Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.26
Peer-review started: October 7, 2016
First decision: November 11, 2016
Revised: November 22, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: February 24, 2017
Processing time: 139 Days and 12.3 Hours
The calcineurin inhibitor (CNI) tacrolimus (TAC) is an integral part of the immunosuppressive regimen after solid organ transplantation. Although TAC is very effective in prevention of acute rejection episodes, its highly variable pharmacokinetic and narrow therapeutic window require frequent monitoring of drug levels and dose adjustments. TAC can cause CNI nephrotoxicity even at low blood trough levels (4-6 ng/mL). Thus, other factors besides the TAC trough level might contribute to CNI-related kidney injury. Unfortunately, TAC pharmacokinetic is determined by a whole bunch of parameters. However, for daily clinical routine a simple application strategy is needed. To address this problem, we and others have evaluated a simple calculation method in which the TAC blood trough concentration (C) is divided by the daily dose (D). Fast TAC metabolism (C/D ratio < 1.05) was identified as a potential risk factor for an inferior kidney function after transplantation. In this regard, we recently showed a strong association between fast TAC metabolism and CNI nephrotoxicity as well as BKV infection. Therefore, the TAC C/D ratio may assist transplant clinicians in a simple way to individualize the immunosuppressive regimen.
Core tip: The calcineurin inhibitor tacrolimus (TAC) is the mainstay of the immunosuppressive regimen after solid organ transplantation. Nevertheless, TAC can cause nephrotoxicity even at low blood trough levels. Thus, other factors than the TAC trough level might be responsible for kidney injury. Recently published studies showed a strong association between fast TAC metabolism and nephrotoxicity as well as BK virus infection. The TAC metabolism rate defined as the TAC concentration/dose ratio is a cost neutral tool to identify patients at risk for TAC-associated decline in renal function after transplantation.
- Citation: Thölking G, Gerth HU, Schuette-Nuetgen K, Reuter S. Influence of tacrolimus metabolism rate on renal function after solid organ transplantation. World J Transplant 2017; 7(1): 26-33
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/26.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.26
The calcineurin inhibitor (CNI) tacrolimus (TAC) is a cornerstone of the immunosuppressive regimen after solid organ transplantation. Nevertheless, its highly variable pharmacokinetics and narrow therapeutic window require frequent therapeutic drug monitoring (TDM) and the (nephrotoxic) side effects of TAC might limit its application[1]. In particular dose adjustment after TAC prescription is difficult as many patients often show troughs above or below their target range despite TDM. In order to overcome these limitations, new TAC formulations with different galenics have been developed and different protocols with TAC dose reduction, switch, elimination and combination of reduced TAC and mechanistic target of rapamycin (mTOR) inhibitors have been studied[2-5]. E.g., the recent ATHENA trial evaluates a de novo everolimus (EVR)-based regimen in combination with reduced cyclosporine A (CSA) or TAC vs a standard regimen in patients that underwent renal transplantion (RTx)[6]. Results of this trial are expected soon.
After RTx, low dosed TAC regimens showed superiority regarding the prevention of biopsy-proven acute rejection (BPAR) and preserving the kidney function compared to the CNI CSA and the mTOR inhibitor sirolimus (SRL)[7,8]. Consistently, the present KDIGO guideline recommends TAC-based immunosuppression after RTx[9].
TAC has also become a first choice immunosuppressive drug after liver transplantation (LTx)[10]. Compared to CSA, TAC-treated patients - though experiencing a higher rate of posttransplant diabetes mellitus - showed a significantly reduced mortality at 1- and 3-years post-transplant; rates of graft loss and (steroid-resistant) rejection were lower in these patients[11,12]. In order to avoid CNI nephrotoxicity in LTx patients, several studies have been conducted to evaluate treatment strategies in which standard dosed TAC was either replaced by low dose TAC and mTOR inhibitor or CNI were even completely eliminated from the regimen. In a study with 78 LTx patients renal function recovered slightly after conversion from TAC to an mTOR inhibitor-based regimen[13]. Immunosuppression was switched 31 mo (median) after LTx. Additionally, Fischer et al[14] showed in a prospective, multicenter, open-label study with de novo LTx patients that patients who were randomized to regimen with reduced TAC dose and EVR 30 d after LTx developed lower rates of BPAR and had an improved renal function from randomization to month 36 compared to patients with standard TAC doses. Of note, randomization to the TAC elimination arm in this study was stopped prematurely due to significant higher BPAR rates[15].
In pancreas, heart, lung, or combined organ transplantation, TAC also constitutes an integral part of the immunosuppressive regimen[16-20]. CNI-sparing or -free regimens in these patients are currently investigated but safety of these concepts is still under debate. Notably, none of these CNI-free regimens has yet been shown to provide an immunosuppressive efficacy that equals those of CNIs[21-23].
After pancreas transplantation TAC and mycophenolate mofetil (MMF) maintenance therapy seems to be the most effective immunosuppressive regimen with regard to long term survival and prevention of acute rejection[16,24]. However, occurrence of TAC-related side effects like posttransplant diabetes mellitus or nephrotoxicity has led to increasing efforts to minimize CNI in this cohort. E.g., in one study pancreas transplanted patients were switched from standard immunosuppression with TAC and MMF to low dose TAC and SRL[25]. From the authors view, the low dose TAC and SRL regimen was safe and did not worsen proteinuria and renal function when compared with TAC and MMF. In simultaneous pancreas and kidney transplantation Sageshima et al[17] evaluated the efficacy and safety of TAC and EVR compared to TAC and MMF in a retrospective study. Unfortunately, both studies failed to show relevant advantages of the combined TAC/mTOR regimen.
The introduction of EVR in the maintenance therapy of heart transplant recipients, with reduced CNI, has been shown to significantly improve the renal function during an observational period of at least 5 years[18]. An early renal benefit in lung transplant recipients was lost over the time but long-term immunosuppressive efficacy was maintained[18].
Despite all efforts to minimize TAC exposure and its side effects even in low dose regimens (4-6 ng/mL)[26], TAC, however, remains the mainstay of the immunosuppressive regimen after solid organ transplantation[2,14]. Therefore, transplant physicians need an approach to identify patients at risk to develop TAC-related side effects in clinical routine. We and others proposed that the patient’s individual TAC metabolism type can be used for adaption of the immunosuppressive regimen. We believe that the TAC metabolism rate defined as the TAC blood trough concentration (C) divided by the daily dose (D) is such a convenient predictor for TAC metabolism estimation. Perspectively, C/D tests could probably detect patients at high risk of developing TAC-related complications even before their transplantation.
Due to missing data on the TAC metabolism rate and its value in recipients of other organ transplants than kidney and liver, we herein focus on the impact of the C/D ratio in the latter.
TAC has a narrow therapeutic window and can cause acute and chronic nephrotoxicity. However, some authors even question the concept of a “harmless” therapeutic window. They state that yet to be effective, CNI must operate within their nephrotoxic therapeutic range as can be seen when CNI withdrawal leads to an immediate increase in estimated glomerular filtration rate (eGFR)[1]. Activation of the renin-angiotensin system and increased sympathetic nerve activity causing vasospasm of renal arteries might be involved in this context[27,28]. An imbalance of vasodilatory factors like nitric oxide[29,30] and prostaglandins[30] and vasoconstrictive variables like thromboxane[31] and endothelin[32] is discussed to promote further renal damage.
Acute CNI nephrotoxicity typically appears early after RTx correlating to the period of high CNI exposure. It presents, e.g., with acute arteriolopathy, tubular vacuolization and swelling of endothelial cells and death of myocytes of the tunica media[1]. The prevalence of CNI-associated chronic lesions increases by time[1].
Tubule-interstitial fibrosis/tubular atrophy (IF/TA) is a typical histological finding in chronic CNI-related allograft injury. Tubular microcalcifications, glomerulosclerosis and arteriolar hyalinosis are further chronic manifestations. In contrast to some acute CNI-related kidney injuries which can be resolved within the first months after RTx, chronic CNI-nephrotoxicity observed after the third month after RTx is usually progressive[1].
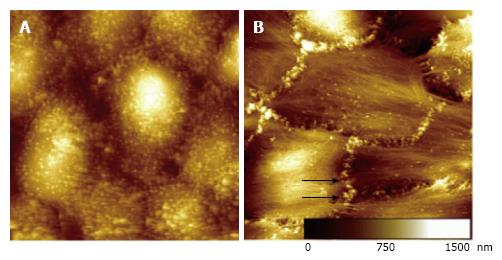
For example, TAC exposure induces epithelial-mesenchymal transition (EMT) by activation of the profibrotic cytokine transforming growth factor-β1 (TGF-β1) pathway in renal tubular cells[33]. TGF-β1 in turn induces cell growth, increases the production of smooth-muscle actin and stress fiber formation in epithelial cells[33,34]. This results in a decrease of cellular surface microvilli and increases stiffness of tubular epithelial cells[35]. During this conversion process, tubular cells loose epithelial characteristics and appear in a mesenchymal shape (Figure 1[35]) (EMT). However, these effects seem to be cell specific. While some cells have the ability to proliferate, others are decomposed by autophagy[33,36].
Early withdrawal or dose reduction of TAC/CNI and introduction of an mTOR inhibitor might stabilize fibrosis[37]. However, the adequate time point for TAC/CNI withdrawal or dose reduction is still elusive and the group of patients who might benefit from this intervention remains yet to be clearly identified[2,3,13,14,38].
TAC metabolism underlies several individual, genetic and clinical, as well as pharmacokinetic factors. Recipient age, gender, body mass index, delayed graft function, hematocrit, serum albumin and absorption have been proposed to be relevant determinants[39-42]. However, some of these factors are still a matter of debate.
Drug interactions interfering with TAC metabolism are of high clinical relevance for physicians. Changes of the TAC pharmacokinetic by other immunosuppressive drugs, such as EVR, SRL and corticosteroids have to be considered in daily routine. Especially, induction of TAC metabolism by high doses of corticosteroids has to be taken into account early after transplantation[40,41,43]. Whether these interactions are of clinical relevance or not remains largely unknown[44].
TAC metabolism is influenced by cytochrome-P450 enzymes CYP3A expression variants, e.g., in the intestine[45,46]. This genetic expression variant determines the first-pass effect of orally administered TAC. This is important, because Sato et al[47] showed that recipients taking their usual dose of TAC in case of diarrhea had significant elevated trough levels and a prolongation of maximum concentrations when compared to the regular situation. It is supposed, that this phenomenon is caused by a shift of the main intestinal areas of TAC metabolism (duodenum and jejunum) to the lower intestine[47,48].
The CYP3A4 and CYP3A5 variants in the liver lead to significant differences in TAC pharmacokinetics[39,41,49]. Predominantly but not exclusively, CYP3A5*1-expressors have been characterized as fast TAC metabolizers, while slow metabolizers mostly express CYP3A5*3[45,50-52]. Early after transplantation, it has been shown that a rapid decline in TAC metabolism is only present in CYP3A5*3/*3 patients while the decline is absent in CYP3A5*1 allele carriers[53,54]. This finding might be explained by high steroid doses and a gradual rise in hematocrit that affect CYP3A5*3/*3 and CYP3A4 activity. In comparison, CYP 3A5 carriers (CYP3A5*1) receive higher TAC doses (fast metabolizers) early after transplantation and continue with a higher or even increased exposure as time after transplantation elapses[41]. In a meta-analysis, Shi et al[55] showed that especially CYP3A4*1B genetic polymorphism may affect TAC metabolism. If the presence of CYP3A5 in the kidney, i.e., in the renal apical tubular plasma membrane impacts, e.g., on the degree of CNI nephrotoxicity is still a matter of debate[56,57].
Unfortunately, the dosage needed to achieve the target TAC level varies in patients with known CYP3A polymorphisms over time[58]. Therefore, genetic testing does not solve the dosing problem and we still have to rely on trough level testing. To end this, genotyping of patients is still far from being a routine test and at present of questionable relevance in the daily transplant setting.
The TAC concentration/dose ratio (C/D ratio) is an established equation to describe the TAC metabolism rate[59-61]: C/D ratio (ng/mL * 1/mg) = [Blood TAC trough concentration (ng/mL)]/[Daily TAC dose (mg)].
We intended to keep the approach very simple and tested if body weight (which was suggested to be included into the equation by others) can be removed from the equation[59,60,62]. Our approach was supported by Kim et al[58] who showed that TAC adverse events in a 5-year follow-up of RTx patients were independent from body weight.
The presented equation provides a simple, cost neutral clinical tool which can be applied without performing additional tests. Standard trough levels from regular therapeutic TAC drug monitoring can be used for C/D ratio calculation of in- as well as outpatients.
We analyzed TAC metabolism using the C/D ratio in a study of 248 RTx patients at our center. Analyzing the outcomes and distribution of recipients’ C/D ratios in our cohort, we calculated a cut off for the TAC C/D ratio of 1.05 for definition of fast metabolizers. After a 24 mo follow-up, patients with a C/D ratio < 1.05 had a lower eGFR, needed more indication biopsies and showed more often biopsy proven CNI nephrotoxicity compared to intermediate and slow TAC metabolizers[60]. In accordance with our data, Kuypers et al[63] showed that CYP3A5*1 genotype carriers (mainly fast metabolizers) had a significantly increased risk for biopsy-proven TAC-induced nephrotoxicity [HR: 2.38 (1.15-4.92), P = 0.01] at 3 mo post-transplant. These results were confirmed by Genvigir et al[64] who also reported that carriage of two or more fast metabolization CYP3A5 alleles is associated with lower eGFR values ninety days after RTx. Rojas et al[65] showed that the weight adjusted C/D ratio in RTx recipients among CYP3A5*1 allele carriers compared with carriers of the CYP3A5*3/*3 genotype was lower and demonstrated that the expresser genotype was associated with a higher risk of acute rejection and chronic nephrotoxicity. Nevertheless, further studies on similar and different ethnical cohorts showed partly contradictory results[66-68]. Thus, until now, the prediction of renal function by CYP3A genotyping still remains ambiguous.
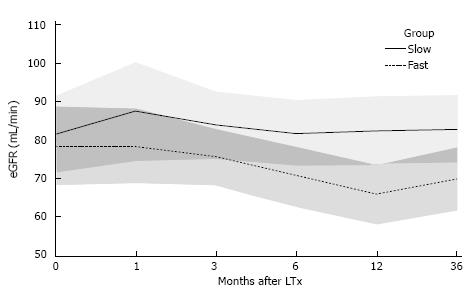
We confirmed our findings in a cohort of LTx patients. During a 36 mo follow-up renal function was lower in fast TAC metabolizers (defined by C/D ratio) than in slow TAC metabolizers (Figure 2)[59]. In this study, fast metabolizers had more TAC side effects like higher rates of assumed CNI nephrotoxicity and had been more often switched from TAC to other immunosuppressive drugs.
It is well known that higher TAC trough levels are more toxic and increase the risk of side effects[69]. Jacobson et al for example calculated a HR of 1.22 for a 1 ng/mL increase of the TAC trough level to develop acute CNI nephrotoxicity after RTx. It is important to note that our RTx patients in the fast metabolizer cohort had lower TAC trough levels at 1, 3 and 6 mo after transplantation compared to slower metabolizers (Table 1[60]). This was confirmed in a cohort of LTx patients[59]. Kuypers et al[53] identified nephrotoxicity to be dependent on the TAC dose. In accordance to these results, in our studies fast metabolizers received higher TAC doses than slow metabolizers 1, 3 and 6 mo after transplantation[59,60]. These findings led us to the hypothesis that CNI nephropathy predominantly seen in fast metabolizers might be related to TAC overexposure during the first hours after TAC intake.
| Fast metabolizers (n = 97) | Interm metabolizers (n = 78) | Slow metabolizers (n = 73) | P value | |
| Tacrolimus mean trough level (ng/mL) | 8.2 ± 1.6 | 9.2 ± 1.8 | 9.5 ± 1.8 | < 0.001a |
| After 1 mo | 9.4 ± 3.2 | 10.5 ± 2.7 | 11.0 ± 3.2 | 0.002a |
| After 3 mo | 7.8 ± 2.1 | 9.1 ± 2.9 | 9.5 ± 2.8 | < 0.001a |
| After 6 mo | 7.2 ± 2.3 | 7.8 ± 2.4 | 8.0 ± 2.8 | 0.079a |
| Tacrolimus mean daily dose (mg) | 11 (6-27) | 8 (4-14) | 6 (2-12) | < 0.001b |
| After 1 mo | 14 (6-40) | 10 (4-22) | 8 (2-20) | < 0.001b |
| After 3 mo | 10 (4-23) | 7 (4-13) | 4 (2-12) | < 0.001b |
| After 6 mo | 9 (3-21) | 5 (2-10) | 3 (2-8) | < 0.001b |
| Prednisolon mean daily dose (mg) | 15 (4-37) | 14 (5-70) | 13 (0-40) | 0.06b |
| After 1 mo | 20 (15-90) | 20 (15-70) | 20 (0-50) | 0.155b |
| After 3 mo | 14 (3-30) | 13 (5-30) | 13 (0-30) | 0.496b |
| After 6 mo | 10 (5-30) | 9 (5-20) | 8 (0-20) | 0.114b |
This hypothesis is supported by the finding that besides increased rates of CNI nephrotoxicity, a higher incidence of BKV nephropathy (BKN) is observed in fast TAC metabolizers[60]. This was confirmed in a second study involving 192 RTx patients (96 BKV positive and 96 BKV negative controls). Patients with BKV infection showed lower Tac C/D ratios at 1, 3 and 6 mo after RTx and were mainly classified as fast TAC metabolizers[62]. Therefore, fast TAC metabolizers seem to be prone to CNI nephrotoxicity and suffer more likely from BKV infection[70].
Although TAC is a cornerstone in the immunosuppressive regimen after solid organ transplantation, nephrotoxic site effects and a narrow therapeutic window may limit its application. Elimination, dose reduction, or replacement of TAC is often foiled by increased rates of BPAR[14,38], occurrence of adverse events[8] or considerable rise in the costs caused by replacing immunosuppressive drugs like belatacept[71]. Due to the fact, that CNI nephrotoxicity can also occur in regimens with low TAC target levels[26], a tool to identify patients at risk for developing an inferior kidney function is desirable.
We were able to demonstrate a strong association between a low TAC C/D ratio (< 1.05 ng/mL*1/mg) and reduced renal function after a follow-up of 24 and 36 mo after RTx and LTx, respectively[59,60]. Furthermore, a low C/D ratio (fast TAC metabolism) led to more indication biopsies, more CNI nephrotoxicity and more BKV infection after RTx[62].
In this context, CYP3A genotyping has improved our knowledge on TAC metabolism and might explain why patients present as slow or fast metabolizers but its predictive value in terms of TAC dose requirement or renal function is still unsatisfactory[58]. Currently, genetic testing does not deliver relevant data and counteracts our simplification strategy in the daily routine.
In conclusion, fast TAC metabolism is associated with a reduced renal function after RTx and LTx. Higher rates of CNI nephrotoxicity and BKV infections/BKVN are assumed to be at least partly responsible for these results. Calculation of the TAC C/D ratio is a simple clinical tool that may assist transplant clinicians in individualizing immunosuppressive regimens.
Controlled, prospective, multicenter trials are needed to confirm the predictive value of the TAC C/D ratio.
| 1. | Nankivell BJ, PNg CH, OConnell PJ, Chapman JR. Calcineurin Inhibitor Nephrotoxicity Through the Lens of Longitudinal Histology: Comparison of Cyclosporine and Tacrolimus Eras. Transplantation. 2016;100:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U, Vanrenterghem Y. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9:1876-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, Schemmer P, Fischer L, Klein CG, Nadalin S. Long-term follow-up of five yr shows superior renal function with everolimus plus early calcineurin inhibitor withdrawal in the PROTECT randomized liver transplantation study. Clin Transplant. 2016;30:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 4. | Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96:191-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Beckebaum S, Iacob S, Sweid D, Sotiropoulos GC, Saner F, Kaiser G, Radtke A, Klein CG, Erim Y, de Geest S. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011;24:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Nashan B, Thaiss F. Design and rationale of the ATHENA study--A 12-month, multicentre, prospective study evaluating the outcomes of a de novo everolimus-based regimen in combination with reduced cyclosporine or tacrolimus versus a standard regimen in kidney transplant patients: study protocol for a randomised controlled trial. Trials. 2016;17:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1417] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 8. | Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 412] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Kidney Disease: Improving Global Outcomes Transplant Work G. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3:S1-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 1158] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 746] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 11. | McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6:1578-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | O’Grady JG, Hardy P, Burroughs AK, Elbourne D; UK and Ireland Liver Transplant Study Group. Randomized controlled trial of tacrolimus versus microemulsified cyclosporin (TMC) in liver transplantation: poststudy surveillance to 3 years. Am J Transplant. 2007;7:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Hüsing A, Schmidt M, Beckebaum S, Cicinnati VR, Koch R, Thölking G, Stella J, Heinzow H, Schmidt HH, Kabar I. Long-Term Renal Function in Liver Transplant Recipients After Conversion From Calcineurin Inhibitors to mTOR Inhibitors. Ann Transplant. 2015;20:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, Duvoux C, Nevens F, Fung JJ, Dong G. Three-year Outcomes in De Novo Liver Transplant Patients Receiving Everolimus With Reduced Tacrolimus: Follow-Up Results From a Randomized, Multicenter Study. Transplantation. 2015;99:1455-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 16. | Gruessner AC, Gruessner RW. Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant. 2016;21:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Sageshima J, Ciancio G, Chen L, Dohi T, El-Hinnawi A, Paloyo S, Misawa R, Ekwenna O, Yatawatta A, Burke GW. Everolimus with low-dose tacrolimus in simultaneous pancreas and kidney transplantation. Clin Transplant. 2014;28:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Gullestad L, Eiskjaer H, Gustafsson F, Riise GC, Karason K, Dellgren G, Rådegran G, Hansson L, Gude E, Bjørtuft Ø, Jansson K, Schultz HH, Solbu D, Iversen M. Long-term outcomes of thoracic transplant recipients following conversion to everolimus with reduced calcineurin inhibitor in a multicenter, open-label, randomized trial. Transpl Int. 2016;29:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Kaczmarek I, Zaruba MM, Beiras-Fernandez A, Reimann R, Nickel T, Grinninger C, Sadoni S, Hagl C, Meiser B. Tacrolimus with mycophenolate mofetil or sirolimus compared with calcineurin inhibitor-free immunosuppression (sirolimus/mycophenolate mofetil) after heart transplantation: 5-year results. J Heart Lung Transplant. 2013;32:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Neurohr C, Huppmann P, Zimmermann G, Leuchte H, Baumgartner R, Hatz R, Frey L, Uberfuhr P, Bittmann I, Behr J. Tacrolimus and mycophenolate mofetil as first line immunosuppression after lung transplantation. Transpl Int. 2009;22:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Kimelman M, Brandacher G. Trends in immunosuppression after pancreas transplantation: what is in the pipeline? Curr Opin Organ Transplant. 2013;18:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6:1039-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 23. | Aliabadi A, Cochrane AB, Zuckermann AO. Current strategies and future trends in immunosuppression after heart transplantation. Curr Opin Organ Transplant. 2012;17:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Schulz T, Pries A, Caliebe A, Kapischke M. Long-term survival after simultaneous pancreas-kidney transplantation with primary function of at least one year--a single-center experience. Ann Transplant. 2014;19:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Kandula P, Fridell J, Taber TE, Sharfuddin A, Yaqub MS, Phillips CL, Chen J, Mujtaba M. Impact of tacrolimus-sirolimus maintenance immunosuppression on proteinuria and kidney function in pancreas transplant alone recipients. Transplantation. 2012;94:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Tsuchiya T, Ishida H, Tanabe T, Shimizu T, Honda K, Omoto K, Tanabe K. Comparison of pharmacokinetics and pathology for low-dose tacrolimus once-daily and twice-daily in living kidney transplantation: prospective trial in once-daily versus twice-daily tacrolimus. Transplantation. 2013;96:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Klein IH, Abrahams AC, van Ede T, Oey PL, Ligtenberg G, Blankestijn PJ. Differential effects of acute and sustained cyclosporine and tacrolimus on sympathetic nerve activity. J Hypertens. 2010;28:1928-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Zuber M, Donnerer J. Effect of FK506 on neurotransmitter content and expression of GAP-43 in neurotoxin-lesioned peripheral sensory and sympathetic neurons. Pharmacology. 2002;66:44-50. [PubMed] |
| 29. | Singh L, Singh G, Sharma A, Sinha A, Bagga A, Dinda AK. A comparative study on renal biopsy before and after long-term calcineurin inhibitors therapy: an insight for pathogenesis of its toxicity. Hum Pathol. 2015;46:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Gossmann J, Radounikli A, Bernemann A, Schellinski O, Raab HP, Bickeböller R, Scheuermann EH. Pathophysiology of cyclosporine-induced nephrotoxicity in humans: a role for nitric oxide? Kidney Blood Press Res. 2001;24:111-115. [PubMed] |
| 31. | Yamada K, Sugisaki Y, Suzuki S, Akimoto M, Amemiya H, Yamanaka N. New morphological changes induced by FK506 in a short period in the rat kidney and the effect of superoxide dismutase and OKY-046 on THEM: the relationship of FK506 nephrotoxicity to lipid peroxidation and change in production of thromboxane A2 in the kidney. Transpl Int. 1992;5 Suppl 1:S564-S567. [PubMed] |
| 32. | Raina A, Horn ET, Benza RL. The pathophysiology of endothelin in complications after solid organ transplantation: a potential novel therapeutic role for endothelin receptor antagonists. Transplantation. 2012;94:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Bennett J, Cassidy H, Slattery C, Ryan MP, McMorrow T. Tacrolimus Modulates TGF-β Signaling to Induce Epithelial-Mesenchymal Transition in Human Renal Proximal Tubule Epithelial Cells. J Clin Med. 2016;5:pii: E50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 34. | Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 823] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 35. | Thoelking G, Reiss B, Wegener J, Oberleithner H, Pavenstaedt H, Riethmuller C. Nanotopography follows force in TGF-beta1 stimulated epithelium. Nanotechnology. 2010;21:265102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Rivelli RF, Gonçalves RT, Leite M, Santos MA, Delgado AG, Cardoso LR, Takiya CM. Early withdrawal of calcineurin inhibitor from a sirolimus-based immunosuppression stabilizes fibrosis and the transforming growth factor-β signalling pathway in kidney transplant. Nephrology (Carlton). 2015;20:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Budde K, Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, Wüthrich RP, Mühlfeld A, Heller K, Porstner M. Five-year outcomes in kidney transplant patients converted from cyclosporine to everolimus: the randomized ZEUS study. Am J Transplant. 2015;15:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Kuypers DR, de Loor H, Naesens M, Coopmans T, de Jonge H. Combined effects of CYP3A5*1, POR*28, and CYP3A4*22 single nucleotide polymorphisms on early concentration-controlled tacrolimus exposure in de-novo renal recipients. Pharmacogenet Genomics. 2014;24:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Stratta P, Quaglia M, Cena T, Antoniotti R, Fenoglio R, Menegotto A, Ferrante D, Genazzani A, Terrazzino S, Magnani C. The interactions of age, sex, body mass index, genetics, and steroid weight-based doses on tacrolimus dosing requirement after adult kidney transplantation. Eur J Clin Pharmacol. 2012;68:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | de Jonge H, Vanhove T, de Loor H, Verbeke K, Kuypers DR. Progressive decline in tacrolimus clearance after renal transplantation is partially explained by decreasing CYP3A4 activity and increasing haematocrit. Br J Clin Pharmacol. 2015;80:548-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Gijsen V, Mital S, van Schaik RH, Soldin OP, Soldin SJ, van der Heiden IP, Nulman I, Koren G, de Wildt SN. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant. 2011;30:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Anglicheau D, Flamant M, Schlageter MH, Martinez F, Cassinat B, Beaune P, Legendre C, Thervet E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18:2409-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Kuypers DR. Influence of interactions between immunosuppressive drugs on therapeutic drug monitoring. Ann Transplant. 2008;13:11-18. [PubMed] |
| 45. | Uesugi M, Masuda S, Katsura T, Oike F, Takada Y, Inui K. Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharmacogenet Genomics. 2006;16:119-127. [PubMed] |
| 46. | Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G, Starzl T. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 614] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 47. | Sato K, Amada N, Sato T, Miura S, Ohashi Y, Sekiguchi S, Satomi S, Okazaki H. Severe elevations of FK506 blood concentration due to diarrhea in renal transplant recipients. Clin Transplant. 2004;18:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Lampen A, Christians U, Gonschior AK, Bader A, Hackbarth I, von Engelhardt W, Sewing KF. Metabolism of the macrolide immunosuppressant, tacrolimus, by the pig gut mucosa in the Ussing chamber. Br J Pharmacol. 1996;117:1730-1734. [PubMed] |
| 49. | Tavira B, Coto E, Díaz-Corte C, Ortega F, Arias M, Torres A, Díaz JM, Selgas R, López-Larrea C, Campistol JM. Pharmacogenetics of tacrolimus after renal transplantation: analysis of polymorphisms in genes encoding 16 drug metabolizing enzymes. Clin Chem Lab Med. 2011;49:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1618] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 51. | Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Gijsen VM, Madadi P, Dube MP, Hesselink DA, Koren G, de Wildt SN. Tacrolimus-induced nephrotoxicity and genetic variability: a review. Ann Transplant. 2012;17:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Kuypers DR, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82:711-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Hesselink DA, van Schaik RH, van Agteren M, de Fijter JW, Hartmann A, Zeier M, Budde K, Kuypers DR, Pisarski P, Le Meur Y. CYP3A5 genotype is not associated with a higher risk of acute rejection in tacrolimus-treated renal transplant recipients. Pharmacogenet Genomics. 2008;18:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Shi WL, Tang HL, Zhai SD. Effects of the CYP3A4*1B Genetic Polymorphism on the Pharmacokinetics of Tacrolimus in Adult Renal Transplant Recipients: A Meta-Analysis. PLoS One. 2015;10:e0127995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Joy MS, Hogan SL, Thompson BD, Finn WF, Nickeleit V. Cytochrome P450 3A5 expression in the kidneys of patients with calcineurin inhibitor nephrotoxicity. Nephrol Dial Transplant. 2007;22:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Metalidis C, Lerut E, Naesens M, Kuypers DR. Expression of CYP3A5 and P-glycoprotein in renal allografts with histological signs of calcineurin inhibitor nephrotoxicity. Transplantation. 2011;91:1098-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Kim IW, Noh H, Ji E, Han N, Hong SH, Ha J, Burckart GJ, Oh JM. Identification of factors affecting tacrolimus level and 5-year clinical outcome in kidney transplant patients. Basic Clin Pharmacol Toxicol. 2012;111:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Thölking G, Siats L, Fortmann C, Koch R, Hüsing A, Cicinnati VR, Gerth HU, Wolters HH, Anthoni C, Pavenstädt H. Tacrolimus Concentration/Dose Ratio is Associated with Renal Function After Liver Transplantation. Ann Transplant. 2016;21:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Thölking G, Fortmann C, Koch R, Gerth HU, Pabst D, Pavenstädt H, Kabar I, Hüsing A, Wolters H, Reuter S. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS One. 2014;9:e111128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Ji E, Choi L, Suh KS, Cho JY, Han N, Oh JM. Combinational effect of intestinal and hepatic CYP3A5 genotypes on tacrolimus pharmacokinetics in recipients of living donor liver transplantation. Transplantation. 2012;94:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Thölking G, Schmidt C, Koch R, Schuette-Nuetgen K, Pabst D, Wolters H, Kabar I, Hüsing A, Pavenstädt H, Reuter S. Influence of tacrolimus metabolism rate on BKV infection after kidney transplantation. Sci Rep. 2016;6:32273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Kuypers DR, Naesens M, de Jonge H, Lerut E, Verbeke K, Vanrenterghem Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit. 2010;32:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 64. | Genvigir FD, Salgado PC, Felipe CR, Luo EY, Alves C, Cerda A, Tedesco-Silva H, Medina-Pestana JO, Oliveira N, Rodrigues AC. Influence of the CYP3A4/5 genetic score and ABCB1 polymorphisms on tacrolimus exposure and renal function in Brazilian kidney transplant patients. Pharmacogenet Genomics. 2016;26:462-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Rojas L, Neumann I, Herrero MJ, Bosó V, Reig J, Poveda JL, Megías J, Bea S, Aliño SF. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2015;15:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Chen JS, Li LS, Cheng DR, Ji SM, Sun QQ, Cheng Z, Wen JQ, Sha GZ, Liu ZH. Effect of CYP3A5 genotype on renal allograft recipients treated with tacrolimus. Transplant Proc. 2009;41:1557-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Quteineh L, Verstuyft C, Furlan V, Durrbach A, Letierce A, Ferlicot S, Taburet AM, Charpentier B, Becquemont L. Influence of CYP3A5 genetic polymorphism on tacrolimus daily dose requirements and acute rejection in renal graft recipients. Basic Clin Pharmacol Toxicol. 2008;103:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 68. | Naesens M, Lerut E, de Jonge H, Van Damme B, Vanrenterghem Y, Kuypers DR. Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol. 2009;20:2468-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900-905. [PubMed] |
| 70. | Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 71. | Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 588] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Friedman EA, Kita K, Nechifor G, Trkulja V S- Editor: Song XX L- Editor: A E- Editor: Lu YJ