Published online Oct 24, 2017. doi: 10.5500/wjt.v7.i5.250
Peer-review started: April 28, 2017
First decision: June 16, 2017
Revised: August 25, 2017
Accepted: September 12, 2017
Article in press: September 13, 2017
Published online: October 24, 2017
Processing time: 178 Days and 6.3 Hours
High-dose therapy followed by autologous hematopoietic stem cell (HSC) transplant is considered standard of care for eligible patients with multiple myeloma. The optimal collection strategy should be effective in procuring sufficient HSC while maintaining a low toxicity profile. Currently available mobilization strategies include growth factors alone, growth factors in combination with chemotherapy, or growth factors in combination with chemokine receptor antagonists; however, the optimal strategy has yet to be elucidated. Herein, we review the risks and benefits of each approach.
Core tip: Obtaining an adequate peripheral blood stem cell yield is essential for the successful outcome of autologous hematopoietic stem cell transplant in multiple myeloma. While growth factor mobilization continues to be largely successful, suboptimal collection rates have been noted, particularly as use of novel therapies continue to increase. Chemomobilization remains toxic and has not been associated with better disease control. The newest mobilizing agent, plerixafor, is capable of overcoming suboptimal mobilization even in patients who are at a high risk of mobilization failure. Each mobilization strategy should be selected based on patient specific variables as well as risk factors for mobilization failure.
- Citation: Wallis WD, Qazilbash MH. Peripheral blood stem cell mobilization in multiple myeloma: Growth factors or chemotherapy? World J Transplant 2017; 7(5): 250-259
- URL: https://www.wjgnet.com/2220-3230/full/v7/i5/250.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i5.250
High-dose therapy followed by autologous hematopoietic stem cell (HSC) transplant (auto-HCT) is considered standard of care for eligible patients with multiple myeloma (MM). MM remains the most common indication for auto-HCT, accounting for over 6000 transplants in the United States alone in 2013[1]. Auto-HCT has been shown to prolong progression-free survival and overall survival in patients with MM[2-4], a benefit that has been maintained even after the availability of immunomodulatory drugs such as thalidomide and lenalidomide[5,6], and proteasome inhibitors like bortezomib. Mobilization and collection of an optimal number of HSC is a fundamental requirement for auto-HCT. The optimal collection strategy should be effective in procuring sufficient HSC while maintaining a low toxicity profile. Currently available mobilization strategies include growth factors alone, growth factors in combination with chemotherapy, or growth factors in combination with chemokine receptor antagonists; however, the optimal strategy has yet to be elucidated. Herein, we review the data surrounding each approach.
Historically, bone marrow (BM) was used as the sole source of HSC for transplantation[7,8]. However, the ability to mobilize HSC to peripheral blood (PB) has eliminated the risk of general anesthesia, intubation, and painful aspirations associated with BM harvesting. Peripheral blood stem cell (PBSC) collection can be performed in the outpatient setting with a shorter recovery time. Additionally, use of PBSC reduces time to hematopoietic reconstitution, hospital stay, and need for transfusions[9-11]. Consequently, PB has largely replaced BM as the source of HSC for auto-HCT[12].
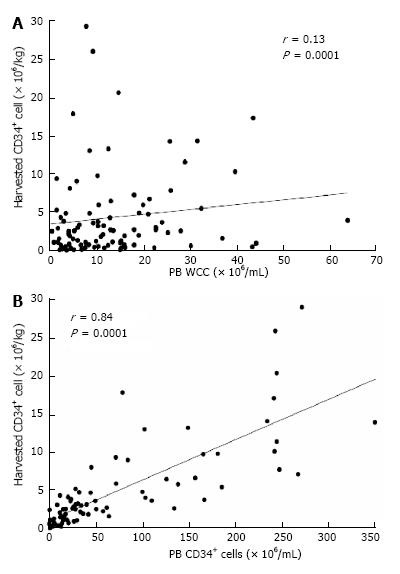
The number of CD34 expressing mononuclear cells in PBSC collection correlates well with engraftment kinetics and thus is used as a surrogate marker of HSC[13-16] (Figure 1). A dose of > 2 million CD34+ cells per kilogram (cells/kg) is considered the minimum acceptable dose for timely engraftment[17]. However, larger cell doses have been associated with a more rapid time to platelet and neutrophil recovery[18,19] and therefore ≥ 3-5 million CD34 cells/kg is considered an optimal target[20,21].
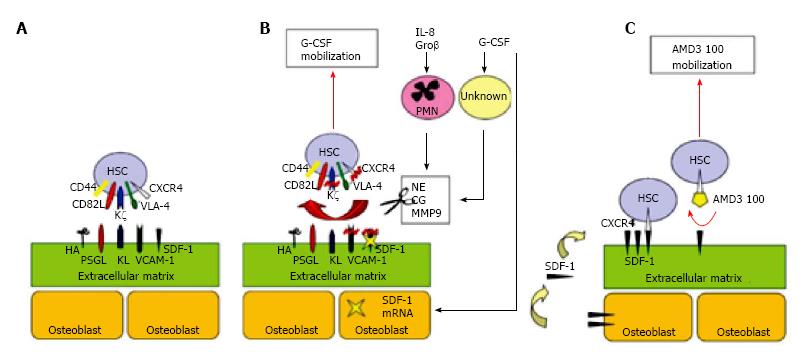
HSC primarily reside in the BM and account for 1%-4% of all mononuclear cells[13,15,22]. Retention of HSC in the BM is dependent on interactions between cell adhesion molecules on the surface of HSC, such as chemokine receptor 4 and very late antigen 4 (VLA4), and BM stromal factors, such as vascular cell adhesion molecule (VCAM-1) and stromal cell-derived factor-1 (SDF-1)[23]. Mobilization of HSC from BM to PB is the result of induced chemical disruption of these interactions between HSC and BM stroma. Cytokines, such as granulocyte-colony stimulating factor (G-CSF), and chemotherapy drugs like cyclophosphamide play an important role in releasing HSC from their niches in the BM[23-25] (Figure 2).
Historically, growth factors alone have been largely successful in mobilizing an adequate cell yield in MM patients undergoing auto-HCT[26,27] (Table 1). G-CSF has well established kinetics as well as favorable toxicity and cost profiles[28-30] but has been associated with suboptimal mobilization in over 20% of MM patients[31-33]. Data regarding a dose-response relationship between G-CSF and CD34+ cell yield is discordant but doses up to 40 μg per kilogram per day (μg/kg per day) have been studied[34-36]. A widely accepted G-CSF dose for PBSC mobilization is 10 μg/kg per day as single or divided doses.
| Ref. | Disease | Collection strategy | n | CD34+ yield (× 10-6 cell/kg): Median (range) | Failure n (%) |
| Desikan et al[26] | MM | G-CSF 10-16 μg/kg per day | 117 | 6.2 (0.6-34.1) | NR |
| Kröger et al[27] | MM | G-CSF 10-24 μg/kg per day | 25 | 3.8 (0.3-17) | 3 (12) |
| Popat et al[31] | MM | G-CSF | 302 | NR | 9% |
| Pusic et al[90] | MM | G-CSF 10 μg/kg per day | 384 | 4.6 | 24 (6.3) |
| NHL HD | G + C | 17 | 8.5 | 1 (5.9) | |
| Weaver et al[34] | BC | G-CSF 10 μg/kg per day | 14 | 0.6 (0.1-2.8) | NR |
| G-CSF 20 μg/kg per day | 13 | 1 (0.2-5.2) | |||
| G-CSF 30 μg/kg per day | 14 | 2.4 (0.6-6.8) | |||
| G-CSF 40 μg/kg per day | 14 | 1.4 (0.1-4.8) | |||
| Weisdorf et al[42] | NHL | GM-CSF 250 μg/m2 per day | 16 | 4.78 (3.02-10.68) | 0 |
| HD | G-CSF 250 μg/m2 per day | 15 | 8.01 (3.17-29) | 0 | |
| Spitzer et [41] | BC GCT | GCSF 10 mcg/kg per day | 26 | 21.45 (1.63-182.91) | NR |
| NHL HD | GCSF 10 mcg/kg per day + | 24 | 13.33 (0.56-102.08) | ||
| MM | GM-CSF 5 mcg/kg per day | ||||
| Hosing et al[39] | MM | PEG 12 mg × 1 | 19 | 8.4 (4.1-15.8) | 0 |
| G-CSF 10 μg/kg per day | 8 | 8.1 (5.17-19.2) | 0 |
Other growth factors such as granulocyte-macrophage- colony stimulating factor (GM-CSF), pegylated G-CSF, and tbo G-CSF have also been studied for PBSC mobilization in MM patients[37-42]. When G-CSF was compared to GM-CSF in MM patients, CD34+ cell yield was similar between the two groups, but GM-CSF-mobilized patients had a longer duration of neutropenia[43]. In-vitro data suggest that combination of G-CSF + GM-CSF may improve PBSC yield[44,45], but clinical trial data has not found a significant difference in CD34+ cell yield or time to hematopoietic recovery with combination therapy[41].
Pegylated (PEG) filgrastim, a covalent conjugate of G-CSF and monomethoxy-polyethylene glycol, has a terminal half-life of 15-80 h, which enables less frequent administration compared to G-CSF. Given as a single 12 mg injection followed by PBSC collection, all MM patients who received PEG filgrastim successfully collected their target CD34+ cells/kg dose[39]. Similarly, a multi-dose regimen of PEG filgrastim had a higher CD34+ cells yield on first apheresis compared to G-CSF, but no differences in overall cell yield was observed[46]. Its convenient dosing schedule makes it an attractive option for PBSC mobilization.
Tbo-filgrastim is a non-glycosylated recombinant methionyl human G-CSF manufactured using the bacterium strain E. coli K802[47]. While not FDA approved for stem cell mobilization, retrospective data in MM patients found no difference in overall cell yield, number of apheresis sessions required for collection, nor need for rescue therapy with plerixafor[38,48].
Transient circulation of PBSC occurs during the recovery phase of chemotherapy-induced pancytopenia[22,49,50] and is augmented by growth factor support[22] (Table 2). This process, chemomobilization (CM), provides not only higher cell yields than G-CSF alone, but also affords anti-myeloma activity[32,51-54]. Cyclophosphamide (CY) 2-4 g/m2, either alone or in combination with other chemotherapeutic agents, is commonly used in CM and has been a successful mobilization technique even in patients who underwent induction therapy with novel agents[31,55-59]. The impact of increased doses of CY on PBSC yields has shown conflicting results but was consistently associated with a longer duration of neutropenia as well as the use of antibiotics and blood products[54,60-64]. No additional impact on cell yield or objective response rate has been seen with the use of combination chemotherapy followed by growth factor[55,65] (Table 3). Furthermore, despite the potential benefit of cytoreduction, CM has not been associated with a better disease control or survival in MM[32,51,52,66-68].
| Ref. | Disease | Collection strategy | n | CD34+ yield (× 10-6 cell/kg): Median (range) | Failure rates n (%) |
| Weaver et al[91] | MM ML BC | G-CSF 6 μg/kg per day | 49 | 12 (0.1-54) | 2 (4.1) |
| GM-CSF 250 μg/m2 per day | 49 | 5.4 (0.02-64) | 4 (8.2) | ||
| GM-CSF × 5 d then G-CSF 6 μg/kg per day | 52 | 10.5 (0.4-96) | 1 (1.9) | ||
| Arora et al[43] | MM | G-CSF 250 μg/m2 per day | 35 | 16.4 (1.1-71.7) | NR |
| GM-CSF 250 μg/m2 per day | 37 | 12.8 (0.4-94.5) | |||
| Tricot et al[46] | MM | PEG 6 mg q7d × 2 | 97 | NR; no difference | NR |
| G-CSF 10 μg/kg per day | 140 | ||||
| Fruehauf et al[92] | MM | PEG 12 mg × 1 | 26 | 9.7 (4.9-40.5) | 3 (11.5) |
| Steidl et al[93] | MM | PEG 12 mg × 1 | 12 | 7.4 (4.9-38) | 0 |
| G-CSF 8.5 μg/kg per day | 12 | 10.8 (5-87) | 0 |
| Ref. | Collection strategy | n | CD34+ yield (× 10-6 cell/kg): median (range) | Hospital days: median (range) | Infection (%) | Transfusions (%) platelet/PRBC |
| Desikan et al[32] | CY 6 g/m2 + G-CSF 6 μg/kg per day | 22 | 33.4 (NR) | No difference | 18 | 86/86 |
| G-CSF 16 μg/kg per day | 22 | 5.8 (NR) | 0 | 18/55 | ||
| Alegre et al[51] | CY 4 g/m2 + GM-CSF | 18 | 6.8 (1.8-34.8) | 21 (16-34) | 11 | 33.3/27.7 |
| G-CSF 10 μg/kg per day | 22 | 4.85 (2.1-10.05) | 0 | 0 | 0/0 | |
| Fitoussi et al[60] | CY 7 g/m2 + HGF | 74 | 8.6 (0.4-166) | 15 (9-34) | 17.6 | 75.7/94.6 |
| CY 4 g/m2 + HGF | 42 | 13.4 (0.7-66.8) | 22 (13-55) | 16.7 | 26.2/52.4 | |
| Jantunen et al[61] | CY 4 g/m2 + G-CSF 5-10 μg/kg per day | 32 | 4.9 (0.8-47.4)1 | 9 (6-14) | NR | 34/53 |
| CY 1.2-2 g/m2 + G-CSF 5 μg/kg per day | 42 | 5.6 (0.9-19)1 | 5 (3-12) | NR | 0/28 | |
| Gojo et al[65] | CY 4.5 g/m2 + G-CSF | 28 | 21.38 (0-106.8) | 8 (4-24) | 25 | 57/NR |
| CY 4.5 g/m2 + VP-16 + G-CSF | 49 | 22.39 (0-114.71) | 7 (3-68) | 53 | 67/NR | |
| Hamadani et al[94] | CY 3-4 g/m2 + G-CSF | 55 | 16.6 (2-82) | 4 (1-9) | NR | 21.8/34.5 |
| CY 1.5 g/m2 + G-CSF | 68 | 7.5 (0-18) | 3 (1-5) | NR | 2.9/8.8 | |
| Hiwase et al[95] | CY 3-4 g/m2 + G-CSF | 26 | 7.71 | 7 (3-22) | 19 | No difference |
| CY 1-2 2 g/m2 + G-CSF | 61 | 5.17 | 6 (3-18) | 5 |
The newest mobilizing agent, plerixafor, rapidly and reversibly inhibits chemokine receptor CXCR4 on HSC, thereby preventing the binding of SDF-1a to CXCR4. Synergistic effect on PBSC mobilization is observed when plerixafor is given in combination with G-CSF[69,70]. A phase III randomized, placebo controlled trial in MM patients compared mobilization with plerixafor + G-CSF to placebo + G-CSF. Use of plerixafor resulted in an increase in proportion of patients that were able to collect a cell yield of ≥ 6 × 106/kg with fewer apheresis procedures compared to the G-CSF only group. Transplant outcomes were similar between groups[71]. Plerixafor can overcome suboptimal mobilization seen with prolonged prior lenalidomide therapy and other conventional chemotherapy agents[72,73]. Following failed attempts to mobilize, MM patients received a combination of G-CSF and plerixafor. In this population, at least 70% of patients were able to achieve a sufficient PBSC yield, without any evidence of tumor mobilization[73,74]. Plerixafor is successful when used as the initial mobilization strategy but at an increased drug acquisition cost and in patients that presumably could have attained an appropriate cell yield with G-CSF alone[75,76].
Risk adaptive strategies use initial mobilization with G-CSF alone and utilize plerixafor only in patients whose PB CD34+ count on day 4 is less than a predetermined threshold (10 × 106/L-10 × 109/L). Such strategies are associated with fewer mobilization failures when compared to traditional mobilization methods and appear to be cost effective[76-79]. Additional methods of cost reduction, namely the use of tbo-filgrastim, in combination with plerixafor has been studied. Prospective data in MM patients found similar overall cell yields without any mobilization failures[80].
Mobilization failure is generally defined as the inability to procure 2 × 106 CD34+ cells/kg in 4 apheresis sessions. Despite recent advances in PBSC collection strategies, failure to obtain an adequate cell dose continues to delay and preclude auto-HCT in otherwise suitable transplant candidates. Factors associated with inadequate HSC mobilization in MM patients include: Thrombocytopenia[81], age > 60 years[36,58,82], extensive treatment course[17], prior radiotherapy, prior exposure to alkylating agents[17,83], and prolonged use of lenalidomide[20,21,31,84,85]. Such factors have been incorporated in consensus guidelines on stem cell mobilization (Table 4).
| Strategy | Recommendations |
| Mobilization | |
| G-CSF alone | Limit use to patients |
| Treated with ≤ 1 line of therapy | |
| Never exposed to melphalan | |
| Received ≤ 4 cycles of lenalidomide | |
| Use doses from 10-16 μg/kg per day | |
| Monitor PB CD34+ count | |
| Chemomobilization + G-CSF | Limit to patients who have not adequately responded to salvage therapy |
| Plerixafor | Suitable for all patients particularly if goals include |
| Highest cell yield obtainable | |
| Fewer apheresis sessions | |
| Remobilization | |
| Plerixafor | P + G-CSF or P + CM + G-CSF |
| Chemomobilization | Acceptable in patients who failed cytokine mobilization |
| Bone marrow harvest | Use as third-line option in patients in whom ASCT is compelling |
Lenalidomide’s impact on cell yield is of particular concern due to its common use in frontline therapy[86]. While known to cause neutropenia and thrombocytopenia, the exact mechanism of lenalidomide induced myelosuppression is not fully known. In one study, lenalidomide was associated with a significant decrease in expression of transcription factor PU. 1, which is critical for myeloid maturation[87]. In another study, lenalidomide-treated patients were found to have decreased BM CD34+ cells after six cycles of therapy[88]. This supports the literature that identifies lenalidomide as a risk factor for suboptimal stem cell collection and suggests that transplant eligible patients receiving lenalidomide should proceed to mobilization as early as feasible.
Despite identification of risk factors for poor mobilization, predictive algorithms have not correctly identified poor mobilizers[89]. The best predictor of adequate CD34+ cell collection is the pre-collection PB CD34+ cell count. A strong correlation exists with PB CD34+ cell count and the final CD34+ cell collection (Figure 1). PB CD34+ count ≥ 20 × 103 CD34+ cells/mL was associated with an adequate HSC collection in 94% of patients[16,90].
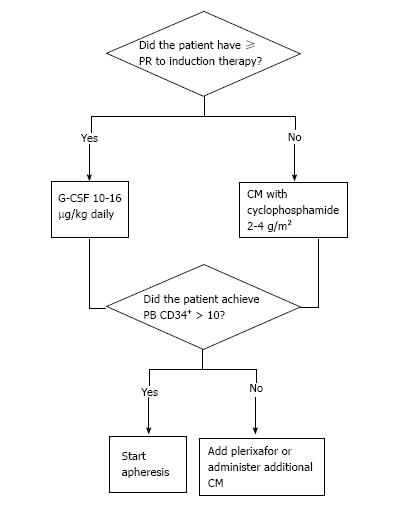
In summary, obtaining an adequate PBSC yield is essential for the successful outcome of auto-HCT in MM. Each mobilization strategy reviewed here has its own advantages and disadvantages (Table 5) and should be selected based on patient specific variables. Current practice at the authors’ institution is detailed in Figure 3; however, practitioners should be cognizant of risk factors for mobilization failure and utilize appropriate algorithms to optimize stem cell collection.
| Mobilization strategy | Advantages | Disadvantages |
| Growth factor | Cost effective | No anti-myeloma effect |
| Successful mobilization in most patients | Multiple injections and collections | |
| Predictable schedule | Potential sub-optimal yield | |
| CM | Anti-myeloma effect | Cytopenias |
| Increased cell yield | Infection risk | |
| Fewer apheresis sessions | Hospital admission | |
| Potential transfusion requirement | ||
| Unpredictable count recovery | ||
| Plerixafor | Rapid kinetics | Higher drug cost |
| Increased cell yield | ||
| Fewer apheresis sessions |
| 1. | Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation. 2014;CIBMTR Summary Slides Available from: http://www.cibmtr.org. |
| 2. | Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2135] [Cited by in RCA: 2062] [Article Influence: 68.7] [Reference Citation Analysis (5)] |
| 3. | Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ; Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1422] [Cited by in RCA: 1399] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 4. | Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, Lauta VM, Bergonzi C, Barbui A, Caravita T. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, Pezzatti S, Caravita T, Cerrato C, Ribakovsky E. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 619] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 6. | Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, Siniscalchi A, Magarotto V, Pour L, Carella A. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 7. | Körbling M, Freireich EJ. Twenty-five years of peripheral blood stem cell transplantation. Blood. 2011;117:6411-6416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Kessinger A, Sharp JG. The whys and hows of hematopoietic progenitor and stem cell mobilization. Bone Marrow Transplant. 2003;31:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Beyer J, Schwella N, Zingsem J, Strohscheer I, Schwaner I, Oettle H, Serke S, Huhn D, Stieger W. Hematopoietic rescue after high-dose chemotherapy using autologous peripheral-blood progenitor cells or bone marrow: a randomized comparison. J Clin Oncol. 1995;13:1328-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 178] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Henon PR, Liang H, Beck-Wirth G, Eisenmann JC, Lepers M, Wunder E, Kandel G. Comparison of hematopoietic and immune recovery after autologous bone marrow or blood stem cell transplants. Bone Marrow Transplant. 1992;9:285-291. [PubMed] |
| 11. | To LB, Roberts MM, Haylock DN, Dyson PG, Branford AL, Thorp D, Ho JQ, Dart GW, Horvath N, Davy ML. Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant. 1992;9:277-284. [PubMed] |
| 12. | Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: 2011 CIBMTR summary slides. Available from: http://www.cibmtr.org. |
| 13. | Andrews RG, Singer JW, Bernstein ID. Monoclonal antibody 12-8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood. 1986;67:842-845. [PubMed] |
| 14. | Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988;81:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 373] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157-165. [PubMed] |
| 16. | Armitage S, Hargreaves R, Samson D, Brennan M, Kanfer E, Navarrete C. CD34 counts to predict the adequate collection of peripheral blood progenitor cells. Bone Marrow Transplant. 1997;20:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Tricot G, Jagannath S, Vesole D, Nelson J, Tindle S, Miller L, Cheson B, Crowley J, Barlogie B. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995;85:588-596. [PubMed] |
| 18. | Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, Gooley T, Demirer T, Schiffman K, Weaver C. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 500] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, West W. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961-3969. [PubMed] |
| 20. | Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, Shaughnessy P, Snyder E, Bensinger W, Copelan E. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 21. | Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, Kumar S, Munshi NC, Dispenzieri A, Kyle R. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia. 2009;23:1904-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni AM. Circulation of CD34+ hematopoietic stem cells in the peripheral blood of high-dose cyclophosphamide-treated patients: enhancement by intravenous recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1989;74:1905-1914. [PubMed] |
| 23. | Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Liu F, Poursine-Laurent J, Link DC. The granulocyte colony-stimulating factor receptor is required for the mobilization of murine hematopoietic progenitors into peripheral blood by cyclophosphamide or interleukin-8 but not flt-3 ligand. Blood. 1997;90:2522-2528. [PubMed] |
| 25. | Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020-3027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 392] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Desikan KR, Tricot G, Munshi NC, Anaissie E, Spoon D, Fassas A, Toor A, Zangari M, Badros A, Morris C. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol. 2001;112:242-247. [PubMed] |
| 27. | Kröger N, Zeller W, Hassan HT, Krüger W, Renges H, Hummel K, Gutensohn K, Lölliger C, Zander AR. Successful mobilization of peripheral blood stem cells in heavily pretreated myeloma patients with G-CSF alone. Ann Hematol. 1998;76:257-262. [PubMed] |
| 28. | Neupogen® [package insert]. Thousand Oaks, CA. Amgen Pharmaceuticals Inc;. 2016; Available from: http://pi.amgen.com/united_states/neupogen/neupogen _pi_hcp_english.pdf. |
| 29. | Grigg AP, Roberts AW, Raunow H, Houghton S, Layton JE, Boyd AW, McGrath KM, Maher D. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 1995;86:4437-4445. [PubMed] |
| 30. | Stroncek DF, Clay ME, Petzoldt ML, Smith J, Jaszcz W, Oldham FB, McCullough J. Treatment of normal individuals with granulocyte-colony-stimulating factor: donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36:601-610. [PubMed] |
| 31. | Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P, Shpall E, McMannis J, Körbling M, Alousi A. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, Munshi N, Singhal S, Mehta J, Tindle S. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Pozotrigo M, Adel N, Landau H, Lesokhin A, Lendvai N, Chung DJ, Chimento D, Riedel E, Chen X, Reich L. Factors impacting stem cell mobilization failure rate and efficiency in multiple myeloma in the era of novel therapies: experience at Memorial Sloan Kettering Cancer Center. Bone Marrow Transplant. 2013;48:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Weaver CH, Birch R, Greco FA, Schwartzberg L, McAneny B, Moore M, Oviatt D, Redmond J, George C, Alberico T. Mobilization and harvesting of peripheral blood stem cells: randomized evaluations of different doses of filgrastim. Br J Haematol. 1998;100:338-347. [PubMed] |
| 35. | Martínez C, Urbano-Ispizua A, Marín P, Merino A, Rovira M, Carreras E, Montserrat E. Efficacy and toxicity of a high-dose G-CSF schedule for peripheral blood progenitor cell mobilization in healthy donors. Bone Marrow Transplant. 1999;24:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | de la Rubia J, Bladé J, Lahuerta JJ, Ribera JM, Martínez R, Alegre A, García-Laraña J, Fernández P, Sureda A, de Arriba F. Effect of chemotherapy with alkylating agents on the yield of CD34+ cells in patients with multiple myeloma. Results of the Spanish Myeloma Group (GEM) Study. Haematologica. 2006;91:621-627. [PubMed] |
| 37. | Bruns I, Steidl U, Kronenwett R, Fenk R, Graef T, Rohr UP, Neumann F, Fischer J, Scheid C, Hübel K. A single dose of 6 or 12 mg of pegfilgrastim for peripheral blood progenitor cell mobilization results in similar yields of CD34+ progenitors in patients with multiple myeloma. Transfusion. 2006;46:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 38. | Elayan MM, Horowitz JG, Magraner JM, Shaughnessy PJ, Bachier C. Tbo-Filgrastim versus Filgrastim during Mobilization and Neutrophil Engraftment for Autologous Stem Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:1921-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Hosing C, Qazilbash MH, Kebriaei P, Giralt S, Davis MS, Popat U, Anderlini P, Shpall EJ, McMannis J, Körbling M. Fixed-dose single agent pegfilgrastim for peripheral blood progenitor cell mobilisation in patients with multiple myeloma. Br J Haematol. 2006;133:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Publicover A, Richardson DS, Davies A, Hill KS, Hurlock C, Hutchins D, Jenner MW, Johnson PW, Lamb J, Launders H. Use of a biosimilar granulocyte colony-stimulating factor for peripheral blood stem cell mobilization: an analysis of mobilization and engraftment. Br J Haematol. 2013;162:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Spitzer G, Adkins D, Mathews M, Velasquez W, Bowers C, Dunphy F, Kronmueller N, Niemeyer R, McIntyre W, Petruska P. Randomized comparison of G-CSF + GM-CSF vs G-CSF alone for mobilization of peripheral blood stem cells: effects on hematopoietic recovery after high-dose chemotherapy. Bone Marrow Transplant. 1997;20:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Weisdorf D, Miller J, Verfaillie C, Burns L, Wagner J, Blazar B, Davies S, Miller W, Hannan P, Steinbuch M. Cytokine-primed bone marrow stem cells vs. peripheral blood stem cells for autologous transplantation: a randomized comparison of GM-CSF vs. G-CSF. Biol Blood Marrow Transplant. 1997;3:217-223. [PubMed] |
| 43. | Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, Weisdorf DJ. Randomized comparison of granulocyte colony-stimulating factor versus granulocyte-macrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Bot FJ, van Eijk L, Schipper P, Backx B, Löwenberg B. Synergistic effects between GM-CSF and G-CSF or M-CSF on highly enriched human marrow progenitor cells. Leukemia. 1990;4:325-328. [PubMed] |
| 45. | Hara H, Namiki M. Mechanism of synergy between granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in colony formation from human marrow cells in vitro. Exp Hematol. 1989;17:816-821. [PubMed] |
| 46. | Tricot G, Barlogie B, Zangari M, van Rhee F, Hoering A, Szymonifka J, Cottler-Fox M. Mobilization of peripheral blood stem cells in myeloma with either pegfilgrastim or filgrastim following chemotherapy. Haematologica. 2008;93:1739-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Granix® [package insert]. North Wales, PA: Teva Pharmaceuticals Inc; 2014. Available from: http://www.granixhcp.com/Pdf/prescribing-information.pdf. |
| 48. | Martino M, Recchia AG, Moscato T, Fedele R, Neri S, Gentile M, Alati C, Vincelli ID, Piro E, Penna G. Efficacy of biosimilar granulocyte colony-stimulating factor versus originator granulocyte colony-stimulating factor in peripheral blood stem cell mobilization in de novo multiple myeloma patients. Cytotherapy. 2015;17:1485-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Bell AJ, Figes A, Oscier DG, Hamblin TJ. Peripheral blood stem cell autografting. Lancet. 1986;1:1027. [PubMed] |
| 50. | Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031-1039. [PubMed] |
| 51. | Alegre A, Tomás JF, Martínez-Chamorro C, Gil-Fernández JJ, Fernández-Villalta MJ, Arranz R, Díaz MA, Granda A, Bernardo MR, Escudero A. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant. 1997;20:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, Gastineau DA, Gertz MA. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Wood WA, Whitley J, Moore D, Sharf A, Irons R, Rao K, Serody J, Coghill J, Gabriel D, Shea T. Chemomobilization with Etoposide is Highly Effective in Patients with Multiple Myeloma and Overcomes the Effects of Age and Prior Therapy. Biol Blood Marrow Transplant. 2011;17:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Dingli D, Gastineau DA, Winters JL, Litzow MR. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43:619-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Gettys SC, Gulbis A, Wilhelm K, Sasaki K, Dinh Y, Rondon G, Qazilbash MH. Modified CVAD and modified CBAD compared to high-dose cyclophosphamide for peripheral blood stem cell mobilization in patients with multiple myeloma. Eur J Haematol. 2017;98:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Green DJ, Bensinger WI, Holmberg LA, Gooley T, Till BG, Budde LE, Pagel JM, Frayo SL, Roden JE, Hedin L. Bendamustine, etoposide and dexamethasone to mobilize peripheral blood hematopoietic stem cells for autologous transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2016;51:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Güner ŞI, Yanmaz MT, Selvi A, Usul C. The High Effect of Chemomobilization with High-Dose Etopside + Granulocyte-Colony Stimulating Factor in Autologous Hematopoietic Peripheral Blood Stem Cell Transplantation: A Single Center Experience. Hematol Rep. 2016;8:6319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Musto P, Simeon V, Grossi A, Gay F, Bringhen S, Larocca A, Guariglia R, Pietrantuono G, Villani O, D’Arena G. Predicting poor peripheral blood stem cell collection in patients with multiple myeloma receiving pre-transplant induction therapy with novel agents and mobilized with cyclophosphamide plus granulocyte-colony stimulating factor: results from a Gruppo Italiano Malattie EMatologiche dell’Adulto Multiple Myeloma Working Party study. Stem Cell Res Ther. 2015;6:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Mark T, Stern J, Furst JR, Jayabalan D, Zafar F, LaRow A, Pearse RN, Harpel J, Shore T, Schuster MW. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008;14:795-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Fitoussi O, Perreau V, Boiron JM, Bouzigon E, Cony-Makhoul P, Pigneux A, Agape P, Nicolini F, Dazey B, Reiffers J. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant. 2001;27:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Jantunen E, Putkonen M, Nousiainen T, Pelliniemi TT, Mahlamäki E, Remes K. Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilisation in patients with multiple myeloma. Bone Marrow Transplant. 2003;31:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Corso A, Arcaini L, Caberlon S, Zappasodi P, Mangiacavalli S, Lorenzi A, Rusconi C, Troletti D, Maiocchi MA, Pascutto C. A combination of dexamethasone, cyclophosphamide, etoposide, and cisplatin is less toxic and more effective than high-dose cyclophosphamide for peripheral stem cell mobilization in multiple myeloma. Haematologica. 2002;87:1041-1045. [PubMed] |
| 63. | Goldschmidt H, Hegenbart U, Haas R, Hunstein W. Mobilization of peripheral blood progenitor cells with high-dose cyclophosphamide (4 or 7 g/m2) and granulocyte colony-stimulating factor in patients with multiple myeloma. Bone Marrow Transplant. 1996;17:691-697. [PubMed] |
| 64. | Sizemore CA, LaPorte J, Sanacore M, Holland HK, Mccollum J, Westerman J, Morris LE, Bashey A, Solomon SR. A Comparison of Toxicity and Mobilization Efficacy Following Two Different Doses of Cyclophosphamide for Mobilization of Hematopoietic Stem Cells in Multiple Myeloma Patients [abstract]. Biol Blood Marrow Tr. 2010;16:S206-S206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Gojo I, Guo C, Sarkodee-Adoo C, Meisenberg B, Fassas A, Rapoport AP, Cottler-Fox M, Heyman M, Takebe N, Tricot G. High-dose cyclophosphamide with or without etoposide for mobilization of peripheral blood progenitor cells in patients with multiple myeloma: efficacy and toxicity. Bone Marrow Transplant. 2004;34:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Stewart AK, Vescio R, Schiller G, Ballester O, Noga S, Rugo H, Freytes C, Stadtmauer E, Tarantolo S, Sahebi F. Purging of autologous peripheral-blood stem cells using CD34 selection does not improve overall or progression-free survival after high-dose chemotherapy for multiple myeloma: results of a multicenter randomized controlled trial. J Clin Oncol. 2001;19:3771-3779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Tuchman SA, Bacon WA, Huang LW, Long G, Rizzieri D, Horwitz M, Chute JP, Sullivan K, Morris Engemann A, Yopp A. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher. 2015;30:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Uy GL, Costa LJ, Hari PN, Zhang MJ, Huang JX, Anderson KC, Bredeson CN, Callander NS, Cornell RF, Perez MA. Contribution of chemotherapy mobilization to disease control in multiple myeloma treated with autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307-1318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 878] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 70. | Liles WC, Broxmeyer HE, Rodger E, Wood B, Hübel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 567] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 71. | DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Früehauf S, Horwitz M. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720-5726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 633] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 72. | Costa LJ, Abbas J, Hogan KR, Kramer C, McDonald K, Butcher CD, Littleton A, Shoptaw K, Kang Y, Stuart RK. Growth factor plus preemptive (‘just-in-time’) plerixafor successfully mobilizes hematopoietic stem cells in multiple myeloma patients despite prior lenalidomide exposure. Bone Marrow Transplant. 2012;47:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Tricot G, Cottler-Fox MH, Calandra G. Safety and efficacy assessment of plerixafor in patients with multiple myeloma proven or predicted to be poor mobilizers, including assessment of tumor cell mobilization. Bone Marrow Transplant. 2010;45:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Kim JS, Yoon DH, Park S, Yoon SS, Cho SG, Min CK, Lee JJ, Yang DH, Kwak JY, Eom HS. Prognostic factors for re-mobilization using plerixafor and granulocyte colony-stimulating factor (G-CSF) in patients with malignant lymphoma or multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy: the Korean multicenter retrospective study. Ann Hematol. 2016;95:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Kim SS, Renteria AS, Steinberg A, Banoff K, Isola L. Pharmacoeconomic impact of up-front use of plerixafor for autologous stem cell mobilization in patients with multiple myeloma. Cytotherapy. 2014;16:1584-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Cooper DL, Medoff E, Patel N, Baker J, Pratt K, Foss F, Seropian SE, Perreault S, Wu Y. Autologous Stem Cell Mobilization in the Age of Plerixafor. Clin Lymphoma Myeloma Leuk. 2016;16:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Costa LJ, Alexander ET, Hogan KR, Schaub C, Fouts TV, Stuart RK. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant. 2011;46:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Smith VR, Popat U, Ciurea S, Nieto Y, Anderlini P, Rondon G, Alousi A, Qazilbash M, Kebriaei P, Khouri I. Just-in-time rescue plerixafor in combination with chemotherapy and granulocyte-colony stimulating factor for peripheral blood progenitor cell mobilization. Am J Hematol. 2013;88:754-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2012;52:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Fiala MA, Schwab D, Vij R, Cashen AF, Stockerl-Goldstein K, Abboud CN. Randomized Trial of Tbo-Filgrastim versus Filgrastim for Autologous Stem Cell Mobilization in Patients with Multiple Myeloma or Non-Hodgkin Lymphoma [abstract]. Blood. 2015;23:516. |
| 81. | Lacativa CP, Lacativa PG, Garnica M, Portugal RD, Schaffel R, Dutra Hdos S, Nogueira CM, Nucci M, Maiolino A. Risk factors for unsuccessful peripheral blood stem cell harvesting using granulocyte-colony stimulating factor mobilization in patients with multiple myeloma. Transfus Apher Sci. 2012;47:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Sinha S, Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Dingli D, Micallef IN, Hogan WJ, Gastineau DA. Majority of patients receiving initial therapy with lenalidomide-based regimens can be successfully mobilized with appropriate mobilization strategies. Leukemia. 2012;26:1119-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Prince HM, Imrie K, Sutherland DR, Keating A, Meharchand J, Crump RM, Girouard C, Trip K, Stewart AK. Peripheral blood progenitor cell collections in multiple myeloma: predictors and management of inadequate collections. Br J Haematol. 1996;93:142-145. [PubMed] |
| 84. | Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, Litzow MR, Fonseca R, Roy V, Rajkumar SV. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 85. | Waterman J, Rybicki L, Bolwell B, Copelan E, Pohlman B, Sweetenham J, Dean R, Sobecks R, Andresen S, Kalaycio M. Fludarabine as a risk factor for poor stem cell harvest, treatment-related MDS and AML in follicular lymphoma patients after autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma (Version 3.2016). Available from: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. |
| 87. | Pal R, Monaghan SA, Hassett AC, Mapara MY, Schafer P, Roodman GD, Ragni MV, Moscinski L, List A, Lentzsch S. Immunomodulatory derivatives induce PU.1 down-regulation, myeloid maturation arrest, and neutropenia. Blood. 2010;115:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Wilk CM, Heinzler N, Boquoi A, Cadeddu RP, Strapatsas T, Dienst A, Majidi F, Deenen R, Bruns I, Schroeder T. Lenalidomide consolidation treatment in patients with multiple myeloma suppresses myelopoieses but spares erythropoiesis. Int J Cancer. 2016;139:2343-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 89. | Costa LJ, Nista EJ, Buadi FK, Lacy MQ, Dispenzieri A, Kramer CP, Edwards KH, Kang Y, Gertz MA, Stuart RK. Prediction of poor mobilization of autologous CD34+ cells with growth factor in multiple myeloma patients: implications for risk-stratification. Biol Blood Marrow Transplant. 2014;20:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, Westervelt P, Vij R, Abboud CN, Stockerl-Goldstein KE. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 91. | Weaver CH, Schulman KA, Wilson-Relyea B, Birch R, West W, Buckner CD. Randomized trial of filgrastim, sargramostim, or sequential sargramostim and filgrastim after myelosuppressive chemotherapy for the harvesting of peripheral-blood stem cells. J Clin Oncol. 2000;18:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Fruehauf S, Ehninger G, Hübel K, Topaly J, Goldschmidt H, Ho AD, Müller S, Moos M, Badel K, Calandra G. Mobilization of peripheral blood stem cells for autologous transplant in non-Hodgkin’s lymphoma and multiple myeloma patients by plerixafor and G-CSF and detection of tumor cell mobilization by PCR in multiple myeloma patients. Bone Marrow Transplant. 2010;45:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Steidl U, Fenk R, Bruns I, Neumann F, Kondakci M, Hoyer B, Gräf T, Rohr UP, Bork S, Kronenwett R. Successful transplantation of peripheral blood stem cells mobilized by chemotherapy and a single dose of pegylated G-CSF in patients with multiple myeloma. Bone Marrow Transplant. 2005;35:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Hamadani M, Kochuparambil ST, Osman S, Cumpston A, Leadmon S, Bunner P, Watkins K, Morrison D, Speir E, Deremer D. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant. 2012;18:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Hiwase DK, Bollard G, Hiwase S, Bailey M, Muirhead J, Schwarer AP. Intermediate-dose CY and G-CSF more efficiently mobilize adequate numbers of PBSC for tandem autologous PBSC transplantation compared with low-dose CY in patients with multiple myeloma. Cytotherapy. 2007;9:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Spyridonidis A, Zhang JJ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ