and split liver grafts on risk of biliary stricture formation. Wan et al[32] demonstrated an OR = 0.64 favoring right split grafts compared to whole grafts in the formation of biliary strictures post liver transplant in adults. Similarly, only 11 patients received a DCD liver graft in out cohort.
In conclusion, the incidence of biliary stricture formation post liver transplant in our center was 15%. Serum total bilirubin levels > 55 μmol/L at Day 7 post-operatively were associated with an increased risk of stricture formation, suggesting that bilirubin could be used to identify those that need closer surveillance following liver transplantation.
Liver transplantation is a lifesaving surgical procedure available to those eligible with end-stage liver failure. Biliary strictures can cause a disruption in the flow of bile and formation post liver transplantation is a frequent cause for patient morbidity and mortality. Due to the significant burden of disease biliary strictures cause, those patients with biliary strictures often require either endoscopic intervention, surgical re-do of the anastomosis or even re-transplantation.
Biliary strictures post liver transplantation can be classified into two categories, non-anastomotic stricture and anastomotic stricture. Non-anastomotic strictures are often difficult to treat. They are associated with worse outcomes as they often present in numbers and are situated anatomically in a difficult to access location outside the biliary tree. Earlier identification and subsequent treatment of biliary strictures post liver transplant have been associated with improved patient outcomes and decrease the need for re-transplant. Current identified risk factors for biliary stricture formation post liver transplant include sub-optimal surgical technique, the presence of bile leak, hepatic artery thrombosis, primary sclerosing cholangitis, donation after circulatory death donors and prolonged cold or warm ischemic time. Identifying risk factors and clinical indicators for the development of biliary strictures would allow clinicians to identify at risk patients and potentially predict stricture formation. This would allow for earlier treatment of strictures, improving clinical patient care and allograft survival.
This study investigates the risk factors and clinical indicators associated with biliary stricture formation post liver transplantation. In order to translate these findings clinically, this study also aimed to describe potential surveillances method for biliary strictures formation post liver transplantation. These clinical tools would allow for the early identification and treatment of biliary strictures, with the aim of improving patient outcomes.
Electronic data for this study was collected retrospectively on all liver donors and recipients in the state of Queensland between 2005 and 2014. Within this data set we analyzed demographic, intra-operative and post-operative characteristics of each procedure. In addition, post-operative liver function tests, serum bilirubin and Tacrolimus levels were collected from post-operative Days 0 to 7. Biliary stricture formation post-operatively was recorded, the interventions used to treat and their timing was also identified. This study was unique in that is used logistical regression to identify potential risk factors and clinical indicators for biliary stricture formation.
This study demonstrated the incidence of biliary strictures post liver transplantation at our center at 15%. Significant risk factors for the formation of biliary strictures post-transplant included primary sclerosing cholangitis as the primary indication for transplant and the presence of hepatic artery thrombosis. As a clinical indicator, Day 7 total serum bilirubin > 55 μmol/L was found to be associated with an increased risk of stricture formation. Investigation into potential mechanisms explaining this rise in bilirubin in patients with strictures would be beneficial.
As well as known risk factors for biliary stricture formation, this study identified Day 7 total serum bilirubin > 55 μmol/L as a significant clinical indicator for the development of biliary strictures post liver transplant. As biliary strictures pose a significant burden of morbidity and mortality on patients post liver transplantation, identifying clinical indicators such as elevated total serum bilirubin for stricture formation is a useful tool to enable clinicians to provide early and more successful care to those transplant recipients more at risk. This study identified previously known risk factors for biliary stricture formation post transplantation including primary sclerosing cholangitis are the primary indication for transplant and the presence of hepatic artery thrombosis. Previous studies have identified elevated bilirubin in the post-operative period as a risk factor for biliary stricture formation. This study adds to this body of evidence as it proposes a specific measure of total serum bilirubin (> 55 μmol/L) that is associated with biliary stricture formation post liver transplant. The results of this study can be translated into clinical practice by applying a clinical algorithm to patients that are considered at higher risk of biliary stricture formation post-transplant. The authors suggest focused surveillance of these patients for biliary stricture formation within the immediate three to six-month post-operative period with a magnetic resonance cholangiopancreatography scan.
The authors would like to acknowledge Cheryl Fourie and Jade Carey from Donate Life and the Australia and New Zealand Organ Donor Registry.
| 1. | Boraschi P, Donati F. Complications of orthotopic liver transplantation: imaging findings. Abdom Imaging. 2004;29:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Axelrod DA, Dzebisashvili N, Lentine KL, Xiao H, Schnitzler M, Tuttle-Newhall JE, Segev DL. Variation in biliary complication rates following liver transplantation: implications for cost and outcome. Am J Transplant. 2015;15:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kienlein S, Schoening W, Andert A, Kroy D, Neumann UP, Schmeding M. Biliary complications in liver transplantation: Impact of anastomotic technique and ischemic time on short- and long-term outcome. World J Transplant. 2015;5:300-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (4)] |
| 6. | Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Holt AP, Thorburn D, Mirza D, Gunson B, Wong T, Haydon G. A prospective study of standardized nonsurgical therapy in the management of biliary anastomotic strictures complicating liver transplantation. Transplantation. 2007;84:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 8. | Kitazono MT, Qayyum A, Yeh BM, Chard PS, Ostroff JW, Coakley FV. Magnetic resonance cholangiography of biliary strictures after liver transplantation: a prospective double-blind study. J Magn Reson Imaging. 2007;25:1168-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Nemes B, Gámán G, Doros A. Biliary complications after liver transplantation. Expert Rev Gastroenterol Hepatol. 2015;9:447-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | Krok KL, Cárdenas A, Thuluvath PJ. Endoscopic management of biliary complications after liver transplantation. Clin Liver Dis. 2010;14:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Ward EM, Kiely MJ, Maus TP, Wiesner RH, Krom RA. Hilar biliary strictures after liver transplantation: cholangiography and percutaneous treatment. Radiology. 1990;177:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med. 2016;4:111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 18. | Cerný V, Daniel M. Occurrence of the tick Ixodes ricinus in a landscape strongly influenced by human activities. Wiad Parazytol. 1986;32:351-353. [PubMed] |
| 19. | Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D’Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 322] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 20. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 935] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 21. | Wagener G, Raffel B, Young AT, Minhaz M, Emond J. Predicting early allograft failure and mortality after liver transplantation: the role of the postoperative model for end-stage liver disease score. Liver Transpl. 2013;19:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Op den Dries S, Sutton ME, Lisman T, Porte RJ. Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation. 2011;92:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Cheng J, Zhou L, Jiang JW, Qin YS, Xie HY, Feng XW, Gao F, Zheng SS. Proteomic analysis of differentially expressed proteins in rat liver allografts developed acute rejection. Eur Surg Res. 2010;44:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Cheng L, Tian F, Tian F, Tang L, Chen G, Luo Z, Ren J, Wang S. Repression of Farnesoid X receptor contributes to biliary injuries of liver grafts through disturbing cholangiocyte bile acid transport. Am J Transplant. 2013;13:3094-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Rerknimitr R, Sherman S, Fogel EL, Kalayci C, Lumeng L, Chalasani N, Kwo P, Lehman GA. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Lemmer ER, Spearman CW, Krige JE, Millar AJ, Bornman PC, Terblanche J, Kahn D. The management of biliary complications following orthotopic liver transplantation. S Afr J Surg. 1997;35:77-81. [PubMed] |
| 29. | Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Wojcicki M, Lubikowski J, Klek R, Post M, Jarosz K, Białek A, Wunch M, Czuprynska M. Reduction of biliary complication rate using continuous suture and no biliary drainage for duct-to-duct anastomosis in whole-organ liver transplantation. Transplant Proc. 2009;41:3126-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles’ heel. Liver Transpl. 2006;12:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Wan P, Li Q, Zhang J, Xia Q. Right lobe split liver transplantation versus whole liver transplantation in adult recipients: A systematic review and meta-analysis. Liver Transpl. 2015;21:928-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Guo JS, Hua YP, Salvadori M, Sugawara Y, Tsoulfas G, Zhang ZH S- Editor: Cui LJ L- Editor: A E- Editor: Yan JL