INTRODUCTION
BK virus (BKV) was first isolated from the urine of a patient with transplant ureteric stenosis[1] in 1971. BKV is a member of the Polyomaviridae family, falling into the Betapolyomavirus subcategory with JC virus and Simian virus 40 (SV40)[2]. BKV and SV40 have approximately 70% similarity within their respective genomes[3,4]. This similarity between the genomes of these viruses enables SV40 to be a marker for immunohistochemical staining, which is vital in diagnosis of BKV-associated nephropathy (BKVAN)[4]. BKV primary infection occurs in early childhood and is asymptomatic in the majority of cases[5,6]. Transmission of BKV is thought to involve respiratory and oral routes[7], and results in a seroprevalence of 82% in adulthood[8]. BKV is a latent infection, which can lie dormant in tissues, the kidney being the most notable. Heritage et al[9] showed that BKV was present in 50% of kidneys based on DNA sequencing of BKV in renal samples. The virus is able to reside in renal tubular epithelial cells and in the uroepithelium. Therefore, another “artificial” route of BKV transmission is through kidney transplantation, particularly on the background of the immunosuppression required in this context to prevent organ rejection. Moreover, although primary infection in non-transplanted patients is generally asymptomatic, viral infection in the context of transplantation and immunosuppression may result in viral replication within epithelial tissues (in this case renal tubules), and the development of inflammation, BKVAN, which resembles other forms of tubule-interstitial nephritis and transplant rejection. If left untreated, these processes progress to result in allograft dysfunction and failure[10]. Indeed, North American and European series suggest that although not the leading overall cause of graft failure, it represents an important and potentially treatable (even preventable) cause in many[11,12]. For many years, BKVAN was mis-diagnosed as rejection, and therefore inappropriately treated, resulting in graft failure in many patients. Nevertheless, greater understanding of BKV has resulted in clinical advances in the field. In this literature review the virology of BKV, the mechanism of BKVAN pathogenesis, and advances in therapeutic strategies will be addressed.
VIROLOGY OF BKV
BKV is a member of the Polyomavirus genus of the Polyomaviridae family. These viruses are 40-45 nm in diameter[1,13] comprising of an icosahedral capsid surrounding double-stranded DNA, which is able to replicate in the host cell nucleus[14]. The BKV genome contains 5153 base pairs that can be translated bi-directionally[6,15,16]. However, recent analysis of BKV from a kidney transplant patient observed a genome size of 5141 base pairs[17]. The BKV genome is divided into three regions: Early, late and regulatory (non-coding control region or NCCR) regions. The early stage encompasses regulatory proteins such as small tumour antigens (tAg) and large tumour antigens (TAg) as well as late structural capsid proteins - viral protein (VP) 1, VP2, VP3 and the agnoprotein[18]. However, these late proteins are produced after the genome of the virus has be replicated[19]. VP1 is the most common protein found on the outer layer of the capsid and contains a small groove used for host cell receptor binding[20]. The NCCR encodes transcriptional control elements, such as the origin of replication and promoters for genes encoded within the early and late regions[11,21]. BKV enters cells via VP1 binding to sialic acid residues of glycoprotein receptors[22,23]. After receptor binding BKV is internalised via a caveolae-mediated endocytosis pathway, the virus then travelling to the cell nucleus to launch either a latent or acute infection[13,24,25]. According to Jin et al[26], four serotypes (I-IV) of BKV are recognised based on the differences between amino acids 61-83 in the region coding for VP1, with the similarity of this region between different serotypes at 61%-70%[27]. Serotype I is the most common within the worldwide population (80%), followed by type IV (15%)[28]. However, Sharma et al[29], using a phylogenetic whole-genome approach, suggested a classification system of BKV which contain serotypes V and VI.
PATHOGENESIS OF BKVAN
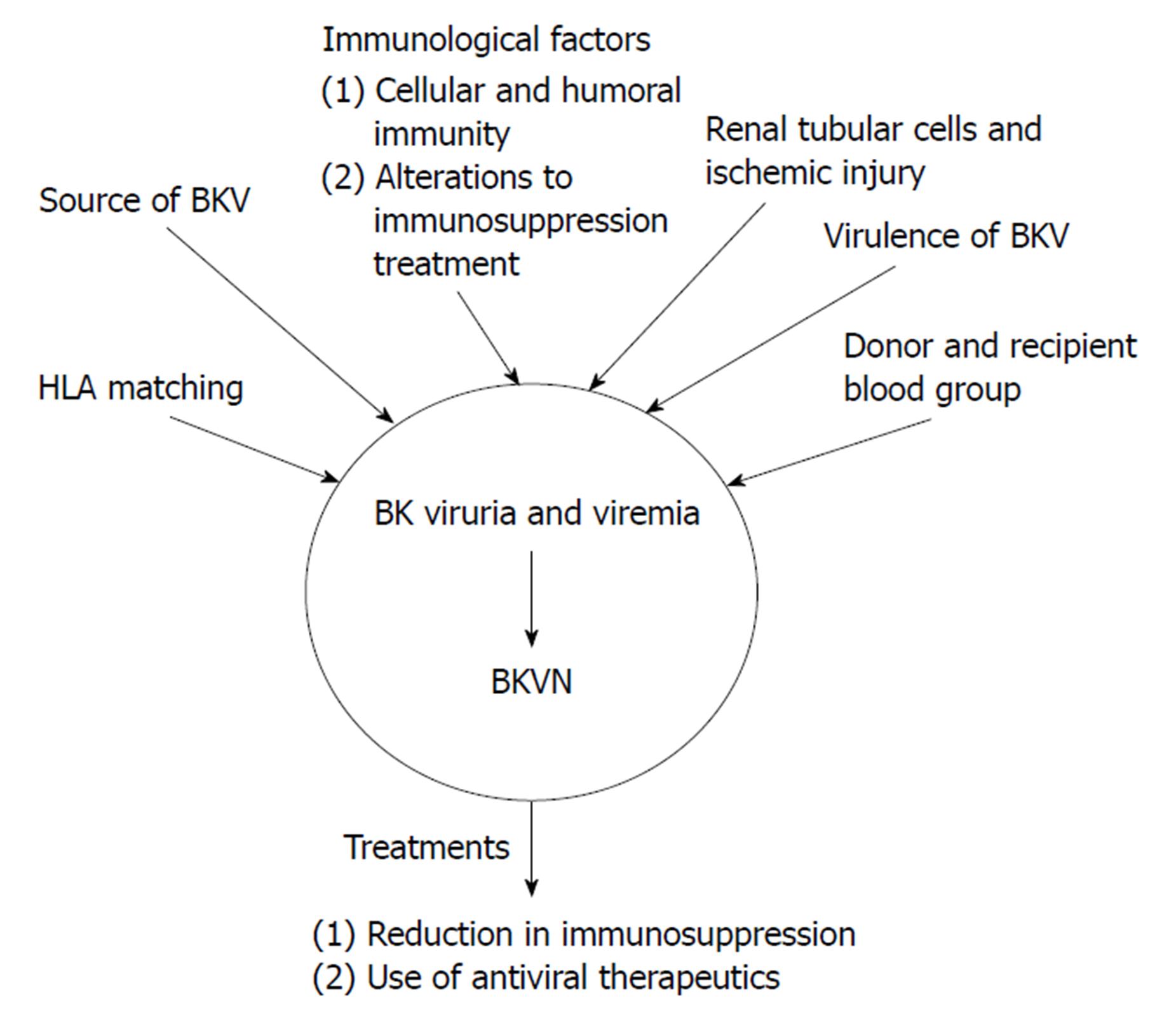
There are many proposed factors relating to the pathogenesis of BKVAN as shown in Figure 1: Source of the viral infection; host cellular immunity to BKV; the influence of immunosuppression; HLA matching; recipient and donor blood group matching.
Figure 1 Proposed mechanisms for the pathogenesis of BK virus-associated nephritis after BK virus infection has occurred resulting in BK viruria or BK viremia.
These mechanisms include immunological factors, such as alterations to immunosuppressive therapy and cellular and humoral immunity, the source of BKV, either from the recipient or the donor, HLA matching, donor and recipient blood group. The two main treatment options for BKVN are a reduction in immunosuppression and the use of antiviral therapies. These treatments can also be used for BK viruria and viremia in order to prevent progression to BKVAN. BKV: BK virus; BKVAN: BK virus-associated nephritis.
Source: Donor vs recipient
BKVAN within transplanted kidneys arises from either primary infection from the transplanted kidney itself, or following reactivation from latency in the patient’s native urinary tract. Epidemiological work has striven to understand the predominant mechanism in this context. Andrews et al[30] were the first to show that recipients of transplants from seropositive donors (either deceased or living), was associated with increased rate of BKV infection within the transplant kidney, thereby suggesting the importance of donor-derived infection in the pathogenesis of BKVAN. This was supported by data from Bohl et al[31] who observed BKV infection in 25 of 54 (46%) recipients of kidneys from seropositive donors compared with 4 of 27 (15%) recipients of kidneys from seronegative donors (P = 0.007). These authors also noted that the rate at which BK viruria occurred was faster in patients receiving kidneys from seropositive vs seronegative donors (median onset 45 d vs 370 d respectively; P < 0.001). The duration of BK viruria was also longer in the context of seropositive donors (median duration 157 d vs 7 d, P = 0.009). The authors also recorded that none of the 27 recipients from seronegative donors developed viremia or sustained viremia, whereas these numbers were 7 and 4 respectively in recipients from seropositive donors. In addition, this study demonstrated donor BKV antibody titre inversely correlated with the time to onset of post-transplant viruria (P = 0.001), and was positively correlated with duration of viruria (P = 0.014) and peak urine viral titres (P = 0.005). These studies did not evaluate the importance of recipient BKV serostatus, but this was done in a study of paediatric recipients[32], which suggested the importance of recipient BK seronegativity. In this study, all patients developed BKV viruria, with recipient seronegativity strongly associated with the development of nephropathy (P = 0.01). Finally, Saundh et al[33] studied 112 renal transplant patients before and after transplantation, and conducted a phylogenetic analysis of VP1 sequences and serotypes. Twelve patients developed BKV viremia, and 8 had a sufficiently high viral load to allow amplification of VP1. Based on this analysis the authors concluded that donor-derived infection was responsible for the majority of cases of BKV infection. A single patient had two differing VP1 subgroups present (Ib-1 after 6 mo followed by Ia after 12 mo post transplantation), perhaps suggesting a potential switch between donor and recipient strains, and means that cases of BKV infection due to reactivation from the recipient may be a real phenomenon. However, the burden of evidence from this, and the other aforementioned studies, is that donor-derived infection represents the major risk confronting the kidney transplant recipient.
Cellular and humoral immunity
Humoral and cellular immunity is thought to be implicated with the pathogenesis of BKVAN, and both CD8+ and CD4+ T cells are involved in the recognition and clearance of viruses such as BKV. The lack of BKV specific IgG may be important in the development of BKVAN[12]. As mentioned above, there is a greater risk for patients that are BKV seronegative at the time of their transplant, as they will have no BKV specific antibody; patients with previous exposure and who have developed immunity to BKV, may not develop the infection[34]. Yet the presence of a BKV-specific antibody response is clearly not protective, and it is likely that cellular immunity plays the central role in viral control. Certainly, patients with BKV specific antibodies remain at risk of developing BKVAN[35]. Comoli et al[36] showed that patients that had BKVAN had fewer BKV-specific lymphocytes that secreted interferon-γ (IFN-γ), with the mean frequency of BKV specific, IFN-γ being 151 × 106 cells. This was approximately 10 times less than other viruses related to transplantation, such as EBV. The researchers concluded that, based on their data that there is reduced BKV immunity, which in turn would increase the rate of active BKV infection.
Immunosuppression burden
Immunosuppression is required to ameliorate rejection of the transplanted kidney by the host immune system. However, with developments in immunosuppressive drugs such as mycophenolate mofetil (MMF) and tacrolimus (Tac), the reduction in rejection rate has been inversely paralleled by an increased incidence of BKV infection. Calcineurin inhibitor (CNI) based therapies have been shown to increase the risk of BKVAN and subsequent nephrotoxicity following renal transplant[37]. However, a recent study by Jacobi et al[38] observed no significant change in the number of patients with BKV infection (n = 352) when using CNI of either tacrolimus or cyclosporine A (CyA). This led to the conclusion that CNI immunosuppressants were not associated in BK viremia.
Mengel et al[39] suggested particularly increased risk of BKV nephropathy with a combination of Tac and MMF. Similarly, Brennan et al[40] showed that viruria was most common with a drug combination of Tac-MMF (46%) and lowest with cyclosporine-MMF (13%). These authors also confirmed the association between viruria and subsequent BKV viremia. In addition, when surveillance for BK viremia was undertaken for the purposes of this study, a reduction in immunosuppression in response to detectable viremia resulted in reductions in viral load in 95% of patients, and without increased risk of rejection. A more recent randomized study also supports the role of immunosuppression in this context. In a study of 682 patients, the combination of Tac and MMF was associated with greater rates of viremia at 6 and 12 mo post-transplantation than the combination of cyclosporine and MMF (16.3% vs 10.6%, P = 0.048 and 12.1% vs 4.8%, P = 0.004 respectively). Cumulative steroid dose up to month 3 was also a risk factor for viremia in this study, highlighting the role of overall immunosuppression burden in this disease[41]. Clinical observations of campth therapy were made by Kayler et al[42], they administered campth to two patients with BKV viruria and one with nephropathy. In all cases, there was increased viral replication and one of the patients with viruria developed nephropathy. The authors concluded that campth treatment does not permanently remove immune cells that are able to respond against BKV and that the therapy does not prevent stop the clearance of BKV from the blood.
Recent attention has focused on the role of lytic antibody induction, and particularly the role of alemtuzumab (anti-CD52 monoclonal antibody; “Campath”) which is undergoing more recent widespread usage. A large study (“3C study”) demonstrated double the incidence of BKV infection with alemtuzumab compared with basiliximab induction. However, absolute incidence was low (8% vs 4%), and this difference was driven by BK viremia (7% vs 3%) rather than BKV nephropathy, the rates of which were very low in both alemtuzumab and basiliximab arms of the study (1% and 2% respectively)[43]. Conversely, it has been shown that alemtuzumab did not remain significant risk factor after the adjusted hazard ratio for each variable had been calculated[44].
A United States OPTN database review showed that there was an increased risk of BKV infection with an induction therapy using thymoglobulin (P < 0.0001)[44]. In a study by Ott et al[45] renal transplants patients, under either basiliximab (n = 22) or thymoglobulin (n = 27) treatment regimens, were assessed for complication in a mean follow up period of three years. Of the 27 patients treated with thymoglobulin, two developed BKVAN whereas no patients had a BKV infection when treated with basiliximab. However, CMV infections were observed in both patient cohorts, with four and three patients infected for basiliximab and thymoglobulin therapies, respectively. This indicated that treatments using thymoglobulin carry a greater risk of BKV infection to renal transplant patients post transplant.
Effect of HLA matching
The adaptive immune response to viral infection is dependent on T-cell recognition of viral antigen presented in the context of self-MHC. In other transplant settings, it has been shown that immune responses to viral antigen presented in the context of donor-derived MHC (in this case cytomegalovirus) do not develop[46]. The donor-derived nature of BKV may therefore impair the magnitude or timing of effective immune clearance. In keeping with this concept, a study by Lee et al[47] used a mouse model to show ineffective clearance of BKV in the context of MHC mismatching. Clinical data also supports this concept of increased HLA mismatch as a risk factor for BKVAN[48-50]. However, in contrast, a clinical study from Drachenberg et al[51] found lesser degrees of HLA matching was actually associated with maintained graft function in patients with established BKVAN. This led to the proposal that even though reduction in HLA matching would decrease the recipient’s ability to mount an effective immune response to BKV, there would be less tissue damage, thus reduced risk of graft loss. This in turn raises questions in regard to the mechanism of viral clearance in this context, and whether this is dependent on the T-cell response or other viral elimination mechanisms such as NK and NKT cell activation. Clinical data from other cohorts may also serve to clarify the current understanding.
Aside from HLA matching, the question arises as to whether BKV infection and nephropathy is associated with specific (donor or recipient) HLA alleles. Awadalla et al[50] found no such association(s), but another study from Bohl et al[31] found a possible link between HLA C7 and the severity of BKV infection. Although there was no association between BK viremia and either donor or recipient HLA-A, -B or -DR type, all 11 transplant recipients with persistent BK viremia received kidneys from HLA C7 negative donors, and 10 of these 11 recipients also lacked HLA C7. The possible mechanism underlying this observation is unclear. However, if confirmed, this raises implications for the relevance of HLA-C typing in transplantation, which is not currently recommended or undertaken, but which may identify individuals at greater risk of refractory infection.
Donor and recipient blood group
A fundamental feature of transplantation is the matching of blood groups of donor and recipient in order to avoid the risk of hyper-acute rejection. Nevertheless, with intensified preconditioning and antibody removal, blood group-incompatible transplantation is now commonplace[52]. However, Sharif et al[53] suggest a high rate of BKVAN in such patients. In a study of 62 blood group incompatible transplantations between 1998 and 2010, the risk of BKVAN was 17.7% (compared to 3% risk among blood group compatible patients). This data has been replicated in a different cohort by Bentall et al[54]. While we may infer this is due to desensitization and/or heightened immunosuppression for incompatible patients, the authors actually observed a lower risk among a cotemporaneous group of 221 HLA antibody incompatible transplants (5.9%, P = 0.008) who also underwent intensified preconditioning and received stronger induction therapy (ATG) compared to either blood group incompatible or compatible patients (Basiliximab). Therefore, pre-conditioning or heightened immunosuppression cannot be the sole explanation for this observation. Interestingly, the authors identified the lack of a typical accommodation-like phenotype (defined as C4d deposition in the absence of any micro-circulation inflammation) among blood-group incompatible transplant recipients with BKVAN compared to those without BKVAN (40.0% vs 75.8% respectively, P = 0.04). However, it is unclear from this data whether lack of accommodation-like phenotype development increases the risk for BKVAN or whether blood-group incompatible patients with BKVAN lose their accommodation-like phenotype but further studies are warranted to research this further.
OTHER RISK FACTORS
There are also a number of risk factors between the donor and recipient that can increase the risk of a BKV infection, including gender, race, age, diabetes mellitus or where the organ was sourced from a deceased donor[39,44,55,56].
CLINICAL FEATURES AND DIAGNOSIS OF BKVAN
The median time to clinically apparent BKVAN is within the first year after transplantation[57,58]. The recipient is characteristically asymptomatic, with the infection presenting as progressively worsening renal function, usually in the absence of significant or new-onset proteinuria[59]. This presentation generally results in a “for cause” biopsy, which shows the characteristic features of BKVAN. A number of histological grading systems have attempted to classify BKVAN[60-63], and whilst differences exist between these alternative systems, recurring themes are:
The separation into stages of BKVAN depending on the presence of viral infection in the absence of inflammation or significant chronic damage (Grade A), with inflammation dominating over chronic damage (Grade B), and with chronic damage (fibrosis and tubular atrophy) as a notable component, with or without inflammation (Grade C).
That prognosis is correlated with these stages of BKVAN, and especially with the presence of significant chronic damage (Grade C nephropathy).
In simultaneous biopsy cores, discordant findings (i.e., the lack of evidence of BKVAN in one of the cores) was found in around a third of cases. Of note, in the core without evidence of virus, interstitial inflammation and/or acute tubular injury were frequent findings (approximately 80%), raising implications for the clinical interpretation of kidney biopsy specimens where only one core is retrieved, where the sampling is inadequate, and where there is collateral evidence that BKVAN may be a diagnosis[64].
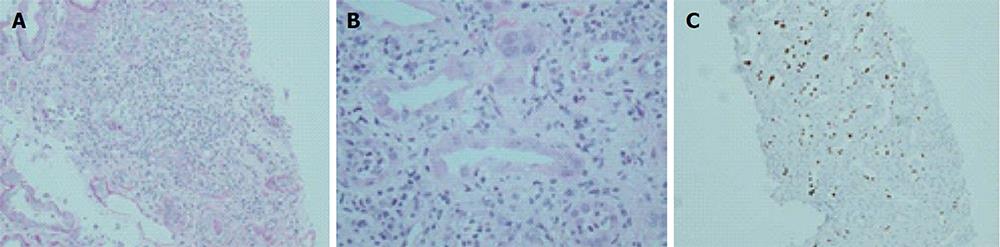
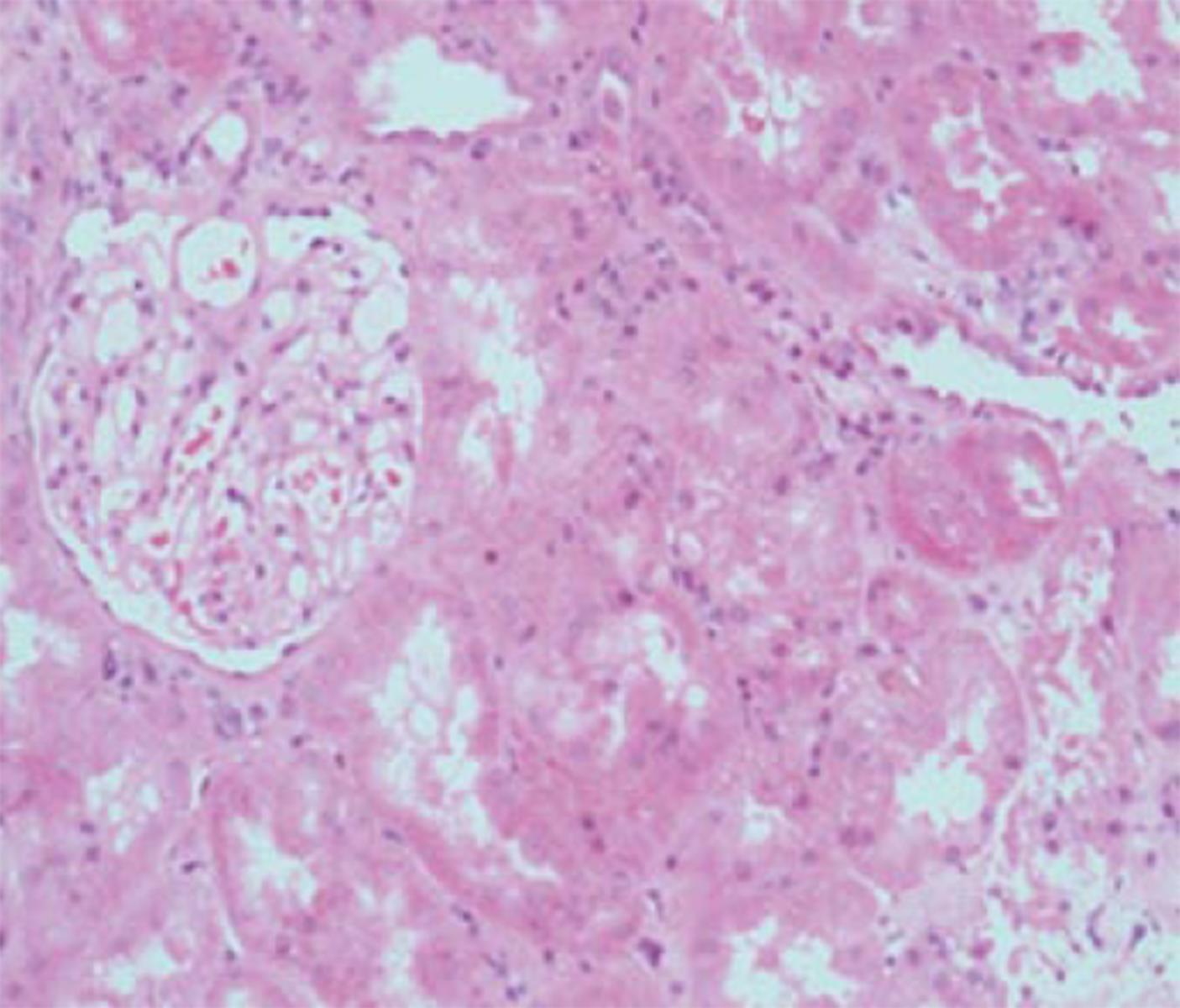
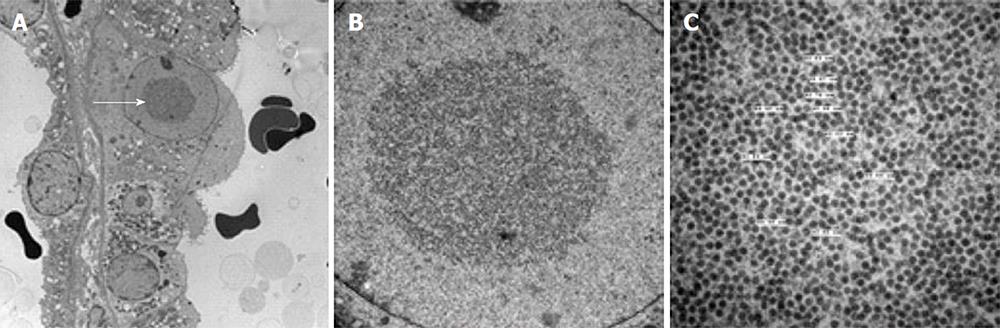
Whilst concurrent viremia is almost universal with the finding of BKVAN on microscopy, the magnitude of circulating viral load seems to have little or no relationship with the extent of nephropathy[64]. Representative examples of the histological appearances of BKVAN are shown in Figures 2-4.
Figure 2 Histological features of BK virus nephropathy by light microscopy.
A: Tubule-interstitial infiltrate and tubulitis classical for BKVN, but also compatible with any other form of interstitial nephritis such as acute cellular rejection; B: Higher power view of same biopsy sample, with characteristic viral inclusions seen within epithelial cells (circled); C: Positive SV40 immunoperoxidase staining on same specimen, confirming diagnosis of BKVN. BKVN: BK virus nephropathy.
Figure 3 Kidney with preserved tubular architecture, without significant chronic damage or interstitial inflammation, but with BK virus nephropathy confirmed by virtue of positive SV40 staining (as shown in insert taken from immunoperoxidase sample from same biopsy specimen).
Figure 4 Histological features of BK virus nephropathy by electron microscopy.
A: Electron microscopy evidence of viral inclusions (arrow) within epithelial cells, equivalent to those seen and circled in the light microscopy sample shown in Figure 2B; B: Higher power magnification of epithelial viral inclusions; C: Highest magnification demonstrating characteristic appearance and size (labelled) of BK virions.
For many years, BKVAN was confused with acute rejection, as both have the appearance of an “interstitial nephritis”. With more widespread recognition of BKVAN, the availability of blood and urine testing for viral load, and the utility of SV40 staining on biopsy samples, the pathological diagnosis of BKVAN has become more straightforward. However, acute rejection and BKVN are not mutually exclusive, and particularly in the period following BKVAN treatment (see below), the two may coincide, and it may be unclear which represents the dominant process. Despite efforts, there remains no consensus in regard to the most accurate way to separate these entities, although the presence of macro- or micro-vascular inflammation points to rejection as a component at least.
Aside from the classical presentation described above, BKVAN may present in a “subclinical” manner, as is seen with other forms of transplant-related renal injury including “subclinical rejection”. Recent clinical studies have used protocol renal biopsies to test for the presence of BKV in this setting. Buehrig et al[65] concluded that allograft biopsy allowed earlier detection of BKVAN, and the potential for this to enable earlier treatment, although this proposed approach has not yet been evaluated. Whether such a strategy translates into improved overall clinical outcome (and justifies the risk, inconvenience and cost of the biopsy) remains to be seen.
SCREENING FOR BKV INFECTION
Established BKV screening methods include testing urine for decoy cells, viral particles by electron microscopy, and viral DNA by PCR. However, plasma PCR for detection of viremia remains a more common approach to screening[66]. It has also been suggested that circulating viral loads above certain thresholds (approximately > 4 log copies/mL) can be considered presumptive of nephropathy even in the absence of histological evidence (see above). Whilst inter-laboratory standardization of such PCR assays is awaited, such discrete values remain subject to interpretation by individual centres.
A more recent study carried out by Singh et al[67] investigated whether the qualitative detection of three-dimensional aggregates of polyomavirus (Haufen crystals) within a patient’s urine could be used as a diagnostic test for patients BKVAN. Of 21 patients known to have BKVAN 77 of the 143 samples taken contained Haufen. During follow up, the presence or absence of Haufen matched the course of renal disease. All control samples (194) were negative. The predictive values of Haufen for BKVAN were 97% for positive and 100% for negative. This leads to the conclusion that Haufen testing in urine is a more accurate approach than detection of viral DNA in urine or plasma, although the reproducibility and generalizability of these findings requires further clarification.
Finally, the role of urine profiling for VP1 (BK capsid protein) mRNA is under investigation, with exploratory[68] and validation studies[69] suggesting potential utility in predicting nephropathy, albeit in small patient numbers Further generalisation may yet provide additional important information in this regard.
CURRENT AND FUTURE TREATMENTS FOR BKV INFECTION
The first step in the treatment of BKV infection is reduction in immunosuppression. Certainly, this approach is not disputed for cases of nephropathy, although evidence guiding the order with which the component immunosuppressant are withdrawn is lacking. Less clear is whether immunosuppression should be altered in the face of viremia and in the absence of overt nephropathy. This question was addressed in a large and important study of 200 patients by Brennan et al[40], where reduction in immunosuppression in response to detectable viremia on protocolised plasma samples resolved 95% cases of viremia with no signal towards graft rejection, dysfunction or loss. Other smaller studies also support this approach of “pre-emptive” therapy[70]. The efficacy of this strategy is also supported by Saad et al[71]. In this study, MMF and/or Tac doses were reduced for the patients, who in this study were not restricted to those with viremia (24 patients: 66% BKVN, 34% Viremia). Overall, a decline in BKV viral load was seen. However, three patients developed acute cellular rejection, albeit with successful treatment with intravenous bolus steroids. One patient experienced BKVN relapse during pregnancy and lost the graft. Seventeen patients maintained or improved their graft function following this reduction in immunosuppression. In summary, the evidence from these studies support decreasing immunosuppression as the first line of treatment for patients that present with BKVAN, and possibly with detectable viremia, although clearly the risk of rejection needs careful consideration. Controlled studies are required to solidify these findings, although in the meantime the recommendation from Kidney Disease: Improving Global Outcomes (KDIGO) expert panel is to reduce immunosuppression when plasma viral loads exceed a certain threshold (10000 copies per mL, whilst accounting for inter-laboratory variation)[72].
BKV interacts with the AKT/mammalian target of rapamycin (mTOR) pathway[73]. Everolimus and sirolimus are examples of mTOR inhibitors (mTOR-i)[73-76]. Everolimus was observed by Polanco et al[75] to increase renal function in BKVAN positive patients that had their treatments converted from tacrolimus to everolimus, with a suspension of mycophenolate. This study involved 15 patients, all presenting with BKVAN of which 9 underwent the immunosuppressant conversation. The serum creatinine of these patients decreased from 2 (± 0.21) mg/dL at the time of conversion to 1.6 (± 0.39) mg/dL at the final follow up. BK viremia became negative in 5 of the 9 patients and the remain 4 had a > 95% decrease in BKV. This decrease in BKVAN is also seen in conversion to sirolimus. In a recent single centre retrospective study by Tohme et al[77], patients were either placed on a tacrolimus or sirolimus based immunosuppression therapy. If the patients were < 62 years old they were converted from tacrolimus to sirolimus. Clinically significant BK viremia fell when converting from tacrolimus (P = 0.04) to sirolimus (P = 0.02), 17.9% to 4.3%, respectively. However, the hazard ratio for the male gender was also associated with the incidence of BK viremia (P = 0.03). Discontinuation of the sirolimus treatment occurred in 34% of patients due to various side effects. Thus, the use of mTOR-i as a treatment option of not only provides immunosuppression, reducing the risk of acute rejection, but also due to its behaviour as a metabolic pathway inhibitor for BKV it can also aid in the reduction in viral load, hence a lower risk of developing BKVAN.
The next question is whether detection of virus in urine (rather than waiting for it to appear in plasma) might represent a more efficient screening and intervention biomarker. In this regard, the clinical data is less optimistic. Specifically a series of retrospective studies have suggested increased rates of (or episodes of) acute rejection in the presence of viruria, even in the absence of viremia[78-80]. Whilst a proportion of these episodes were likely a response to immunosuppression weaning, there were clearly others which were unrelated, and which may potentially be a manifestation of low grade viral reactivation and inflammation inciting a secondary alloimmune response. However, irrespective of the mechanism, these observations (although limited by study design and interpretation) suggest that immunosuppression weaning in the context of viruria should not be recommended until and unless further information comes to light.
Even with successful treatment with immunosuppression reduction, the timeline of viral clearance is variable, although the reported median time to complete plasma clearance is 9 mo[81]. Serial renal histopathology following treatment is interesting, although reports are limited due to the nature of such studies. Of relevance though, the report from Menter et al[81] suggests that a self-limited “interstitial nephritis” is common during the phase of viral clearance and that this may represent an appropriate antiviral response rather than alloimmunity.
Specific antiviral therapy is generally used as a secondary line of treatment for BKVAN, and although an attractive approach, the role(s) of multiple agents remain unproven and unclear. Although better recognized as antibacterial agents, the quinolone antibiotics do display in vitro activity against polyoma viruses. Arroyo et al[82] retrospectively investigated the effects of ciprofloxacin on patients with BK viruria and viremia, after clinical failure with prior reduction in immunosuppression. The study showed that there were no adverse effects of ciprofloxacin and that out of the nine patients that received the treatment, three showed complete clearance of the virus and another three had the viral load in the plasma reduced by ≥ 50%. Unfortunately, a subsequent randomized controlled trial of 3 mo levofloxacin (from post-operative day 5) in 154 kidney transplant recipients showed no effect on the development of BKV viruria compared with the control group (29% vs 33%)[83]. In addition, an increased incidence of antibiotic resistance to bacterial isolates, and also a signal towards increased tendonitis was seen in the levofloxacin treated arm. Observational data also comes from Jung et al[84], this time studying the effect of leflunomide on biopsy-proven BKVN in paediatric patients. Tac dosage was reduced and leflunomide and intravenous immunoglobulin treatment was instituted. Viral load then decreased and remained below 100 copies/mL over an 18 mo period with no loss of renal function, from a value of 474140 copies/mL of BKV viral load in patient serum. Intravenous immunoglobulin in the absence of adjunctive antiviral agents has also been reported as a treatment for BKV infection[85], and observational data supports a potential role for another antiviral agent, cidofovir[86]. Yet in the absence of more robust data, few conclusions can be drawn; it is also relevant to highlight the conclusion of a 2010 systematic review, which found no evidence of an effect for either leflunomide or cidofovir in treating this infection[87]. Adoptive cell therapy in the context of transplant-associated infections is perhaps best known in the context of EBV and post-transplant lymphoproliferative disease. Whilst no data exists for this strategy in the setting of BKV infection, it is possible this approach might hold promise. In the context of infection with the related polyoma virus, JC virus, a report describes a positive clinical response to this form of therapy in a patient following hematopoietic cell transplantation[88]. Intuitively, the same approach may be worthwhile for BKV infection.
CONCLUSION
This review focuses on the pathogenesis, risk factors, presentation and treatment of BKV infection in the setting of kidney transplantation, which remains clearly the most common scenario in which this polyoma virus infection is encountered. Whilst important understanding has accumulated over recent years, and has certainly led to improved recognition of this infection and clinical management of patients, there is much more to be discovered and studied. We believe the most important tasks at hand are now to: (1) more accurately risk-stratify patients prior to (and also following) transplantation, with aim of individualizing immunosuppression and reducing the risk of (or duration/consequences of) BKV infection. This may include developing understanding of, and then monitoring strategies for, cell-mediated immune responses to this virus, which can then be interpreted in combination with peripheral blood and renal biopsy measures of viral load and (admittedly currently unavailable) standardised assays of alloreactivity to garner a more “holistic” understanding of the overall and antigen-specific immunosuppressive burden; and (2) to enhance the sizeable observational experience of treatment strategies with controlled studies of immunosuppression weaning and/or adjunctive antiviral agents. It is not unconceivable that with such refined approaches BKV infection (whilst not eradicated) may present a far less sinister complication for kidney transplant patients in the future.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Esposito P, Friedman EA, Moens U, Salvadori M S- Editor: Ji FF L- Editor: A E- Editor: Yan JL