Published online Dec 24, 2016. doi: 10.5500/wjt.v6.i4.697
Peer-review started: April 28, 2016
First decision: July 5, 2016
Revised: September 1, 2016
Accepted: September 21, 2016
Article in press: September 23, 2016
Published online: December 24, 2016
Processing time: 229 Days and 22.8 Hours
To compare the impact of tacrolimus (FK) and cyclosporine (CYA) on acute rejection and graft survival and to assess the predominant causes of graft loss between patients receiving these two calcineurin inhibitors (CNIs).
Retrospective review of 1835 patients who received a kidney transplant (KTX) between 1999-2012. Patients were grouped based on initial CNI utilized: 1195 in FK group, 640 in CYA group. Data on baseline characteristics, clinical outcomes, and causes of graft loss in both groups were analyzed.
Cumulative acute rejection rates were 14% in the FK vs 24% in the CYA group. Despite more marginal donor characteristics in the FK group, these patients had better graft survival rates compared to the CYA group. Three and five year graft survival rates were 88% and 84% respectively in the FK group compared to 79% and 70% respectively in the CYA group (P < 0.001). After multivariate analysis, which controlled for confounders, FK use was a strong predictor for lower acute rejection rates [odds ratio (OR) 0.60, 95%CI: 0.45-0.79] and better renal allograft survival (OR 0.740, 95%CI: 0.58-0.94). Death with a functioning graft was the most common cause of graft loss in both groups. Common causes of death included cardiovascular disease, infections, and malignancies. Chronic allograft nephropathy was also found to be an important cause of graft loss, being more prevalent in the CYA group.
The use of FK-based maintenance immunosuppression therapy is associated with a significantly lower rate of acute rejection and better graft survival compared to CYA-based regimen. Individualizing immunosuppression through risk-stratified CNI choice may lead to improved outcomes across all spectra of KTX patients.
Core tip: Tacrolimus (FK) has surpassed cyclosporine (CYA) as the calcineurin inhibitor (CNI) of choice for the vast majority of kidney transplant (KTX) programs. Yet, CYA continues to be an important alternative for patients intolerant to FK. FK is associated with significantly lower rate of acute rejection and better graft survival compared to CYA. Individualizing immunosuppression through risk-stratified CNI choice may lead to improved outcomes across all spectra of KTX patients.
- Citation: Kamel M, Kadian M, Srinivas T, Taber D, Posadas Salas MA. Tacrolimus confers lower acute rejection rates and better renal allograft survival compared to cyclosporine. World J Transplant 2016; 6(4): 697-702
- URL: https://www.wjgnet.com/2220-3230/full/v6/i4/697.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i4.697
Calcineurin inhibitors (CNIs) are the main immunosuppressive agents utilized in kidney transplantation[1]. Cyclosporine (CYA) and tacrolimus (FK) are currently the most widely used maintenance immunosuppressants for prevention of acute rejection in kidney transplant recipients. CYA-based regimen was more common in the era of 1990 until 2002, after which FK-based regimen became more commonly used in most transplant programs. In our transplant center, FK became the primary CNI of choice in 2005. FK and CYA show variable side effect profiles. Hypertension, hyperlipidemia, gum hypertrophy, and hirsutism occur more frequently with CYA use. On the other hand, a higher incidence of post-transplant diabetes mellitus is observed with FK therapy. Prolonged use of CNI may result in nephrotoxicity.
FK use is associated with less acute rejection compared to CYA, as documented in different studies[2,3]. Mayer et al[2] found that among 448 renal transplant recipients who were on triple therapy (FK or CYA + Azathioprine + Prednisone), patients who were in the FK group had a significant reduction in the frequency of acute rejection at 12 mo (FK 25.9% vs CYA 45.7%; P < 0.001). Ekberg et al[3] also found that at 12 mo post-transplant, the use of FK-based regimen is associated with less biopsy-proven acute rejection compared to CYA use (12.3% vs 25.8%, P < 0.01).
FK is frequently preferred in patients with high immunologic risk (highly sensitized, ABO-incompatible organ recipients), delayed graft function, and African American race. Data regarding graft survival based on the use of FK vs CYA is controversial with most studies showing similar graft survival rates with the use of either agent[4]. Vincenti et al[5] showed comparable patient (79.1% vs 81.4%; P = 0.472) and graft (64.3% vs 61.6%; P = 0.558) survival between treatment arms at 5 years of follow-up among FK and CYA-treated patients. However, after accounting for patients initially on CYA who crossed over to FK, the authors found significantly reduced graft failure in the FK group[5]. Gonwa et al[6] showed that among 223 kidney transplant recipients who experienced delayed graft function, patients who used FK-based therapy had a better 3-year graft survival compared to CYA use (84.1% vs 49.9%, P = 0.02). Given these conflicting findings, this study aims to compare rates of acute rejection and graft loss among patients who receive FK and CYA.
This was a retrospective cohort study of 1835 patients who received a KTX between 1999-2012 at a single center. Patients were grouped based on the type of CNI they were prescribed: 1195 patients utilized FK-based immunosuppression whereas 640 patients were on a CYA-based regimen. All patients received an antimetabolite and prednisone in combination with CNI. The initial CYA dose was 4-5 mg/kg PO BID. Target CYA levels were 350-400 ng/mL for weeks 1-4, 250-350 ng/mL for weeks 5-12, 200-300 ng/mL within the first year post-transplant, and 100-200 ng/mL thereafter. Initial FK doses were given at 0.025-0.05 mg/kg PO BID. Target FK levels were kept between 8-12 ng/mL within the first four weeks post-transplant, then 6-10 ng/mL within the first year post-transplant, and 4-6 ng/mL subsequently. Characteristics of recipients (age, race, sex, BMI, etiology of kidney disease, history of heart disease, diabetes, hypertension, years on dialysis, panel reactive antibody, preemptive transplant, living donor transplant), and donors [age, race, kidney donor risk index (KDRI)] were compared between groups. Characteristics of the kidney transplant (cold ischemia time, induction agent) as well as clinical outcomes (cumulative acute rejection rate, delayed graft function, three, and five year graft survival) were also analyzed. The Banff ’97 criteria were used to define the different grades of rejection. Based on center protocol, Banff 1A and 1B rejection episodes were treated with Methylprednisolone IV. Rejection episodes with Banff 2A grade or higher were treated with anti-thymocyte globulin. Subset analysis was conducted on subjects who had graft loss to retrospectively investigate the factors leading to graft loss. For patients who died, causes of death were presented as overall prevalence of infections (encompassing sepsis, bacterial, fungal, CMV, and other viral infections), malignancies (encompassing solid organ tumors, hematologic malignancies, and post-transplant lymphoproliferative disorder), and cardiovascular diseases (encompassing acute myocardial infarction and cerebrovascular accident). Cause of death classified under “other” includes accidents, unknown, or undocumented. Non-adherence was defined as documentation in the medical record by a provider that a patient was not taking their immunosuppressive regimen as prescribed. Under immunosuppression was defined as evidence of kidney transplant injury related to rejection that led or contributed to graft loss.
The statistical review was performed by a clinician with advanced biostatistical training and experience.
Two-sided independent student’s t-test was used to compare continuous data while the χ2 test was used to compare categorical data. A two-sided P-value of less than 0.05 was considered statistically significant.
Multivariate survival analysis, using both logistic and Cox regression, was used to assess the association between CNI choice and acute rejection (logistic), graft survival (Cox), and patient mortality (Cox), while controlling for additional transplant variables known to influence outcomes or those that differed across CNI choice. In a subset analysis of patients who had graft loss, causes of graft loss, and causes of death were compared between the two groups using standard univariate comparative statistics. All analyses were conducted using SPSS version 21.0 (IBM Corp, Armonk, NY).
Table 1 displays demographic characteristics of the two groups. Mean recipient age, race, BMI, etiology of kidney disease, comorbidities, and dialysis vintage, were similar between the two groups. Patients on FK had higher PRA compared to patients on CYA group (17% vs 5%, P < 0.01). Rates of living donor transplants were similar between the two groups. Among patients who received a deceased donor transplant, KDRI was higher in those who received FK. More patients in the FK group received induction agent with depleting antibodies (46% vs 11%, P < 0.01).
| Parameter | Cyclosporine n = 640 | Tacrolimus n = 1195 | P-value |
| Mean recipient age (yr) | 49 ± 12 | 50 ± 13 | 0.059 |
| Race | 0.96 | ||
| Non-African American | 281 (44) | 526 (44) | |
| African-American | 359 (56) | 669 (56) | |
| Sex | 0.78 | ||
| Male | 371 (58) | 693 (58) | |
| Female | 269 (42) | 502 (42) | |
| BMI | 26 ± 7 | 28 ± 5 | 0.462 |
| Etiology of kidney disease | |||
| DM | 172 (26.9) | 375 (31.4) | 0.044 |
| HTN | 317 (49.5) | 559 (46.8) | 0.26 |
| FSGS | 36 (5.6) | 78 (7.3) | 0.177 |
| IgA nephropathy | 24 (3.8) | 34 (2.8) | 0.291 |
| Polycystic kidney | 63 (9.8) | 89 (7.4) | 0.076 |
| History of DM | 186 (29) | 394 (33) | 0.092 |
| History of HTN | 595 (93) | 1135 (95) | 0.122 |
| History of heart disease | 134 (21) | 227 (19) | 0.38 |
| Years on dialysis | 3 ± 2.4 | 3 ± 2.9 | 0.01 |
| PRA | 5% | 17% | < 0.010 |
| Preemptive transplant | 122 (19) | 239 (20) | 0.49 |
| Living donor transplant | 122 (19) | 179 (15) | 0.27 |
| CIT (h) | 13 ± 9 | 16 ± 9 | 0.621 |
| Mean donor age (yr) | 31 ± 18 | 36 ± 16 | < 0.010 |
| KDRI | 0.9 ± 0.6 | 1.3 ± 0.4 | < 0.010 |
| African-American donor | 122 (19) | 203 (17) | 0.27 |
| Induction therapy | < 0.010 | ||
| Cytolytic agents | 70 (11) | 550 (46) | |
| IL-2 receptor antagonist | 570 (89) | 645 (54) |
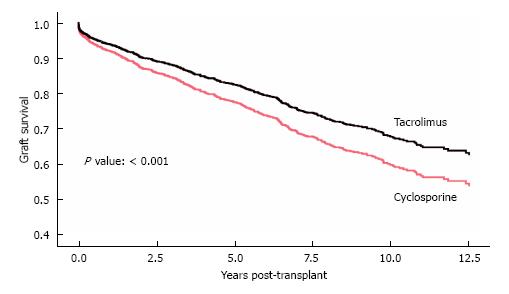
Patients in the FK group had better clinical outcomes in terms of delayed graft function (DGF) rate (15% vs 18%, P = 0.049), cumulative biopsy proven acute rejection rates for Banff 1A and higher, as well as antibody-mediated rejection (14% vs 24%, P < 0.01), three year graft survival (88% vs 79%, P < 0.010), and five year graft survival (84% vs 70%, P < 0.01) (Table 2). FK was a strong predictor of lower acute rejection rates. After multivariate analysis, which accounted for recipient immunologic risks (age, gender, re-transplant, PRA, HLA mismatches, cold ischemic time, induction), donor characteristics (deceased status, ECD, age, race) and delayed graft function, FK continued to be strongly associated with lower acute rejection rates, as compared to CYA (OR = 0.60, 95%CI: 0.45-0.79; P < 0.001) (Table 3). Further analysis showed that even after controlling for all other variables, including delayed graft function and acute rejection, FK remained a strong and statistically significant predictor of graft survival (OR = 0.740, 95%CI: 0.58-0.94; P = 0.012) (Table 4) (Figure 1).
| Parameter | Tacrolimus n = 1195 | Cyclosporine n = 640 | P-value |
| Mean glomerular filtration rate | 56 ± 19 | 46 ± 17 | 0.09 |
| Delayed graft function n (%) | 179 (15) | 115 (18) | 0.049 |
| Acute rejection (biopsy proven) n (%) | 167 (14) | 154 (24) | < 0.010 |
| Three years graft survival | 88% | 79% | < 0.010 |
| Five years graft survival | 84% | 70% | < 0.010 |
| Variable | Hazard ratio | 95%CI | P-value |
| CNI tacrolimus | 0.6 | 0.45-0.79 | < 0.001 |
| Retransplant | 1.43 | 0.91-2.24 | 0.123 |
| PRA | 1 | 0.99-1.00 | 0.529 |
| Cytolytic induction | 0.5 | 0.36-0.69 | < 0.001 |
| Variable | Hazard ratio | 95%CI | P-value |
| CNI tacrolimus | 0.74 | 0.58-0.94 | 0.012 |
| History of DM | 1.41 | 1.13-1.76 | 0.002 |
| History of HTN | 0.56 | 0.34-0.94 | 0.029 |
| Delayed graft function | 2.1 | 1.66-2.66 | < 0.001 |
| Acute rejection | 1.59 | 1.26-2.01 | < 0.001 |
During the study period, there were 106 patients in the FK group and 123 in the CYA group who had graft loss. Death with a functioning graft was the cause of graft loss in the majority of these patients. The leading causes of death among the patients include cardiovascular disease, infections, and malignancies (Table 5). The contribution of non-adherence and underimmunosuppression in patients who had graft loss was not significantly different between the FK and CYA groups.
| Parameter | Tacrolimus n = 106 | Cyclosporine n = 123 | P-value |
| Death with functioning graft | 61 (58) | 66 (54) | 0.55 |
| Cause of death | 0.85 | ||
| Cardiovascular disease | 19 (18) | 19 (15) | |
| Infections | 10 (9) | 9 (7) | |
| Malignancy | 10 (9) | 9 (7) | |
| Others | 33 (31) | 41 (33) | |
| Causes of graft loss | 0.44 | ||
| Chronic allograft nephropathy | 18 (17) | 29 (24) | |
| Acute rejection | 14 (13) | 11 (9) | |
| Acute on chronic rejection | 8 (8) | 13 (11) | |
| Recurrent disease | 1 (1) | 1 (1) | |
| Death | 63 (59) | 68 (55) | |
| Component of non- adherence | 15 (14) | 20 (16) | 0.65 |
| Component of underimmunosuppression | 21 (20) | 25 (20) | 0.92 |
The utilization of potent immunosuppressive medications such as CYA and FK has led to progressive improvement in renal allograft survival. Two large studies on kidney transplant recipients showed that the incidence of acute rejection is much lower with FK-based immunosuppression compared to CYA-based regimen[2,3]. Our study demonstrated similar findings of lower acute rejection rates in patients using FK compared to those on CYA. Acute rejection rate was significantly lower in the FK group despite the relatively higher degree of sensitization, as evidenced by higher PRA, in this group. Multivariate analysis showed that FK was a strong predictor for lower acute rejection rates while controlling for recipient, donor, and transplant characteristics.
The shortage of deceased donor kidneys and the growing number of patients on the waiting list has driven the increased utilization of organs with relatively marginal donor characteristics. Donor factors affect initial graft function and survival[7]. Donor factors that may influence graft survival include age, gender, hypertension, and cardiovascular disease[8]. The KDRI is a comprehensive metric that was recently developed to assess the relative risk of graft failure associated with various combinations of donor characteristics. Kidneys with the highest KDRI quintile are associated with lower graft survival[9]. Although many trials have shown similar graft survival outcomes with FK when compared with CYA-based regimen[4], some studies showed better survival and outcomes with FK-based immunosuppression[6]. Our study showed that although patients in the FK group received kidneys from more marginal donors (higher KDRI), the three year and five year graft survival was still more superior in this group compared to the CYA group (Figure 1).
The risk of infections after kidney transplant depends on the net state of immunosuppression. As FK was shown to be associated with less acute rejection compared to CYA[10], it may concurrently cause more intense immunosuppressive effects compared to CYA. Thus, risk of infections after kidney transplant may be higher with FK compared to CYA. This may be exemplified by the higher incidence of polyomavirus (BK) viremia in patients on FK-based regimen compared to CYA[11]. Progression of BK viremia may lead to BK nephropathy, which can then eventually cause premature renal allograft failure[11]. However, in our subjects who had graft loss, we did not observe a significant difference in the prevalence of infections (including BK) in the FK and CYA groups.
The use of maintenance immunosuppressive medications among transplant recipients increases the long-term risk of malignancy, compared with that of the general population. The overall level of immunosuppression appears to be the principal factor that increases the risk of post-transplant malignancy. Both FK and CYA are associated with an increased risk of malignancy following kidney transplant[12,13]. No direct comparison between these two agents has been reported regarding the incidence of malignancy following kidney transplant. However, FK was found to have higher incidence of de novo malignancy after liver transplant compared to CYA[14]. In our study, we did not find a significant difference in the prevalence of malignancies between the two groups.
Cardiovascular disease is a leading cause of mortality among kidney transplant recipients. Death from cardiovascular disease is the most common cause of renal allograft loss[15]. CNIs potentially contribute to increased risk of cardiovascular events indirectly by the development of new-onset diabetes mellitus, hypertension, and hyperlipidemia. Clinical trials have shown a higher incidence of post-transplant diabetes mellitus with FK. However, the risk of hypertension and hyperlipidemia is slightly higher with CYA than FK. No direct comparison has been done between FK and CYA regarding the incidence of cardiovascular disease. In our study, we found that FK was associated with a slightly higher prevalence of cardiovascular disease compared to CYA, although the difference was not statistically significant.
In conclusion, FK is associated with lower prevalence of acute rejection compared to CYA. It confers better three and five year graft survival even with the use of organs with marginal deceased donor characteristics. An individualized approach to the choice of CNI needs to be employed in order to achieve the best possible outcome while minimizing adverse effects. The use of either FK or CYA should be individualized according to the patient’s comorbid conditions and immunological risk.
Calcineurin inhibitors (CNIs) [cyclosporine (CYA) and tacrolimus (FK)] are currently the most widely used maintenance immunosuppressants for prevention of acute rejection following kidney transplantation. However, data on the impact of these CNIs on acute rejection rate and graft survival have remained equivocal.
The choice of immunosuppressive regimen that will achieve the best renal allograft outcomes remains an important focus in the care of kidney transplant recipients.
The data showed lower acute rejection rates and better graft survival in patients on FK compared to those on CYA.
The use of either FK or CYA should be individualized based on patient’s comorbidities and immunological risk.
FK: Tacrolimus; CYA: Cyclosporine; CNI: Calcineurin inhibitor; DGF: Delayed graft function; DM: Diabetes mellitus; HTN: Hypertension; FSGS: Focal segmental glomerulosclerosis; PRA: Panel reactive antibody; CIT: Cold ischemia time; KDRI: Kidney donor risk index; GFR: Glomerular filtration rate.
The authors are presenting their experience in the use of CNIs in the immunosuppression post renal transplantation.
| 1. | Ponticelli C. Calcineurin-inhibitors in renal transplantation. Too precious to be abandoned. Nephrol Dial Transplant. 2000;15:1307-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Mayer AD. Chronic rejection and graft half-life: five-year follow-up of the European Tacrolimus Multicenter Renal Study. Transplant Proc. 2002;34:1491-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1415] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 4. | Woodward RS, Kutinova A, Schnitzler MA, Brennan DC. Renal graft survival and calcineurin inhibitor. Transplantation. 2005;80:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation. 2002;73:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 275] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, Hardy M, Metzger R, Shield C, Rocher L. Randomized trial of tacrolimus + mycophenolate mofetil or azathioprine versus cyclosporine + mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation. 2003;75:2048-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Moreso F, Serón D, Gil-Vernet S, Riera L, Fulladosa X, Ramos R, Alsina J, Grinyó JM. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant. 1999;14:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Verran DJ, deLeon C, Chui AK, Chapman JR. Factors in older cadaveric organ donors impacting on renal allograft outcome. Clin Transplant. 2001;15:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 824] [Article Influence: 48.5] [Reference Citation Analysis (1)] |
| 10. | Knoll GA, Bell RC. Tacrolimus versus cyclosporin for immunosuppression in renal transplantation: meta-analysis of randomised trials. BMJ. 1999;318:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, Scheuermann EH, Klinger M, Russ G, Pescovitz MD. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13:136-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Imao T, Ichimaru N, Takahara S, Kokado Y, Okumi M, Imamura R, Namba Y, Isaka Y, Nonomura N, Okuyama A. Risk factors for malignancy in Japanese renal transplant recipients. Cancer. 2007;109:2109-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Shihab FS, Bennett WM, Isaac J, Yi H, Andoh TF. Nitric oxide modulates vascular endothelial growth factor and receptors in chronic cyclosporine nephrotoxicity. Kidney Int. 2003;63:522-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Wimmer CD, Angele MK, Schwarz B, Pratschke S, Rentsch M, Khandoga A, Guba M, Jauch KW, Bruns C, Graeb C. Impact of cyclosporine versus tacrolimus on the incidence of de novo malignancy following liver transplantation: a single center experience with 609 patients. Transpl Int. 2013;26:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16:496-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Donkov II, Gheith O, Sheashaa HA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ