Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.49
Peer-review started: August 26, 2016
First decision: October 20, 2016
Revised: December 14, 2016
Accepted: January 2, 2017
Article in press: January 4, 2017
Published online: February 24, 2017
Processing time: 180 Days and 13.4 Hours
To investigate the incidence and the determinants of cardiovascular morbidity in Greek renal transplant recipients (RTRs) expressed as major advance cardiac event (MACE) rate.
Two hundred and forty-two adult patients with a functioning graft for at least three months and available data that were followed up on the August 31, 2015 at two transplant centers of Western Greece were included in this study. Baseline recipients’ data elements included demographics, clinical characteristics, history of comorbid conditions and laboratory parameters. Follow-up data regarding MACE occurrence were collected retrospectively from the patients’ records and MACE risk score was calculated for each patient.
The mean age was 53 years (63.6% males) and 47 patients (19.4%) had a pre-existing cardiovascular disease (CVD) before transplantation. The mean estimated glomerular filtration rate was 52 ± 17 mL/min per 1.73 m2. During follow-up 36 patients (14.9%) suffered a MACE with a median time to MACE 5 years (interquartile range: 2.2-10 years). Recipients with a MACE compared to recipients without a MACE had a significantly higher mean age (59 years vs 52 years, P < 0.001) and a higher prevalence of pre-existing CVD (44.4% vs 15%, P < 0.001). The 7-year predicted mean risk for MACE was 14.6% ± 12.5% overall. In RTRs who experienced a MACE, the predicted risk was 22.3% ± 17.1% and was significantly higher than in RTRs without an event 13.3% ± 11.1% (P = 0.003). The discrimination ability of the model in the Greek database of RTRs was good with an area under the receiver operating characteristics curve of 0.68 (95%CI: 0.58-0.78).
In this Greek cohort of RTRs, MACE occurred in 14.9% of the patients, pre-existing CVD was the main risk factor, while MACE risk model was proved a dependable utility in predicting CVD post RT.
Core tip: Cardiovascular disease being the leading cause of death with a functioning graft following renal transplantation. The aim of this study was to investigate the incidence and the determinants of cardiovascular morbidity in prevalent Greek renal transplant recipients (RTRs) expressed as major adverse cardiac event (MACE) rate. Additionally, we examined the applicability of a recently developed risk prediction model in our population. According to our results older age of recipient and pre-existing cardiovascular disease were the main risk factors for MACE. The applied risk model can be used for risk stratification in this database of RTRs.
- Citation: Anastasopoulos NA, Dounousi E, Papachristou E, Pappas C, Leontaridou E, Savvidaki E, Goumenos D, Mitsis M. Cardiovascular disease: Risk factors and applicability of a risk model in a Greek cohort of renal transplant recipients. World J Transplant 2017; 7(1): 49-56
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/49.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.49
Renal transplantation is the treatment of choice for patients with end stage renal disease (ESRD), as it enhances survival and quality of life and is also cost-effective. Nevertheless, cardiovascular disease (CVD) is the leading cause of death with functioning graft in renal transplant recipients (RTRs)[1,2]. Cardiovascular mortality rates in RTRs are significant lower than in an age stratified dialysis population but remain at least twice as high as in an age-stratified sample of the general population[3-5]. Although, successful renal transplantation results in the removal of the hemodynamic and uremic abnormalities associated with dialysis along with the improvement of cardiovascular indices such as left ventricular hypertrophy[6,7], by the time of renal transplantation, the majority of patients already have a heavy burden of atherosclerosis[8].
Knowledge of responsible cardiovascular risk factors has improved in RTRs but precise risk calculation and realistic prediction of a subsequent cardiovascular fatal or non-fatal event still remains a challenge among transplant physicians. In this direction, risk prediction models for cardiovascular events, based on traditional cardiovascular risk factors, have been validated and applied in the general population but their validity remains controversial in RTRs. Accordingly, the Framingham risk score which is a simple and easily accessible tool for the prediction of the risk of a coronary event within the following 10 years has been shown to underestimate cardiovascular risk in RTRs[9]. Given this gap in prediction, transplant-related risk factors have been investigated in large multicenter databases of RTRs, showing that cardiovascular comorbid conditions and risk factors linked to graft function explain much of the variation in coronary heart disease after kidney transplantation[10].
More recently, Soveri et al[11] developed and internally validated major adverse cardiac event (MACE) and mortality risk calculators for prevalent RTRs by using Assessment of Lescol in Renal Transplantation (ALERT) data from the extension trial. The same group of investigators subsequently externally validated the risk equation in an international transplant database using RTRs from the patient outcomes in renal transplantation (PORT) cohort and successfully applied the risk estimator in the Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial (BENEFIT) and BENEFIT-EXT ended criteria donors trial (BENEFIT-EXT)[12].
In our study, we sought to investigate the incidence and the determinants of cardiovascular morbidity in Greek RTRs expressed as MACE rate. Additionally, we examined the applicability of a validated risk prediction model for MACE in our population.
The full database consisted of 293 RTRs. Adult patients with a functioning graft for at least three months and available data that were followed up on the August 31, 2015 at the two transplant centers of the 6th District Health (Renal Transplant Units of the University Hospital of Patras and University General Hospital of Ioannina), were included in this study. The final analysis included 242 RTRs as for the rest of the patients detailed data regarding coronary heart events and potential CVD risk factors were insufficient.
Recipients’ data elements included demographics, clinical characteristics, time on dialysis prior to transplant, history of comorbid conditions such as diabetes [including new onset diabetes after transplantation (NODAT)], hypertension, cardiac ischemic heart disease [myocardial infarction (MI) based on electrocardiography or troponin rise, coronary angioplasty or artery bypass grafting], congestive heart failure, cerebrovascular accident, transient ischemic attack and peripheral artery disease, pre- and post-transplant smoking status and immunosuppression therapy. Laboratory parameters included renal function markers [serum creatinine, 24 h urine protein content (UPR, mg/24 h)], glucose, hemoglobin, lipid profile [total cholesterol (TChol) and low density lipoprotein-(LDL)], C-reactive protein (CRP) and mineral bone disease markers [calcium, phosphate, parathyroid hormone (PTH)]. Estimated glomerular filtration rate (eGFR) was calculated using the four variable modification of diet in renal disease study equation (MDRD)[13]. Clinical characteristics, laboratory parameters, cardiovascular disease and immunosuppressive medications recorded closest to 3 mo post-transplant were used in the analysis. All data were collected retrospectively and were obtained from the patients’ medical files.
Major adverse cardiac event was strictly defined as one or more of nonfatal MI and/or invasive coronary artery revascularization (angioplasty or coronary artery bypass grafting), that occurred 3 mo post-transplant in a RTR with a functioning allograft on the cross-sectional database review as of August 31, 2015. Follow-up data regarding MACE occurrence were collected retrospectively from the patients’ records. Time to event was defined as time from transplant to the earliest date of MACE.
For prediction of a subsequent MACE, the MACE risk calculator, recently described by Soveri et al[11], was applied in the study. It is a seven variable calculator using age, previous cardiac event, history of diabetes mellitus (DM) including NODAT, pre- and post-transplantation smoking habits, number of renal grafts received, serum creatinine and LDL levels to predict 7-year risk of MACE. The area under the receiver operator curve (ROC) in the original study was 0.738[11]. The MACE risk was calculated for all 242 participants (http://www.medsci.uu.se/forskning/Inflammation_och_autoimmunitet/Njurmedicin/Projekt/risk-calculator/).
This study was approved by the Institutional Scientific Committee and the Review Board of the University General Hospital of Ioannina, 6th District Health (Peloponnese, Ionian Islands, Epirus and Western Greece), Greece.
Data are expressed as mean and standard deviation (for normally distributed data), median and interquartile range (IQR) (for not-normally distributed data), or as percentage frequency (for binary variables). Differences in baseline characteristics of RTRs without (group A) and with MACE (group B) were compared by using the Mann Whitney U test for continuous variables and the chi-square test for categorical variables.
Univariate and multivariate Cox proportional hazards models were used to assess effects of potential risk factors on the primary outcome, first MACE. Tested covariates in the univariate analysis included, age, sex, pre- and post-transplant smoking status, hypertension, systolic blood pressure (BP), DM, pre-existing CVD, total time on dialysis and transplantation, number of grafts, serum creatinine, UPR, TChol, LDL, PTH, CRP and calculated MACE risk. Risk factors with a P value ≤ 0.1 in the univariate analysis were included in the multivariate model. In the Cox analysis data were expressed as hazard ratio (b), 95%CI and P value.
The validation for discrimination was performed externally using the Greek cohort of RTRs. The discriminatory power of MACE risk model for identifying patients with from those without the primary outcome was assessed by calculating the area under the ROC curve (c-statistics). A value of AUC of 50% is considered as the threshold of prognostic usefulness.
All the statistical analyses were performed by using a standard statistical package (IBM SPSS Statistics for Windows, version 22.0).
Demographics, clinical characteristics and laboratory parameters of the 242 RTRs overall and classified in the two groups are shown in Table 1. In the whole group, the mean age was 53 years and 63.6% were males. The vast majority of RTRs were hypertensive patients (87.6%), 29.4% of them were diabetics (including NODAT) and 47 patients (19.4%) had a positive history of CVD before transplantation. The percentage of active smokers in the whole cohort was almost halved after transplantation (previous smokers 35.1% vs current smokers 17.8%, P < 0.001). The mean time on dialysis before transplantation was 4.8 ± 3.9 years. Most of the patients received one renal graft (90%), while 23 patients received two grafts and one patient three grafts. The mean eGFR of the functioning graft was 52 ± 17 mL/min per 1.73 m2 and the median UPR level was 309 mg/24 h (IQR, 167-600 mg/24 h). Immunosuppression regimen was effectively recorded in 209 patients (Table 2). In total, out of the 209 RTRs, 196 (93.8%) received a three-drug regimen (steroids + Calcineurin inhibitor or Everolimus + Mycophenolate mofetil), while 13 received a two-drug regimen.
| Total | Group A | Group B | P | |
| No. of patients (n, %) | 242 | 206 (85.1) | 36 (15) | |
| Age (yr) | 53 ± 12 | 52 ± 12 | 59 ± 10 | < 0.001 |
| Male sex (n, %) | 154 (63.6) | 126 (61.2) | 28 (77.8) | 0.056 |
| Previous smoker (n, %) | 85 (35.1) | 69 (33.5) | 16 (44.4) | 0.2 |
| Current smoker (n, %) | 43 (17.8) | 37 (17.5) | 7 (19.4) | 0.77 |
| Hypertension (n, %) | 212 (87.6) | 178 (86.4) | 34 (94.4) | 0.56 |
| Systolic BP (mmHg) | 140 ± 18 | 141 ± 18 | 137 ± 19 | 0.25 |
| Diabetes mellitus (n, %) | 71 (29.3) | 57 (27.7) | 14 (38.8) | 0.17 |
| Previous CVD (n, %) | 47 (19.4) | 31 (15) | 16 (44.4) | < 0.001 |
| Time on dialysis (yr) | 4.8 ± 3.9 | 4.7 ± 3.6 | 5.6 ± 3.8 | 0.16 |
| Received allografts > 1 (n, %) | 24 (9.9) | 22 (10.7) | 2 (5.6) | 0.6 |
| Time since transplant (mo) | 9.8 ± 5.3 | 9.7 ± 5.3 | 10.5 ± 5.2 | 0.43 |
| Creatinine (mg/dL) | 1.45 ± 0.6 | 1.45 ± 0.57 | 1.44 ± 0.45 | 0.95 |
| eGFR-MDRD (mL/min per 1.73 m2) | 51.9 ± 17.2 | 51.9 ± 17.3 | 52.1 ± 17.2 | 0.97 |
| Urine protein (mg/24 h) | 309 (167-600) | 325 (166-604) | 290 (189-374) | 0.76 |
| Total cholesterol (mg/dL) | 209 ± 33 | 212 ± 34 | 194 ± 25 | 0.08 |
| LDL (mg/dL) | 107 ± 35 | 107 ± 37 | 103 ± 27 | 0.56 |
| Haemoglobin (g/dL) | 13.1 ± 1.7 | 13.1 ± 1.7 | 13.3 ± 1.7 | 0.61 |
| Calcium (mg/dL) | 9.56 ± 0.62 | 9.6 ± 0.7 | 9.5 ± 0.4 | 0.88 |
| Phosphate (mg/dL) | 3.06 ± 0.95 | 3.1 ± 0.9 | 2.7 ± 1.3 | 0.08 |
| PTH (pg/mL) | 118 ± 89 | 117 ± 88 | 127 ± 96 | 0.55 |
| Glucose (mg/dL) | 99 ± 27 | 98 ± 24 | 102 ± 39 | 0.44 |
| CRP (mg/L) | 0.8 (0.3-3) | 0.8 (0.3-2.6) | 0.8 (0.3-3) | 0.78 |
| Total RTRs | Group A | Group B | P | |
| Steroids | 199 (95.2) | 167 (95) | 32 (97) | 0.61 |
| Mycophenolate mofetil | 207 (99) | 175 (99.4) | 32 (97) | 0.18 |
| Tacrolimus | 56 (26.8) | 49 (27.8) | 7 (21.2) | 0.43 |
| Cyclosporine | 146 (69.9) | 122 (69.3) | 24 (72.7) | 0.69 |
| Everolimus | 6 (2.9) | 4 (2.3) | 2 (6.1) | 0.23 |
| CCB | 134 (55.4) | 116 (56.3) | 18 (50) | 0.65 |
| Beta-adrenergic blockers | 151 (62.4) | 128 (62.1) | 23 (63.9) | 0.86 |
| ARBs/ACEi | 131 (54.1) | 117 (56.7) | 14 (38.9) | 0.35 |
| Diuretics | 56 (23.1) | 46 (21.8) | 10 (27.8) | 0.58 |
| Other antihypertensive drugs | 53 (21.9) | 48 (23.3) | 5 (13.9) | 0.46 |
| Hypolipidemic drugs | 154 (63.6) | 134 (65) | 20 (55.6) | 0.49 |
Of the 242 RTRs, with a mean time since transplantation 9.8 ± 5.3 years, 36 patients (14.9%) suffered a MACE with median time to MACE being 5 years. Recipients who sustained a MACE (group B) compared to recipients with no MACE (group A) post transplantation had a significantly higher mean age (59 years vs 52 years, P < 0.001), had a higher prevalence of CVD before transplantation (44.4% vs 15%, P < 0.001) and, with a marginal significance, were more likely to be men (77.8% vs 61.2%, P = 0.056) (Table 1). Patients among the two groups did not differ significantly as for the other clinical characteristics including smoking, hypertension, diabetes, time on dialysis, number of renal grafts, time with functioning graft, renal function markers and assessed laboratory parameters as well as immunosuppression, antihypertensive and hypolipidemic drugs (Tables 1 and 2).
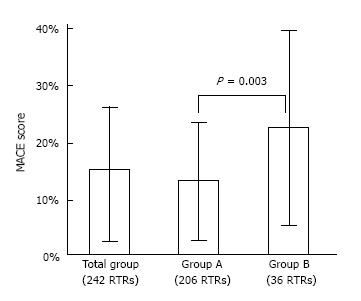
The 242 RTRs included in the study had a mean follow-up of 9.8 years, and 69% of the patients had at least 7 years of follow-up with a functioning graft. Thirty six patients (14.9%) experienced a MACE (1.52 events/100 patient-years) before graft loss with a median time to event 5 years (IQR 2.2-10 years). The 7-year predicted mean risk for MACE by using the 7-variable calculator was 14.6% ± 12.5% in the whole cohort of 242 RTRs. In RTRs who experienced a MACE the predicted risk was 22.3% ± 17.1% and was significantly higher than in RTRs without a subsequent event 13.3% ± 11.1% (P = 0.003) (Figure 1).
Table 3 provides the results of the univariate and multivariate analysis with MACE as the dependent variable of interest. In the univariate Cox regression analysis we found that the calculated MACE risk (HR = 1.04, 95%CI: 1.02-1.06) was associated with a higher risk of a subsequent event. When the risk factors of the model and other factors were tested separately, older age (HR = 1.05, 95%CI: 1.02-1.10), male sex (HR = 0.45, 95%CI: 0.20-0.99) and pre-existing CVD (HR = 3.63, 95%CI: 1.88-7.01) were associated with an increased risk of MACE. In the multivariate model, pre-existing CVD was the main independent predictor for the occurrence of MACE (HR = 2.86, 95%CI: 1.45-5.62), while older age (HR = 1.05, 95%CI: 1.01-1.08) was associated with an increased risk of MACE as well.
| Variables (units of increase) | Univariate | Multivariate | ||
| b (95%CI) | P | b (95%CI) | P | |
| MACE risk (1%) | 1.04 (1.02-1.06) | < 0.001 | ||
| Age (1 yr) | 1.05 (1.02-1.10) | 0.001 | 1.05 (1.01-1.08) | 0.005 |
| Sex (male reference) | 0.45 (0.20-0.99) | 0.05 | 0.58 (0.28-1.37) | 0.18 |
| Previous smoker | 1.51 (0.73-2.92) | 0.21 | ||
| Current smoker | 1.0 (0.44-2.29) | 0.99 | ||
| Systolic BP (1 mmHg) | 1.01 (0.99-1.02) | 0.61 | ||
| DM | 1.53 (0.78-2.98) | 0.21 | ||
| Previous CVD | 3.63 (1.88-7.01) | < 0.001 | 2.86 (1.45-5.62) | 0.006 |
| Number of grafts (first graft reference) | 0.50 (0.12-2.02) | 0.33 | ||
| Tοtal time on dialysis and transplantation (1 yr) | 0.99 (0.92-1.01) | 0.33 | ||
| Creatinine (1 mg/dL) | 0.90 (0.48-1.68) | 0.74 | ||
| Urine protein (1 mg/24 h) | 0.99 (0.99-1.00) | 0.28 | ||
| Total cholesterol | 0.99 (0.99-1.00) | 0.3 | ||
| (1 mg/dL) | ||||
| LDL (1 mg/dL) | 0.99 (0.98-1.01) | 0.46 | ||
| Hemoglobin (1 g/dL) | 1.14 (0.93-1.40) | 0.21 | ||
| PTH (1 pg/mL) | 1.00 (0.99-1.00) | 0.25 | ||
| CRP (1 mg/L) | 1.01 (0.92-1.09) | 0.88 | ||
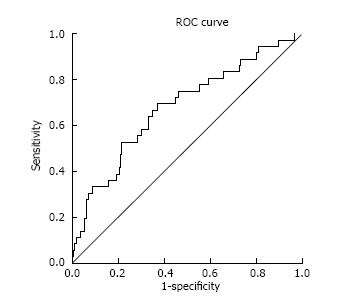
The discrimination ability of the model in the Greek cohort of RTRs was good with an area under the ROC curve of 0.68 (95%CI: 0.58-0.78) (Figure 2).
The incidence of MACE before graft loss in our clinical database of RTRs was 14.9% with a median time to event 5 years. Recipients who suffered a MACE were older and had higher prevalence of pre-existing CVD. The first attempt to apply an externally validated risk MACE model in a Greek cohort of RTRs showed that the model can be used for risk stratification in this population.
Disproportionate increased cardiovascular burden is true since the early stages of chronic kidney disease, further increases during dialysis and although renal transplantation removes hemodynamic and uremic abnormalities associated with dialysis, the vast majority of RTRs with a functioning graft die due to a MACE. In our study, RTRs with a functioning graft who suffered a MACE had higher prevalence of CVD before transplantation, with pre-existing CVD being the most significant risk factor for MACE in this cohort. As regards traditional cardiovascular risk factors such as smoking, hypertension, diabetes and lipid profile their prevalence did not significantly differ between the two groups in our database of RTRs and separately each one could not predict the occurrence of a MACE. Our findings are in accordance with the results of an early study by Kasiske et al[14] showing that the strongest risk factors were pre-existing coronary heart disease, cerebrovascular and peripheral vascular, which were associated with an increase of three to nine times in cardiovascular risk. In this study, there was not a relation between traditional risk factors (smoking, hypertension, or dyslipidemia) and CVD in 1000 RTRs. In the more recent PORT study, a large scale clinical database of 23575 RTRs, it was found that among the significant predicting factors for MACE were age, male sex and pre-existing CVD, whereas traditional modifiable cardiovascular risk factors were very poor predictors of cardiac events[10]. On the other hand, the investigators of the ALERT study used post-hoc analyses and identified the determinants of specific cardiovascular endpoints such as MI being associated with age, hyperlipidemia and diabetes[8].
Unconventional and transplant-related risk factors, including immunological and non-immunological ones further increase the risk of CVD after transplantation[10,15]. In particular, the large multicentre PORT study found that a number of transplant-specific variables, such as delayed graft function, acute rejection and eGFR could predict cardiac events[10]. However, interventional studies which tried to normalize unconventional modifiable risk factors, such as haemoglobin and homocysteine, failed to reduce occurrence of CVD in RTRs[16,17]. Moreover, immunosuppressive drugs prescribed to RTRs, mainly corticosteroids and calcineurin inhibitors (cyclosporine, tacrolimus), which possess diabetogenic and atherogenic side effects exacerbate established cardiovascular risk factors such as dyslipidemia, hypertension, and diabetes[18].
Given the fact that traditional, non-traditional and transplant-related risk factors separately only partly can explain the increased burden of CVD and that the interplay between all these factors seems to be the core of the increased cardiovascular risk in RTRs many groups of investigators have tried to apply established risk models or to create new risk calculators in order to accurate predict a subsequent cardiovascular event in this population. In particular, the use of the Framingham risk score in RTRs underestimates cardiovascular risk, although the addition of renal function in the Framingham equation was shown to improve the prediction of MACE[9,19]. More recently, Soveri et al[11] used data from the ALERT trial[8], a large scale multicenter trial and constructed a seven year, seven variable MACE risk equation with an area under the ROC curve of 0.738[11]. Subsequently they externally validated the 7-year risk calculator for discrimination and calibration in the PORT study database, which was an observational study[10]. Although the calculator was derived from the ALERT trial, a transplant population with moderate CVD risk, it was validated in the high risk RTRs of the PORT study and found suitable for this population with an area under the ROC curve of 0.740[12].
In this study we applied the MACE risk calculator in our cohort of RTRs from two transplant centers in Western Greece. According to the results the predicted risk was significantly higher in RTRs who experienced a MACE than in RTRs without a subsequent event and the calculator by preserving the discrimination ability is suitable for risk stratification in our population. The incidence of MACE in our database was 14.9%, while the incidence of MACE in ALERT trial was 11.8%. It should be noted that there were important differences in the composition of populations among the two studies as ALERT trial included moderate CVD risk RTRs from North Europe and Canada.
Nevertheless, our study has potential limitations which should be taken into consideration. First of all, this is a retrospective study conducted in a small sample population. Additionally, we did not report on data about graft survival and patients’ cardiovascular and total mortality as we included only RTRs with a functioning kidney graft at the time of the cross-sectional database review. Finally, we did not assess the possible effect of transplant-related risk factors, such as delayed graft function, acute rejection, on the occurrence of MACE.
In conclusion, pre-existing CVD was found to be the most important risk factor of a subsequent MACE, which necessitates holistic approach prevention strategies of CVD starting early in the course of chronic kidney disease. In our study, a validated MACE risk calculator was successfully tested in a Greek cohort of RTRs and was found to be suitable for the prediction of MACE in this patient group. Considering the fact that RTRs are a heterogenous population as well as the identification of new emerging transplant related risk factors, patient approach should always be individualized. Nevertheless, the application of cardiovascular risk prediction equations potentiates increased level of alertness among caregivers as well as improved interventional strategies in high risk patients.
We would like to thank Mr Vasilis Koutlas and Ms Eirini Tzalavra, Transplant Coordinators of the Renal Transplant Unit of University Hospital of Ioannina, for helping with the data collection. We also would like to thank Ms Eufrosuni Mplathra, for helping with the collection and record of data.
Kidney transplantation offers a significant improvement in all the cardiovascular parameters of end stage renal disease (ESRD) patients, reduces mortality risk and boosts quality of life.
To determine the risk factors for cardiovascular disease after kidney transplantation and validate a major advance cardiac event (MACE) risk model to a Greek renal transplant recipients (RTRs) cohort.
In this study, the authors found that older age, pre-existing cardiovascular disease (CVD) and MACE risk score, were significant predictors of post-transplant cardiovascular risk. So long as, there are modifiable components to the risk factors/scores, it is the belief that prevention of CVD early in chronic kidney disease along with control of these factors in ESRD patients and RTRs, could possible reduced cardiovascular burden to some degree.
The externally validated equation can be used in any appropriate RTR population to calculate MACE risk.
MACE was defined as one or more of nonfatal myocardial infarction and/or invasive coronary artery revascularization (angioplasty or coronary artery bypass grafting).
It is a well-written study about the event of cardiovascular disease after renal transplantation.
| 1. | Morales JM, Marcén R, del Castillo D, Andres A, Gonzalez-Molina M, Oppenheimer F, Serón D, Gil-Vernet S, Lampreave I, Gainza FJ. Risk factors for graft loss and mortality after renal transplantation according to recipient age: a prospective multicentre study. Nephrol Dial Transplant. 2012;27 Suppl 4:iv39-iv46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Ma MK, Lim WH, Craig JC, Russ GR, Chapman JR, Wong G. Mortality among Younger and Older Recipients of Kidney Transplants from Expanded Criteria Donors Compared with Standard Criteria Donors. Clin J Am Soc Nephrol. 2016;11:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2364] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 4. | Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 598] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Dimény EM. Cardiovascular disease after renal transplantation. Kidney Int Suppl. 2002;78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Dounousi E, Mitsis M, Naka KK, Pappas C, Lakkas L, Harisis C, Pappas K, Koutlas V, Tzalavra I, Spanos G. Differences in cardiac structure assessed by echocardiography between renal transplant recipients and chronic kidney disease patients. Transplant Proc. 2014;46:3194-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Vaidya OU, House JA, Coggins TR, Patil H, Vaidya A, Awad A, Main ML. Effect of renal transplantation for chronic renal disease on left ventricular mass. Am J Cardiol. 2012;110:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Jardine AG, Fellström B, Logan JO, Cole E, Nyberg G, Grönhagen-Riska C, Madsen S, Neumayer HH, Maes B, Ambühl P. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis. 2005;46:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Silver SA, Huang M, Nash MM, Prasad GV. Framingham risk score and novel cardiovascular risk factors underpredict major adverse cardiac events in kidney transplant recipients. Transplantation. 2011;92:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, Kasiske BL. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant. 2010;10:338-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Soveri I, Holme I, Holdaas H, Budde K, Jardine AG, Fellström B. A cardiovascular risk calculator for renal transplant recipients. Transplantation. 2012;94:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Soveri I, Snyder J, Holdaas H, Holme I, Jardine AG, L’Italien GJ, Fellström B. The external validation of the cardiovascular risk equation for renal transplant recipients: applications to BENEFIT and BENEFIT-EXT trials. Transplantation. 2013;95:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3805] [Cited by in RCA: 4307] [Article Influence: 215.4] [Reference Citation Analysis (0)] |
| 14. | Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. J Am Soc Nephrol. 1996;7:158-165. [PubMed] |
| 15. | Jardine AG, Gaston RS, Fellstrom BC, Holdaas H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet. 2011;378:1419-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, Selhub J, Jacques PF, Cole E, Gravens-Mueller L. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011;123:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Rigatto C. Anemia, renal transplantation, and the anemia paradox. Semin Nephrol. 2006;26:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Stoumpos S, Jardine AG, Mark PB. Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int. 2015;28:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Kiberd B, Panek R. Cardiovascular outcomes in the outpatient kidney transplant clinic: the Framingham risk score revisited. Clin J Am Soc Nephrol. 2008;3:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amiya E, Friedman EA, Salvadori M, Yong D, Yorioka N S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ