Published online Jun 24, 2017. doi: 10.5500/wjt.v7.i3.203
Peer-review started: November 23, 2016
First decision: December 29, 2016
Revised: January 21, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: June 24, 2017
Processing time: 216 Days and 3.7 Hours
To identify objective predictive factors for donor after cardiac death (DCD) graft loss and using those factors, develop a donor recipient stratification risk predictive model that could be used to calculate a DCD risk index (DCD-RI) to help in prospective decision making on organ use.
The model included objective data from a single institute DCD database (2005-2013, n = 261). Univariate survival analysis was followed by adjusted Cox-regressional hazard model. Covariates selected via univariate regression were added to the model via forward selection, significance level P = 0.3. The warm ischemic threshold was clinically set at 30 min. Points were given to each predictor in proportion to their hazard ratio. Using this model, the DCD-RI was calculated. The cohort was stratified to predict graft loss risk and respective graft survival calculated.
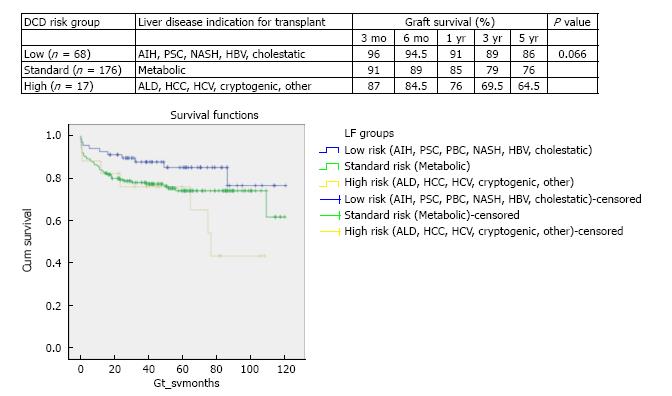
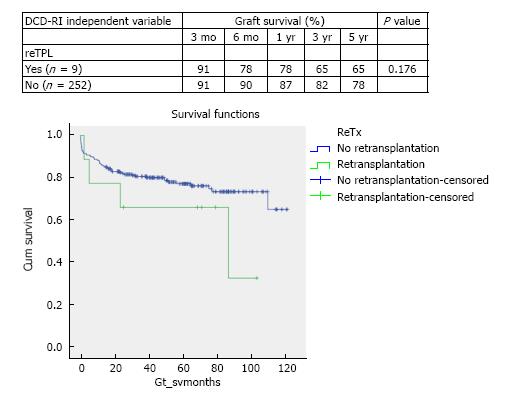
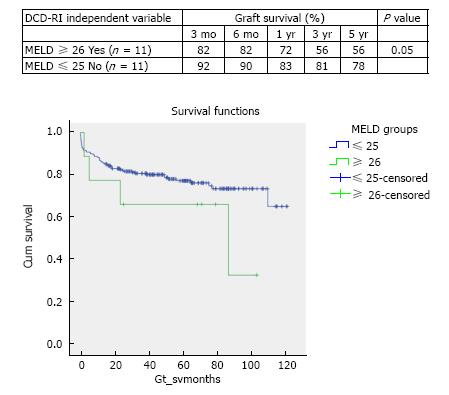
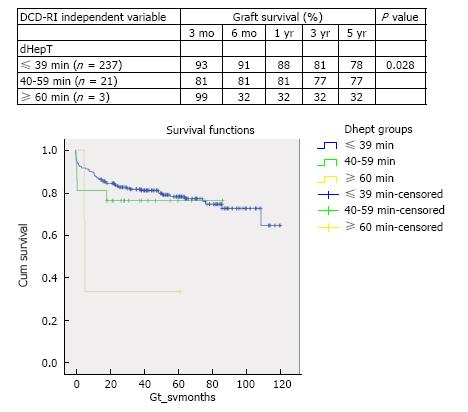
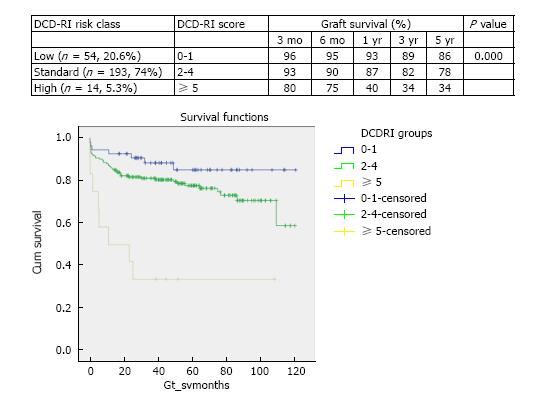
DCD graft survival predictors were primary indication for transplant (P = 0.066), retransplantation (P = 0.176), MELD > 25 (P = 0.05), cold ischemia > 10 h (P = 0.292) and donor hepatectomy time > 60 min (P = 0.028). According to the calculated DCD-RI score three risk classes could be defined of low (DCD-RI < 1), standard (DCD-RI 2-4) and high risk (DCD-RI > 5) with a 5 years graft survival of 86%, 78% and 34%, respectively.
The DCD-RI score independently predicted graft loss (P < 0.001) and the DCD-RI class predicted graft survival (P < 0.001).
Core tip: Calculating the donor after cardiac death (DCD) Risk Index score using objective variables from the donor (cold ischemic time, warm ischemic time, donor hepatectomy time) and from the selected recipient (primary indication for transplant, model for end-stage liver disease, retransplantation) can help rationalize the risk of using a DCD liver in a given recipient in order to produce good results.
- Citation: Khorsandi SE, Giorgakis E, Vilca-Melendez H, O’Grady J, Heneghan M, Aluvihare V, Suddle A, Agarwal K, Menon K, Prachalias A, Srinivasan P, Rela M, Jassem W, Heaton N. Developing a donation after cardiac death risk index for adult and pediatric liver transplantation. World J Transplant 2017; 7(3): 203-212
- URL: https://www.wjgnet.com/2220-3230/full/v7/i3/203.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i3.203
The continuing organ shortage combined with an expanding transplant waiting list is the main determinant of death on the waiting list. From UNOS and Eurotransplant data, death on the waiting list stands at 12% and 27% respectively[1,2]. This has driven the need and usage of the marginal liver or extended criteria organ, a term that encompasses the donor after cardiac death (DCD) liver. DCD liver transplantation has grown exponentially in countries that have utilized this form of donation[3]. Institutionally, DCD donation accounts for over 20% of transplants performed[4]. However, reports of poor patient and graft survival highlight that this is an organ with risks. A number of different factors have been identified as contributing to good outcome after DCD liver transplantation including donor factors of age, cold ischemic time (CIT), warm ischemic time (WIT), donor weight > 100 kg[5-8] and recipient factors of retransplantation (reTPL), on Intensive Therapy Unit (ITU) at time of transplant or renal dysfunction. Additionally, the use of a low risk DCD into a low risk recipient, can produce a good outcome that is equivalent to donor after brainstem death (DBD) liver transplantation[5].
Balancing the risk in DCD liver transplantation to achieve good results is still poorly understood and is both subjectively and experience driven. The aim of this study was to identify objective predictive factors for DCD graft loss and to use these factors to develop a donor recipient stratification risk predictive model that could be used to calculate a DCD risk index (DCD-RI) score to help in prospective decision making on DCD use.
Institutionally the DCD programme started in 2001 and practice has been relatively consistent. In brief, recipient eligibility for DCD liver transplantation is decided at the liver transplant listing multidisciplinary meeting. Recipients offered a DCD liver are typically primary transplants for chronic liver disease (CLD) +/- hepatocellular cancer (HCC). The DCD liver was normally avoided in acute liver failure (ALF) or where a prolonged/difficult recipient hepatectomy was anticipated such as redo transplantation, young adult extrahepatic biliary atresia or the presence of an extensive portomesenteric venous thrombosis, as it would be anticipated to add to the CIT. Consent specifically for DCD transplantation would be obtained from the recipient.
For procurement a modified super rapid Casavilla technique was used[9]. Withdrawal of the DCD donor would either occur in the anaesthetic room or ITU depending on donor hospital preference. After declaration of cardiac death, there would be a 5 min stand off before the donor was brought into the operating room, where the donor team would be scrubbed and ready. After making a thoraco-abdominal incision, venting of blood would be in the chest, followed in sequence, by aortic cannulation, cross clamp in the chest and then portal/superior mesenteric vein cannulation. Adherence to WIT limits was consistent and a DCD liver would be discarded if the WIT exceeded 30 min[10]. The WIT was defined as the time from systolic of 50mmHg or oxygen saturations of 70%, depending on which agonal donor observation occurred first, to time of aortic cannulation.
Once perfusion had started, the gall bladder would be flushed until clear of bile followed by copious in situ flushing of the bile duct with chilled (4 °C) normal saline. Topically, sterile crushed ice would then be placed around the organs to be retrieved. For perfusion in situ 4 L (aortic) and 2 L (portal) University of Wisconsin (UW) with 20000 IU heparin/L would be used. Pressure bags would only be used if flow by gravity was not sufficient. Attention to rapid donor hepatectomy was encouraged. On the back bench the portal vein (500 mL), hepatic artery (250 mL) and bile duct (250 mL) would be flushed further with chilled UW. Finally, the organ would be bagged for cold static storage.
Before proceeding with transplant the liver would be assessed on the backbench by the implanting surgeon. A severely steatotic liver on visual inspection would be discarded. If need be, a fresh frozen trucut liver biopsy would be taken to assess degree of steatosis or to exclude donor pathology. DCD liver steatosis > 30% led to non-usage. Implantation technique was typically piggyback with a temporary portocaval shunt. The majority of livers were re-perfused via the portal vein. Standard immunosuppression was calcineurin inhibitor (tacrolimus) and steroid based. The cold ischemic time was the time from aortic cannulation in the donor to reperfusion in the recipient. Donor hepatectomy time (dHepT) was from the start of donor aortic perfusion to completion of hepatectomy. Model for end-stage liver disease (MELD) was defined as laboratory MELD and exception points have not been applied. The diagnosis of primary ischemic cholangiopathy (PIC) was based on review of biliary imaging by two consultant radiologists that demonstrated diffuse intrahepatic stricturing with no associated hepatic artery thrombosis (HAT).
The data analysed was extracted from a prospectively populated DCD database of a single institute, with a minimum follow up of 2 years (January 2005 - January 2013, n = 261). The pediatric age group was ≤ 16 years. The study had full ethical approval in accordance with the declaration of Helsinki. Descriptive statistics were calculated for objective variables of the donor and the recipient, and for the calculated DCD-RI score. The developed DCD-RI model only included objective donor and recipient data. So subjectively assessed factors, such as liver steatosis were excluded from the analysis and the model. The primary end point was DCD graft loss. Survival analysis was performed using a Cox proportional hazard model and Kaplan-Meier estimator. Donor and recipient variables were tested independently to assess their uncontrolled effect on DCD graft survival. Significant predictive factors were then further analyzed separately with Kaplan-Meier and their respective ranges adjusted according to their level of significance. Similarly, primary indication for liver transplant was divided into 3 groups of high, standard and low risk according to their representation on the Kaplan-Meier survival curves.
For the development of the prediction model, etiology of liver disease was used as first indicator, which was then controlled for selected variables. Variables were added to the Cox regression model using forward selection with a significance level entry set at P = 0.3. Points were given to each variable in proportion to their calculated hazard ratio. WIT threshold was clinically set at 30 min and retained in the model. Using this model, the DCD-RI score was calculated for the study DCD cohort (n = 261). The DCD cohort was then stratified according to predicted graft loss risk as defined by the calculated DCD-RI score into three risk classes of low, standard and high. Respective predicted graft survivals were then calculated using Kaplan-Meier. Internal validation of the developed DCD-RI score was undertaken by performing a retrospective analysis on an earlier DCD cohort n = 37 (04/2001-12/2004), the experience of which has been previously published[11]. The receiver operator curve (ROC) and the area under the curve (AUROC) or c-statistic were then calculated to assess the performance of the DCD-RI score. The DCD-RI ROC curve was also compared to other scoring systems that have been used to predict graft survival after transplant. Statistical analysis was performed using SPSS® IBM® Statistics V22.0.
Table 1 summarizes the objective donor and recipient variables for the DCD study cohort (n = 261). The mean DCD recipient age was 49.45 ± 15.36 years, of which 15 (5.7%) were ≤ 16 years. The mean DCD donor age was 46.1 ± 17.9 years, of which 18 (6.9%) were in the pediatric age group. Redo liver transplantation (reTPL) was a small component of the DCD programme accounting for 3.4% of activity in the period of study. The DCD liver was only used in a few cases of ALF (1.5%) and split/reduction (2.3%) was uncommon (Table 1). In the DCD study cohort the incidence of primary non function (PNF) was 3.4% (n = 9), HAT 5% (n = 13), anastomotic biliary stricture 11.1% (n = 29) and PIC 3.5% (n = 9). Overall, there were 15% (n = 39) deaths and 3.5% (n = 9) retransplants.
| DCD donor and recipient variables | All (n = 261) | ||
| Donor | dAge (yr) | 46.1 ± 17.9 | |
| dBMI | 26 ± 4.9 | ||
| ITU Stay (d) | 3.9 ± 5.8 | ||
| COD (CVA: Other: HBI: Trauma) | 52.5:13.8:16.9:16.9 | ||
| dSodium (mmol/L) | 144.51 ± 11.8 | ||
| dBilirubin (μmol/L) | 9.81 ± 6.88 | ||
| Split/reduced (%) | 2.30% | ||
| WIT (min) | 16.7 ± 9.8 | ||
| dHepT (min) | 24.3 ± 10.6 | ||
| Liver Weight (g) | 1518.28 ± 397.507 | ||
| CIT (min) | 431 ± 118 | ||
| Recipient | rAge (yr) | 49.45 ± 15.36 | |
| rGender | 70.1%M/39.9%F | ||
| rBMI | 25.9 ± 4.7 | ||
| ALF (%) | 1.50% | ||
| rBilirubin (mmol/L) | 89.36 ± 116.38 | ||
| rINR | 1.89 ± 1.88 | ||
| MELD | 14.8 ± 6.4 | ||
| Location (inpatient/home) | 20.3%/79.6% | ||
| Prior abdominal surgery (yr) | 13.40% | ||
| reTPL (yr) | 5.70% | ||
| Indication for TPL | Low | 68 (26%) | |
| Standard | 176 (67.5%) | ||
| High | 17 (6.5%) | ||
Univariate analysis of independent donor and recipient variables was initially performed to determine which variables were associated with DCD graft loss. Recipient variables analyzed were age (rAge), gender (rGender), weight (rWeight), BMI (rBMI), MELD, primary indication for transplant, patient location (home/hospital), reTPL, prior abdominal surgery and ALF/CLD. The donor variables analyzed were age (dAge), weight (dWeight), BMI (dBMI), cause of death (COD), sodium, CIT, WIT, liver weight, hepatectomy time (dHepT) and length of ITU stay (see Table 1).
On univariate analysis, the donor and recipient variables that were found to have a significant effect on DCD graft survival were MELD > 25 (χ2 3.8 log-rank P = 0.05) and dHepT > 60 min (χ2 4.8 log-rank P = 0.028). The variables that reached the significance level of entry into the forward selection regression model (P = 0.3) were primary indication for liver transplant (χ2 5.1 log-rank P = 0.066), reTPL (χ2 1.8 log-rank P = 0.176) and CIT > 10 h (χ2 1.1 log-rank P = 0.292).
On grouping of survival curves based on primary liver disease indication for transplant, three DCD risk groups were defined of low, standard and high. Better survival was demonstrated when a DCD liver was used in a low risk indication for transplant (86% graft survival at 5 years) and poorer survival was found when the DCD liver was used in a high risk indication for transplant (64.5% graft survival at 5 years) (Figure 1). Low DCD risk indications for transplant included autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), non-alcoholic steatohepatitis (NASH), hepatitis B virus (HBV) and cholestatic liver disease. The cholestatic low risk indications for transplant encompassed primary familial intrahepatic cholestasis (PFIC), extrahepatic biliary atresia (EHBA) and Crigler Najjar. The standard risk indications for transplant were metabolic diseases that included Wilson’s, Hemochromatosis and Familial Amyloid Polyneuropathy. The high risk indications for DCD transplant were alcohol related liver disease (ALD), HCC, hepatitis C virus (HCV), cryptogenic and Budd Chiari. Survival analysis for these three DCD risk groups as defined by primary indication for transplant is illustrated in Figure 1 (χ2 5.1 log-rank P = 0.066).
In the period of study, the use of DCD for reTPL was rare, 5.7% (n = 9). In the cases that DCD was used for reTPL, there was significantly worse DCD graft survival. At 5 years DCD graft survival in reTPL was 65% compared to 78% when used in primary liver transplant (see Figure 2 for survival curves, χ2 1.8 log-rank P = 0.176). Use of DCD in recipients with higher MELDs ≥ 26 (n = 11) was also found to be associated with worse DCD graft survival (χ2 3.8 log-rank P = 0.05), with a 5-year survival of 56% compared to 78%, when used in recipients with a MELD < 25 (Figure 3 for DCD survival curves according to MELD). Additionally, the donor hepatectomy time (dHepT) was found to be a determinant of DCD graft survival (χ2 4.8 log-rank P = 0.028), with a dHepT ≥ 60 min associated with early graft loss and a poor 5 year graft survival of 32% (Figure 4 for survival curves according to the dHepT groups).
Clinically, the warm ischemic threshold was set at 30 min and the WIT was retained in the DCD-RI model, despite not being found significant on univariate analysis, as it is institutionally regarded as a constant variable in determining outcome in DCD transplantation. After serial Kaplan-Meier analysis the CIT threshold was statistically set at ≥ 10 h (n = 13) and < 10 h (n = 248) (χ2 1.1 log-rank P = 0.292). However, many programmes are more stringent aiming for shorter CIT < 8 h (17). For the WIT, Kaplan-Meier analysis produced a cut off value of 25 min (n = 240) that had the lowest P value (χ2 0.589 log-rank P = 0.443) and was the value incorporated into the developed DCD-RI model.
There was no difference in DCD graft survival between adult (n = 243) and pediatric (n = 18) donors (HR = 0.819, CI: 0.343-1.958, P = 0.653). Similarly, there was no difference in DCD graft survival between adult (n = 246) and pediatric (n = 15) recipients (HR = 1.268, CI: 0.389-4.132, P = 0.699). Therefore for the developed DCD-RI model adult and pediatric age groups have been combined.
Using primary indication for liver transplant as the primary indicator adjusted for the identified donor (WIT, CIT, dHepT) and recipient variables (MELD, reTPL) multivariate Cox regression analysis was undertaken. For the DCD-RI model points were given to each variable in proportion to the calculated hazard ratio (Table 2). According to the DCD-RI score three DCD-RI risk classes were defined, low risk (DCD-RI < 1), standard risk (DCD-RI 2-4) and high risk (DCD-RI > 5). Transplantation with a high risk DCD-RI score > 5 produced a 1 year graft survival of 40% and at 5 years 34% (Figure 5 for the survival curves according to DCD-RI score, Log-Rank and Breslow pooled over strata P < 0.001).
| Donor/recipient predictor variables | HR (CI) | Points |
| Primary indication for transplant | ||
| Low (P = 0.07) | ||
| Standard (P = 0.05) | 2 (1-4.04) | 2 |
| High (P = 0.04) | 2.83 (1.04-7.24) | 3 |
| reTPL (P = 0.26) | 1.87 (0.63-5.58) | 2 |
| MELD > 25 (P = 0.04) | 2.75 (1.04-7.24) | 3 |
| CIT > 10 h (P = 0.6) | 1.37 (0.4-4.04) | 1 |
| WIT > 25 min (P = 0.4) | 1.48 (0.6-3.63) | 1 |
| dHepT | ||
| 40-60 min (P = 0.5) | 1.36 (0.53-3.53) | 1 |
| > 60 min (P = 0.05) | 4.4 (1.02-19.04) | 4 |
For internal validation of the developed DCD-RI score a retrospective analysis of an earlier DCD cohort 04/2001-12/2004 n = 37 were undertaken. Table 3 for summary of actual and predicted survival using the DCD-RI class subdivision. There was good concordance between actual graft survival and predicted DCD-RI survival, with actual graft survival falling within the confidence interval of the DCD-RI risk class predicted survival.
| DCD Graft Survival (mo) | DCD-RI class | ||
| DCD-RI ≤ 1, low (n = 10/27%) | DCD-RI 2-4, standard (n = 8/21.6%) | DCD-RI ≥ 5, high (n = 19/51.4%) | |
| 3 | |||
| Actual | 100 | 92.6 | 75 |
| Predicted | 96 (100-83.8) | 90 (100-76.8) | 80 (96.2-63.8) |
| 6 | |||
| Actual | 100 | 85.2 | 75 |
| Predicted | 95 (100-83.7) | 90 (100-83.8) | 75 (91.2-58.8) |
| 12 | |||
| Actual | 100 | 77.8 | 75 |
| Predicted | 93 (100-76.8) | 87 (100-70.8) | 40 (56.2-23.8) |
| 60 | |||
| Actual | 100 | 63 | 50 |
| Predicted | 86 (100-83.8) | 78 (94.2-61.8) | 34 (50.2-17.8) |
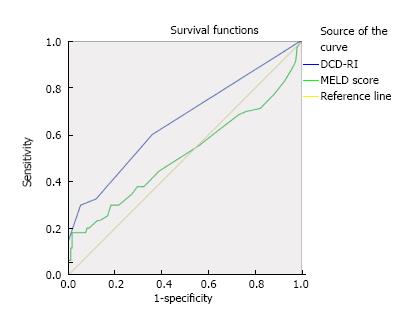
Based on the DCD-RI ROC, a DCD-RI score 1.5 cut off had a good positive predictive value (PPV = 0.993). A low risk DCD-RI score ≤ 1.5, graft survival was predicted with 99.3% sensitivity and 98.3% specificity. Whereas, with a high risk DCD-RI score > 5, specificity was better than sensitivity (Figure 6 for DCD-RI ROC curve). To determine how the DCD-RI score compared to other transplant predictive scoring systems for graft outcome, the DCD-RI ROC curve was compared to other systems (see Figure 6). Based on the c-statistic (or AUROC) the DCD-RI (c-statistic = 0.657) was found to be better than MELD (c-statistic = 0.514) and better than the donor risk index (DRI) (c-statistic = 0.53) in predicting DCD graft loss when applied to the validation cohort.
The DCD liver is regarded as an extended criteria donor graft, in terms of the poorer outcomes that have been reported in the literature. Particular, concerns with this organ are the increased occurrence of PNF (0%-12%), early graft dysfunction (20%-30%), and PIC (15%) that result in the higher rates of graft loss and recipient death[5,6,12-18]. A large component in determining good results in DCD liver transplantation is the ability to balance risk through judicious matching of the donor and recipient. The aim of this work was to develop a formula, the DCD-RI that is valid and easy to apply with readily available objective variables relating to the donor and the recipient to help rationalize this risk balance.
The objective recipient variables that were found to have a significant effect on DCD graft survival were the primary indication for transplant, MELD and reTPL. While from the donor, CIT and hepatectomy time (dHepT) were important. These five variables combined with the fundamental determinant of DCD graft outcome of WIT[7] formed the basis of the developed DCD-RI score. According to primary indication for transplant, three DCD risk groups of low, standard and high were defined (Figure 1). By applying this stratification, good graft survival of over 86% at 5 years was found when the DCD liver was used in recipients with low risk indications for transplant of AIH, PSC, PBC, NASH, HBV, and cholestatic diseases that included PFIC, EHBA and Crigler Najjar. While in the standard risk indication for transplant of metabolic diseases that encompassed Wilson’s, Haemochromatosis and Amyloid, 5 year survival fell by 10%, to 76%. Similarly, 5 year DCD graft survival fell a further 10% to 64%, in the high risk recipient group of HCV, HCC, ALD, cryptogenic and Budd Chiari (Figure 1). When MELD was used to define DCD risk, recipients with a MELD ≤ 25 were found to have a graft survival of 76% at 5 years, with survival falling a further 20% in higher MELD recipients. The use of DCD in reTPL was uncommon, accounting for 3.5% of DCD usage and graft survival was poorer (65% vs 78% at 5 years reTPL v primary transplant). Additionally, a donor hepatectomy time over one hour resulted in poor graft survival of 32% at 5 years. Allocating points, to the risk associated with each of these variables produced the DCD-RI score (Table 2).
A DCD-RI score over 5 was high risk for early graft loss, and predictive for poor long term survival of 34% at 5 years (Figure 5). In order to minimize DCD graft loss, the ideal is to aim for a DCD-RI score less than 5, which can be achieved either by minimizing the risk from the donor by selecting/aiming for a short WIT < 25 min, short CIT < 10 h, dHepT < 60 min, or by negating the DCD risk of the donor by selecting a low risk recipient, i.e., MELD < 25, not a reTPL or belonging to the low risk primary indication group for DCD transplant. Internal validation of the DCD-RI on an earlier cohort supported the validity of the developed DCD-RI score by its ability to accurately predict graft survival for that cohort. Additionally, comparison, of the DCD-RI score showed it to out perform other predictive scoring systems such as the DRI and MELD.
Calculating the DCD-RI score helps to provide a framework to rationalize some of the risks involved in DCD liver transplantation. However, there are limitations to the data that were used to build the DCD-RI scoring system. The transplanted DCD livers whose data was used to design the DCD-RI score were already highly selected[10], automatically introducing bias into the study. By preselecting good quality DCD livers[21] as reflected by young donor age and short ischemic times (cold and warm), the majority (94.6%) of DCD transplants used to develop the DCD-RI belong to the low and standard DCD-RI score risk classes, while high risk DCD-RI transplants (DCD-RI score > 5) were rare in the programme. But by being stringent in DCD selection good outcomes can be achieved, comparable to DBD in both the short and long term[22].
Another factor that is well recognized to be a determinant of outcome in DCD transplantation but was not included in the DCD-RI was steatosis. The main reason for exclusion was assessment of liver steatosis by the surgeon is highly subjective[23] and histological assessment of the donor liver pre-perfusion is not routinely performed in transplant, and is in itself, a subjective assessment. Institutionally, the steatotic (> 30%) DCD liver is not used which may explain why donor BMI and donor liver weight, both surrogate markers of liver steatosis, were not found to have any bearing on graft survival. Neither, donor or recipient age, were included in the DCD-RI model as they were not found to be determinants of outcome in the data analyzed. This again reflects institutional practice, which is not to use donor/recipient age on its own, as a reason for DCD non consideration. Therefore, the developed DCD-RI has been able to combine adult and pediatric data. However, donor age has been identified in other series as a risk factor for graft failure[18] but older donors can be a valuable source of organs, and the risk from age can be balanced by reducing the risk from an alternative donor or recipient factor(s), e.g., CIT[24,25].
Other predictive models for outcome after liver transplantation have been explored but they all, as does the DCD-RI, have various limitations. The donor recipient index (DRI) considers only donor factors[26]. While, the MELD score, that is the foundation of liver allocation on transplant waiting lists[27-29] is a poor predictor of outcome after transplant[30]. A number of other complex models detailing interactions between donor and recipient risk profiles have been developed to predict graft and patient survival after liver transplantation[30-39]. But none consider DCD in isolation and it is well recognized, that the DCD liver is a different type of graft in comparison to DBD, and DBD predictors of outcome have not been found to be applicable to DCD[40].
Only one other group has tried to design a DCD prognostic scoring system, admittedly with smaller numbers (n = 81), in adults only and is yet to be validated[41]. However, they found similar variables to that of the present DCD-RI to be important predictors of DCD graft survival, such as primary indication for transplant, retransplantation, donor warm ischemic time and cold ischemic time (< 6 h). But with their DCD data, unlike the present data, they found recipient BMI (> 30) and donor HBV core antibody status influenced DCD graft outcomes. They did not consider donor hepatectomy time.
In conclusion, the developed DCD-RI score helps to rationalize and balance the risk between the donor and the recipient in DCD liver transplantation, in order to achieve good graft survival. To determine the true utility of the system it will need to be prospectively validated in other large volume DCD programmes.
Sue Landymore, who maintains and updates the liver transplant database.
In Liver Transplant programmes in the West, there is a reliance on cadaveric donors over living related. The availability of cadaveric organs is insufficient to meet transplant need. Therefore, more marginal cadaveric organs have to be used, one such organ, is the donor after cardiac death (DCD) liver. However, this is a high risk organ and difficult to use with good results, most of which depends on subjective experience driven knowledge. The aim of this work was to create a scoring system using pre transplant objective data points from the donor and recipient to rationalize this risk.
There is a lack of data on how to achieve the balance of risk between objective clinical variables from the DCD liver and selected recipient to produce good results. The present work aims to address this lack of information.
The DCD Risk Index (DCD-RI) score that was developed using objective variables from the donor and recipient was able to predict graft loss and DCD-RI class predicted graft survival.
The DCD-RI is a tool that can help in decision making on whether to use a given DCD liver in the selected recipient to produce good results.
Both internal and external was performed in order for this manuscript to be accepted for publication.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kang KJ, Quak SH, Salvadori M, Sugawara Y S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Hart A, Smith JM, Skeans MA. OPTN/SRTR annual data report. Am J Transplant. 2016;16:11-46. |
| 2. | Eurotransplant International Foundation. Annual Report 2013. Available from: http: //www.eurotransplant.org/cms/mediaobject.php?file=AR20135.pdf. |
| 3. | Monbaliu D, Pirenne J, Talbot D. Liver transplantation using Donation after Cardiac Death donors. J Hepatol. 2012;56:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Khorsandi SE, Yip VS, Cortes M, Jassem W, Quaglia A, O’Grady J, Heneghan M, Aluvihare V, Agarwal K, Menon K. Does Donation After Cardiac Death Utilization Adversely Effect Hepatocellular Cancer Survival? Transplantation. 2016;100:1916-1924. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, Fong TL, Sher L, Jabbour N, Aswad S. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Lee KW, Simpkins CE, Montgomery RA, Locke JE, Segev DL, Maley WR. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Taner CB, Bulatao IG, Willingham DL, Perry DK, Sibulesky L, Pungpapong S, Aranda-Michel J, Keaveny AP, Kramer DJ, Nguyen JH. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl. 2012;18:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Fung JJ, Starzl TE. Liver and kidney transplantation from non-heart beating donors: the Pittsburgh experience. Transplant Proc. 1995;27:710-712. [PubMed] |
| 10. | Davila D, Ciria R, Jassem W, Briceño J, Littlejohn W, Vilca-Meléndez H, Srinivasan P, Prachalias A, O’Grady J, Rela M. Prediction models of donor arrest and graft utilization in liver transplantation from maastricht-3 donors after circulatory death. Am J Transplant. 2012;12:3414-3424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Muiesan P, Girlanda R, Jassem W, Melendez HV, O’Grady J, Bowles M, Rela M, Heaton N. Single-center experience with liver transplantation from controlled non-heartbeating donors: a viable source of grafts. Ann Surg. 2005;242:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Abt PL, Desai NM, Crawford MD, Forman LM, Markmann JW, Olthoff KM, Markmann JF. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 275] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D’Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 15. | Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, Shames BD, Becker YT, Odorico JS, Knechtle SJ. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 17. | Jay CL, Lyuksemburg V, Kang R, Preczewski L, Stroupe K, Holl JL, Abecassis MM, Skaro AI. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Ann Surg. 2010;251:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, Fontes P, Marsh JW. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, Reyes JD, Larson AM, Levy AE. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 20. | Axelrod DA, Dzebisashvilli N, Lentine KL, Xiao H, Schnitzler M, Tuttle-Newhall JE, Segev DL. National assessment of early biliary complications after liver transplantation: economic implications. Transplantation. 2014;98:1226-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Andrews PA, Burnapp L, Manas D. Summary of the British Transplantation Society guidelines for transplantation from donors after deceased circulatory death. Transplantation. 2014;97:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | DeOliveira ML, Jassem W, Valente R, Khorsandi SE, Santori G, Prachalias A, Srinivasan P, Rela M, Heaton N. Biliary complications after liver transplantation using grafts from donors after cardiac death: results from a matched control study in a single large volume center. Ann Surg. 2011;254:716-722; discussion 722-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Yersiz H, Lee C, Kaldas FM, Hong JC, Rana A, Schnickel GT, Wertheim JA, Zarrinpar A, Agopian VG, Gornbein J. Assessment of hepatic steatosis by transplant surgeon and expert pathologist: a prospective, double-blind evaluation of 201 donor livers. Liver Transpl. 2013;19:437-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Cescon M, Grazi GL, Cucchetti A, Ravaioli M, Ercolani G, Vivarelli M, D’Errico A, Del Gaudio M, Pinna AD. Improving the outcome of liver transplantation with very old donors with updated selection and management criteria. Liver Transpl. 2008;14:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Firl DJ, Hashimoto K, O’Rourke C, Diago-Uso T, Fujiki M, Aucejo FN, Quintini C, Kelly DM, Miller CM, Fung JJ. Impact of donor age in liver transplantation from donation after circulatory death donors: A decade of experience at Cleveland Clinic. Liver Transpl. 2015;21:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1520] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 27. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3767] [Article Influence: 150.7] [Reference Citation Analysis (2)] |
| 28. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2102] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 29. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1892] [Article Influence: 82.3] [Reference Citation Analysis (1)] |
| 30. | Desai NM, Mange KC, Crawford MD, Abt PL, Frank AM, Markmann JW, Velidedeoglu E, Chapman WC, Markmann JF. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 32. | Ghobrial RM, Gornbein J, Steadman R, Danino N, Markmann JF, Holt C, Anselmo D, Amersi F, Chen P, Farmer DG. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg. 2002;236:315-322; discussion 322-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Avolio AW, Agnes S, Gasbarrini A, Nure E, Siciliano M, Castagneto M. Prognostic value of MELD score and donor quality in liver transplantation: implications for the donor recipient match. Transplant Proc. 2006;38:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 35. | Zamboni F, Franchello A, David E, Rocca G, Ricchiuti A, Lavezzo B, Rizzetto M, Salizzoni M. Effect of macrovescicular steatosis and other donor and recipient characteristics on the outcome of liver transplantation. Clin Transplant. 2001;15:53-77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Haydon GH, Hiltunen Y, Lucey MR, Collett D, Gunson B, Murphy N, Nightingale PG, Neuberger J. Self-organizing maps can determine outcome and match recipients and donors at orthotopic liver transplantation. Transplantation. 2005;79:213-218. [PubMed] |
| 37. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 38. | Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 39. | Briceño J, Cruz-Ramírez M, Prieto M, Navasa M, Ortiz de Urbina J, Orti R, Gómez-Bravo MÁ, Otero A, Varo E, Tomé S, Clemente G, Bañares R, Bárcena R, Cuervas-Mons V, Solórzano G, Vinaixa C, Rubín A, Colmenero J, Valdivieso A, Ciria R, Hervás-Martínez C, de la Mata M. Use of artificial intelligence as an innovative donor-recipient matching model for liver transplantation: results from a multicenter Spanish study. J Hepatol. 2014;61:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 924] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 41. | Hong JC, Yersiz H, Kositamongkol P, Xia VW, Kaldas FM, Petrowsky H, Farmer DG, Lipshutz G, Markovic D, Hiatt JR. Liver transplantation using organ donation after cardiac death: a clinical predictive index for graft failure-free survival. Arch Surg. 2011;146:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |