Published online Dec 24, 2016. doi: 10.5500/wjt.v6.i4.689
Peer-review started: June 29, 2016
First decision: September 28, 2016
Revised: November 12, 2016
Accepted: November 27, 2016
Article in press: November 29, 2016
Published online: December 24, 2016
Processing time: 170 Days and 5.7 Hours
To analyze the clinical impact of preformed antiHLA-Cw vs antiHLA-A and/or -B donor-specific antibodies (DSA) in kidney transplantation.
Retrospective study, comparing 12 patients transplanted with DSA exclusively antiHLA-Cw with 23 patients with preformed DSA antiHLA-A and/or B.
One year after transplantation there were no differences in terms of acute rejection between the two groups (3 and 6 cases, respectively in the DSA-Cw and the DSA-A-B groups; P = 1). At one year, eGFR was not significantly different between groups (median 59 mL/min in DSA-Cw group, compared to median 51 mL/min in DSA-A-B group, P = 0.192). Moreover, kidney graft survival was similar between groups at 5-years (100% in DSA-Cw group vs 91% in DSA-A-B group, P = 0.528). The sole independent predictor of antibody mediated rejection (AMR) incidence was DSA strength (HR = 1.07 per 1000 increase in MFI, P = 0.034). AMR was associated with shortened graft survival at 5-years, with 75% and 100% grafts surviving in patients with or without AMR, respectively (Log-rank P = 0.005).
Our data indicate that DSA-Cw are associated with an identical risk of AMR and impact on graft function in comparison with “classical” class I DSA.
Core tip: The clinical importance of preformed antiHLA-Cw donor-specific antibodies (DSA) in kidney transplant patients remains controversial, so we performed a retrospective study comparing 12 patients with DSA exclusively antiHLA-Cw with 23 patients with preformed DSA antiHLA-A and/or B. Antibody-mediated rejection occurrence and graft survival frequency, respectively, at one and at five years of follow-up, were comparable between groups. Our data support a similar deleterious impact considering DSA-Cw or DSA-A/-B in terms of risk of AMR and impact on graft function.
- Citation: Santos S, Malheiro J, Tafulo S, Dias L, Carmo R, Sampaio S, Costa M, Campos A, Pedroso S, Almeida M, Martins LS, Henriques C, Cabrita A. Impact of preformed donor-specific antibodies against HLA class I on kidney graft outcomes: Comparative analysis of exclusively anti-Cw vs anti-A and/or -B antibodies. World J Transplant 2016; 6(4): 689-696
- URL: https://www.wjgnet.com/2220-3230/full/v6/i4/689.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i4.689
In kidney transplantation the presence of preexisting anti human leukocyte antigen (HLA) donor-specific antibodies (DSA) has impact on graft outcomes. Their presence is associated with an augmented risk of antibody-mediated rejection (AMR)[1] and worst graft survival[2].
Classically, antibodies against major HLA Class I (A and B) and Class II (DR and DQ) antigens are considered to be responsible for most cases of AMR. AntiHLA-Cw are considered less immunogenic when are paralleled to other class I antiHLA antibodies, mainly due to minor HLA-Cw antigen expression on cell surface[3]. Indeed, some studies found that the incidence of antiHLA-Cw antibodies in sensitized patients was lesser than that for HLA-A or HLA-B antibodies[4-6].
However, the progress of additional sensitive assays that identify HLA antibodies, namely solid-phase immunoassays, demonstrated that HLA-C locus may induce an antibody reaction comparable to the other usually tested loci[4,5,7,8]. In 2012, Ling et al[5] showed that kidney transplantation in patients with isolated antiHLA-Cw antibodies was effective (no rejections occurred) when using induction treatment with anti-thymocyte globulin (ATG) and IVIG. Another study evaluated 22 patients with pretransplant DSA antiHLA-Cw in comparison with 88 patients allosensitized but with no detectable preformed DSA and concluded that they seem to be at superior risk for AMR occurrence[9]. Recently, Bachelet et al[10] in their retrospective and multicenter study showed that antiHLA-Cw DSA have the same effect on graft outcome as DSA against “classical” HLA loci (A, B, DR, DQ), suggesting that antiHLA-Cw should also be considered in transplant allocation procedures and in immunologic risk stratification of patients.
As this subject remains controversial, we decided to conduct a retrospective study in kidney transplant patients to investigate the clinical impact of preformed antiHLA-Cw DSA comparing them to DSA against the other HLA class I loci, namely antiHLA-A and/or B.
From the database of our Histocompatibility Center 35 adults who received a kidney transplant since 2007 were identified as having pretransplant donor specific antibodies (DSA) exclusively antiHLA-A and/or -B or exclusively antiHLA-Cw. Twenty-three patients had DSA antiHLA-A and/or antiHLA-B: 6 with DSA antiHLA-A only; 11 with DSA antiHLA-B only and 6 with DSA antiHLA-A and -B. This group was designated DSA-A-B. Twelve patients had DSA exclusively antiHLA-Cw, and this group was designated DSA-Cw. The patients were all transplanted with a negative T- and B-cell cytotoxic crossmatch (standard NIH technique). The Institutional Review Board at Hospital Santo António, CHP approved this study.
Patients in the waiting list were examined for antiHLA IgG by multiplex microsphere based on Luminex X-
map® Technology (LABScreen® Mixed kit, OneLambda, Canoga Park, CA, United States). The cut-off for positive samples was the Normalized Background (NBG) ratio advocated by the manufacturer and executed by the HLA fusion® software (One Lambda Inc.). To determinate the specificity of the HLA antibodies, single-antigen bead (SAB) assays (LabScreen Single Antigen Beads®, OneLambda, Canoga Park, CA) were executed in patients with a positive screening, using the same pretransplant sera. The mean fluorescence intensity (MFI) was measured using LABScan™ 100 flow analyzer (Luminex®, Austin, TX, United States). The analysis was performed using HLA fusion® software (One Lambda Inc.) and a cut-off for a positive reaction were set in MFI value of ≥ 1000.
Samples of all deceased donors were routinely typed before recipient selection in loci HLA-A*, B*, Cw* and DRB1* using polymerase chain reaction (PCR) amplification with specific sequence primers (SSP; Olerup SSP® low resolution HLA typing kits, Stockholm, Sweden). After donor HLA typing, using that information, a virtual crossmatch (virtual XM) was executed. The strength of each single DSA was based on the MFI of one SAB. In the case of several DSA against different HLA-antigens, we considered the cumulative strength of all DSA by adding the individual MFI values.
Thirty-three of the total of 35 patients (94.3%) received induction therapy: Ten patients with a monoclonal antibody anti-IL-2 receptor (Basiliximab Novartis®, 20 mg twice at day 0 and 4), and 23 patients with polyclonal ATG Fresenius® (3 mg/kg for 5-7 d). All patients had an equivalent maintenance immunosuppression using three oral drugs: A calcineurin inhibitor [tacrolimus (FK-506) in the majority of patients (32/35 patients) or cyclosporine (CsA) in 3 patients], mycophenolate mofetil (MMF) and a corticosteroid. FK-506 was started at a dose of 0.1-0.15 mg/kg per day, and was adjusted to maintain levels between 8 and 12 ng/mL during the first month post-transplant, between 7 and 10 ng/mL the next 2-3 mo and between 5 and 8 ng/mL thereafter. MMF was started at a dose of 2000 mg/d, and decreased based on white blood cells count. Methylprednisolone was administered intravenously at doses of 500, 250 and 125 mg/d on the day of transplantation, days 1-2 and days 3-4 after the operation, respectively. Oral prednisolone was started on day 5 after the operation at the dose of 20 mg, being then tapered to 5-10 mg/d within 2-3 mo after transplant. Living donor recipients (n = 3) were prescribed FK-506 and MMF 7 d before transplant.
Eight patients underwent a desensitization protocol. Five patients received intravenous immunoglobulin (IvIg) 2 g/kg at transplant (0.5 g/kg immediately before transplant, and at day 1, 2 and 3) and 1-mo after transplant (1 g/kg in 2 consecutive days). One patient received a similar dose of IvIg and underwent plasmapheresis every other day (first session immediately before transplant, for a total of 6-9 sessions) and two other patients received additionally a dose of Rituximab (375 mg/m2) on day 3 post-transplant.
The data concerning patients’ characteristics and transplantation variables was collected retrospectively. Estimated glomerular filtration rate (eGFR) was assessed using the 2006 Modification of Diet in Renal Disease (MDRD) equation and dialysis requirement in the first week post-transplant was defined as delayed graft function. Patients were followed until graft failure, death or end of follow-up (five years after transplant or December 31, 2015, which came first). Graft survival was evaluated considering graft failure censored for death with a functioning graft.
Graft biopsies were performed “for cause” only. Allograft rejection was classified according Banff classification (updated in 2013) and defined by biopsy where specimens were evaluated by light microscopy and immunofluorescence (with C4d staining). Mild acute cellular rejection (ACR Banff grade I) was treated with 500 mg methylprednisolone for 3 d and increased maintenance immunosuppression. All other ACR were treated with ATG. AMR patients were treated with plasmapheresis every other day (the number of plasmapheresis sessions was 4 per protocol) and IvIg 100 mg/kg after each session. After the last plasmapheresis session, they received a high-dose IvIg (2 g/kg) divided in four daily doses and the same dose was repeated 1 mo later. If not used at transplant, patients received, additionally, one dose of rituximab (375 mg/m2).
Categorical data were expressed as numbers (frequencies) and continuous data were described using median (interquartile range). Categorical data (demographic and medical characteristics) were compared using Pearson χ2 test or Fisher’s exact test, as appropriate. Continuous variables were compared with Mann-Whitney U test. Predictors of AMR were explored by univariate and multivariable (using a backward elimination method, with a P-value < 0.05 necessary for retention in the model) Cox regression. For graft survival curves was used the Kaplan-Meier method, and the comparison between groups was done by log-rank test.
Baseline characteristics of DSA-Cw and DSA-A-B groups are given in Table 1. DSA-Cw patients tended to be younger compared to patients in DSA-A-B group (respectively, 39 years vs 48 years), (P = 0.061). There was no significant difference between groups concerning gender, history of previous transplant or previous pregnancies. However DSA-Cw patients had significantly higher prevalence of previous blood transfusions (75% vs 39%, P = 0.044).
| DSA-A-Bn = 23 | DSA-Cwn = 12 | P | |
| Recipient | |||
| Age (yr), median (IQR) | 48 (39-55) | 39 (33-49) | 0.061 |
| Female gender, n (%) | 13 (57) | 6 (50) | 0.713 |
| Retransplant, n (%) | 11 (48) | 5 (42) | 0.728 |
| Previous blood transfusions, n (%) | 9 (39) | 9 (75) | 0.044 |
| Previous pregnancies, n (%) | 8 (35) | 8 (33) | 1 |
| Kidney-pancreas transplantation, n (%) | 1 (4) | 1 (8) | 1 |
| Donor | |||
| Age (yr), median (IQR) | 45 (36-56) | 45 (32-54) | 0.542 |
| Female gender, n (%) | 8 (35) | 8 (33) | 1 |
| Living donor, n (%) | 1 (4) | 2 (17) | 0.266 |
| Pretransplant immunological data | |||
| Peak PRA, median (IQR) | 4 (0-80) | 8 (0-52) | 0.472 |
| DSA number, median (range) | 1 (1-3) | 1 (1-2) | 0.056 |
| DSAsum MFI, median (IQR) | 7583 (2320-12395) | 2939 (2529-3650) | 0.11 |
| Transplant | |||
| ABDR HLA mismatches, mean ± SD | 3.22 ± 1.28 | 4.08 ± 1.16 | 0.056 |
| FCXM-T + (n = 29), n (%) | 1 (6) | 3 (27) | 0.139 |
| FCXM-B + (n = 29), n (%) | 2 (11) | 0 | 0.512 |
| ATG induction, n (%) | 14 (61) | 9 (75) | 0.476 |
| Tacrolimus (vs CsA), n (%) | 20 (87) | 12 (100) | 0.536 |
| Desensitized, n (%) | 5 (22) | 3 (25) | 1 |
| IvIg only, n | 2 | 3 | |
| IvIg + PP, n | 1 | 0 | |
| IvIg + Rtx + PP, n | 2 | 0 |
Concerning donor characteristics and pretransplant immunological data, namely donor age, donor gender, type of donor transplant (living vs decease), peak PRA, and DSA number, none of these characteristics significantly differed between groups. Although DSA strength median was higher in DSA-A-B (MFI 7583) in comparison with DSA-Cw group (MFI 2939), this difference was not significant (P = 0.110).
Flow cytometry crossmatch (FCXM) was performed for 29 of 35 patients. Positive T- and/or B- cell FCXM was similarly uncommon between groups. Three (27%) patients had a positive T-cell FCXM in the DSA-Cw group and only one (6%) in the DSA-A-B group (P = 0.139). Only two patients had a positive B-cell FCXM and both belonged to the DSA-A-B group.
Immunosuppression and induction treatment were similar between groups. ATG induction was used in 14 (61%) and 9 (75%) patients from the DSA-A-B and DSA-Cw groups, respectively (P = 0.476). Additionally, 5 patients in the DSA-A-B group were desensitized: 2 of them using only IVIG, 1 with IVIG and plasmapheresis and another 2 combining IVIG, plasmapheresis and rituximab. In DSA-Cw group 3 patients were treated with IVIG.
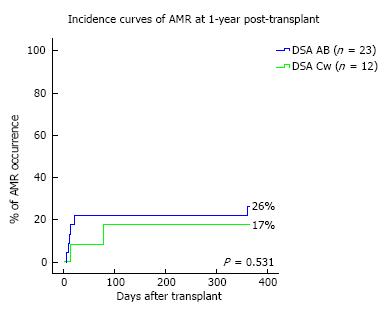
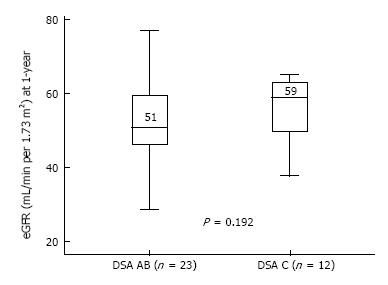
Transplant outcomes are detailed in Table 2. There was no difference in terms of acute rejection at one year between the two groups (6 and 3 cases, respectively in the DSA-A-B and the DSA-Cw groups; P = 1). All cases of acute rejection were diagnosed as AMR in the DSA-A-B group, while in the DSA-Cw group there were 2 cases of AMR and 1 of ACR. Figure 1 shows the incidence of AMR at one-year post-transplant, between DSA-A-B and DSA-Cw patients groups, (respectively, 26% and 17%, Log-rank P = 0.531) with no significant difference being detected. At one year, eGFR tended to be higher in DSA-Cw group (median 59 mL/min) compared to DSA-A-B group (median 51 mL/min), (P = 0.192) (Figure 2). Importantly, follow-up was significantly longer for the DSA-A-B group (median 60 mo) than in the DSA-Cw group (median 18 mo) (P < 0.001). Kidney graft survival at 5-years was also similar between groups (Figure 3, 91% for the DSA-A-B group vs 100% for the DSA-Cw group, P = 0.528).
| DSA-A-Bn = 23 | DSA-Cwn = 12 | P | |
| Delayed graft function, n (%) | 7 (30) | 1 (8) | 0.216 |
| Acute rejection at 1-yr, n (%) | 6 (26) | 3 (25) | 1 |
| AMR at 1-yr, n (%) | 6 (26) | 2 (17) | 0.685 |
| ACR-only at 1-yr, n (%) | 0 | 1 (8) | 0.343 |
| 1 yr-eGFR (mL/min), median (IQR) | 51 (46-60) | 59 (47-64) | 0.192 |
| 1 yr-ProtU, median (IQR) | 0 (0-0.1) | 0.1 (0-0.2) | 0.163 |
| Censored graft failure, n (%) | 2 (9) | 0 | 0.536 |
| Follow-up time (mo), median (IQR) [range] | 60 (45-60) | 18 (11-50) | 0.001 |
| [28-60] | [3-60] |
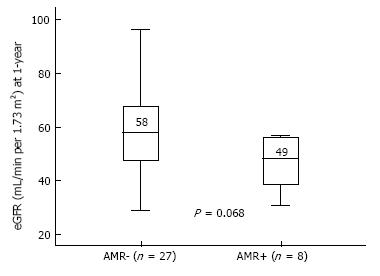
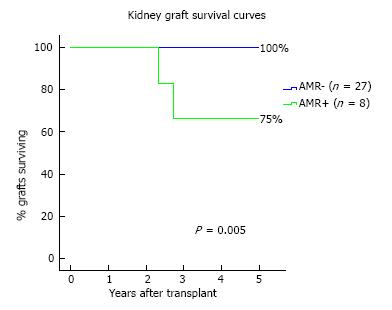
AMR occurred in 8 patients (23%) of the overall cohort. Possible associations between clinical and immunological data and AMR incidence through a Cox regression analysis is shown in Table 3. The sole independent predictor of AMR incidence was the DSA strength, both in uni- and multi-variable analysis (HR = 1.07 per 1000 increase in MFI, P = 0.034). At 1-year, eGFR was lower in AMR+ (median 49 mL/min) in comparison with AMR- patients (median 58 mL/min) (P = 0.068), as shown in Figure 4. At the end of follow-up, kidney graft survival (Figure 5) was 75% in patients that experienced AMR and 100% in those who did not (Log-rank P = 0.005).
| HR for AMR | 95%CI | P | |
| Recipient | |||
| Age (yr), per year | 0.96 | 0.89-1.03 | 0.269 |
| Female (vs male) gender | 0.26 | 0.05-1.26 | 0.094 |
| Retransplant | 2.18 | 0.52-9.13 | 0.287 |
| Previous blood transfusions | 0.5 | 0.12-2.10 | 0.345 |
| Previous pregnancies | 0.24 | 0.03-1.99 | 0.187 |
| Donor | |||
| Age (yr), per year | 1.01 | 0.96-1.06 | 0.684 |
| Living donor | 1.79 | 0.22-14.76 | 0.588 |
| Pretransplant immunological data | |||
| Peak PRA, per unit | 1.01 | 1.00-1.03 | 0.149 |
| DSA Cw (vs AB) | 0.6 | 0.12-2.99 | 0.537 |
| DSAsum MFI, per 10001 | 1.07 | 1.01-1.15 | 0.034 |
| Transplant | |||
| ABDR HLA mismatches, per unit | 0.84 | 0.50-1.41 | 0.512 |
| ATG (vs basiliximab) induction | 1.68 | 0.34-8.34 | 0.527 |
| FCXM + (n = 29) | 0.75 | 0.09-6.21 | 0.787 |
| Desensitized | 1.2 | 0.24-5.97 | 0.825 |
| Delayed graft function | 2.55 | 0.61-10.68 | 0.201 |
This retrospective study demonstrates that patients with preformed DSA solely antiHLA-Cw had a similar impact on post-transplant outcomes comparing to those patients with preformed antiHLA-A/-B DSA. Both groups had a relative high incidence of AMR at one year, 26% in the DSA-A-B group and 25% in DSA-Cw group. Also, the impact on graft outcomes measured by eGFR at one-year and graft survival at the end of follow-up was comparable between groups.
HLA-Cw molecules are scantily expressed at the cell surface compared with HLA-A and HLA-B locus products, but intracellular HLA-A, HLA-B and HLA-Cw alleles are expressed in similar quantities[3,11]. One reason pointed for this low amount at the cell surface is the fact that HLA-Cw alleles interact in a very stable way with the transporter associated with antigen processing (TAP) and they are kept in the endoplasmic reticulum, where they are degraded[11]. Another justification for finding low HLA-Cw at cell proposed by McCutcheon et al[3] is that HLA-Cw heavy chain mRNA is instable and rapidly degraded, resulting in a lower rate of protein. This fact, associated with the modest sensitivity of the lymphocytotocicity-based assays used in the past for identification of HLA-Cw antigens, probably explains why for many years they were considered less immunogenic and neglected in the matching systems of most kidney allocation procedures. Recent studies confirm their lower frequency. Bryan et al[6] in 2010 described in their sensitized transplant patients a 42% positivity to HLA-Cw, which was significantly lesser than sensitization to HLA-A (80%) and HLA-B (83%). In 2012, Ling et al[5], obtained similar results and showed that the frequency of antiHLA-Cw antibodies in sensitized patients was about 56%, lower than HLA-A (79%) and B (86%) antibodies. Our group evaluated 453 sensitized kidney transplantation candidates to determine the presence of antiHLA class I and class II antibodies, comparing how different sensitization events, such as pregnancy, transfusion or previous organ transplantation, affected the degree of HLA alloimmunization[12]. For antiHLA antibodies against class I, if the sensitization event was previous transplant only, the antiHLA antibodies prevalence was 21.2% for -A, 28.8% for -B and 21.1% for -Cw; if the single sensitization event was previous transfusion, the antiHLA antibodies prevalence was 3.9% for -A, 5.5% for -B and 1.6% for -Cw. At last, if the sensitization event was pregnancy only, the antiHLA antibodies prevalence was 13.6% for -A, 11.1% for -B and 6.2% for -Cw.
In spite of their lower frequency, some reports have been published concerning their association with AMR and impact on graft function and survival[8,13,14]. Besides, the recent development of the solid-phase immunoassays, in particular the single-antigen flow bead (SAFB) assays, allowed us to detect and properly identify anti-HLA-Cw antibodies. Tambur et al[15] compared virtual flow-cytometry cross-match to actual cross-match and described that 40% of the cases with a positive actual flow-cytometry cross-match and negative virtual cross-match were explained by the presence of antiHLA-Cw antibodies. Gilbert et al[7] compared two groups of sensitized recipients, one group with only classical HLA-A, -B, -DR, -DQ antibodies (n = 176) and the other group with classical plus HLA-C and/or -DP antibodies (n = 27). They concluded that there was a significant increase in the number of AMR among the group with pre-transplant anti-Cw and -DP antibodies. However, they did not distinguish between pre-transplant anti-DP or anti-Cw antibodies, and they speculated that anti-DP antibodies seemed to be involved more often in poorer graft outcomes. Ling et al[5] investigated the clinical outcomes in kidney transplant patients with isolated Cw-DSA. They identified eight patients with pre-transplant DSA antiHLA-Cw, exclusively. During a median 6 mo of follow-up (range 3-24 mo), patient and graft survival was 100% without any acute rejection occurring. In this group, all the patients had induction therapy with thymoglobulin or basiliximab and additionally all patients received intravenous immunoglobulin, similar to patients with positive FCXM and/or cPRA > 50%. Even so, the median time of follow up was relatively short and may have underestimated the incidence of rejection. Aubert et al[9] evaluated retrospectively 22 renal transplant recipients with isolated antiHLA-Cw DSA at day 0 of renal transplant, comparing them with 88 allosensitized patients with no preformed DSA (control group), and followed for a period of 1 year. Acute AMR was diagnosed in six patients (27.3%) in patients with DSA-Cw vs 9% in those without DSA. In this study, the patients with DSA antiHLA-Cw received less-intensive immunosuppression than the control group of sensitized patients, including ATG induction (only 59.1%), and this may probably be a plausible explanation for this high rate of AMR. However they alert for the necessity of screening pre-transplant DSA HLA-Cw and subsequent modulation of immunosuppression in cases of positivity. More recently, Bachelet el al[10] investigated the clinical effect of DSA antiHLA-Cw and/or -DP, comparing 48 patients transplanted with isolated preformed DSA antiHLA-Cw and/or -DP with a group of HLA-sensitized recipients with no DSA (104 patients) and 47 kidney transplant recipients with preformed DSA antiHLA-A, -B, -DR, and/or -DQ. Two years after transplantation, the groups with DSA (both -Cw/-DP or -A/-B/-DR/-DQ) had similar incidence of AMR and graft survival (and worse than the group with no DSA), showing that preformed DSA anti-HLA-Cw and/or -DP were as deleterious as DSA anti-HLA -A/-B/-DR/-DQ.
Our data reached similar results of these previous studies, confirming that DSA-Cw is associated with a similar incidence of AMR and impact on graft survival in comparison with “classical” DSA against class I[9,10].
We have also shown that patients that experienced AMR had a significant lower kidney graft survival in comparison to patients who did not (respectively, 75% vs 100%, Log-rank P = 0.005), with the sole independent predictor of AMR incidence being DSA strength. The negative impact of DSA for AMR occurrence and adverse results on kidney graft survival has been previously established[2]. Lefaucheur et al[16] stated that it is the occurrence of AMR associated with DSA that has impact on graft survival, since graft survival of DSA-positive patients, in the absence of AMR, is the same as DSA-negative patients. Furthermore, DSA characteristics as number, class or strength may have a negative impact on graft outcomes[1,17-19]. Malheiro et al[20] showed that DSA strength (MFI) had a reasonable ability to predict AMR occurrence, with no cases of AMR occurring below a MFI < 3000. However when the MFI values increased from this value, also did the risk of AMR. Again, Aubert et al[9] in their retrospective study with 22 renal transplant recipients with preformed isolated antiHLA-Cw DSA, showed that the level of DSA at day 0 was predictive for AMR: Measurement of MFI was 4966 (978-17941) in the AMR group and 981 (530-8012) in the group of patients without AMR (P = 0.017).
This study has limitations. First, the small number of patients in the cohort limits our ability to generalize the results. Second, follow-up time difference may have limited the comparative analysis of graft survival according with DSA HLA loci. Contrarily, AMR incidence was not influenced by it, since it was analyzed at 1-year post-transplant. Third, there was no protocol biopsies performed in our patients and it is an important tool for HLA incompatible kidney transplantation[21,22]. Lastly, the limitations of SAB assay are well established and their reported MFI values should be considered for analyzing our results[23].
In summary, our data show that preformed DSA antiHLA-Cw exerts a deleterious effect in presensitized kidney transplant recipients that is similar when compared to antiHLA antibodies against other class I locus (antiHLA-A or -B). Also, the association between AMR occurrence and reduced graft survival is clear, with DSA strength being predictive of rejection. Therefore, HLA-C typing and respective antibody identification will benefit sensitized patients during organ allocation.
Classically, antibodies to major human leukocyte antigen (HLA) Class I (A and B) and Class II (DR and DQ) antigens are considered to be responsible for most cases of AMR. Compared to other class I antiHLA antibodies, antiHLA-Cw are considered less immunogenic.
Preformed antiHLA-Cw donor-specific antibodies (DSA) seem to have the same impact on graft outcome as DSA against “classical” HLA loci (-A, -B, -DR and -DQ), suggesting that it should also be considered in transplant allocation systems and in immunologic risk stratification algorithms.
The clinical relevance of preformed antiHLA-Cw DSA in kidney transplant patients remains controversial, so the authors performed a retrospective study comparing 12 patients with DSA exclusively antiHLA-Cw with 23 patients with preformed DSA antiHLA-A and/or B. Antibody-mediated rejection occurrence and graft survival rates, respectively, at 1 and at 5-years of follow-up, were comparable between groups.
The data show that preformed DSA antiHLA-Cw exerts a deleterious effect in presensitized kidney transplant recipients that is similar when compared to antiHLA antibodies against other class I locus (antiHLA-A or -B). Also, the association between AMR occurrence and reduced graft survival is clear, with DSA strength being predictive of rejection.
HLA: Human leukocyte antigen; DSA: Donor-specific antibodies; AMR: Antibody-mediated rejection.
The topic is very interesting. The authors investigated the possible role of preformed donor-specific antibodies against HLA antigens, specially anti-Cw antibodies compared to standard anti A/B antibodies. The importance of Cw antibodies is still under investigation and this study is valuable about this topic. This article is worthwhile for publication.
| 1. | Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 666] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 2. | Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | McCutcheon JA, Gumperz J, Smith KD, Lutz CT, Parham P. Low HLA-C expression at cell surfaces correlates with increased turnover of heavy chain mRNA. J Exp Med. 1995;181:2085-2095. [PubMed] |
| 4. | Duquesnoy RJ, Marrari M. Detection of antibodies against HLA-C epitopes in patients with rejected kidney transplants. Transpl Immunol. 2011;24:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Ling M, Marfo K, Masiakos P, Aljanabi A, Lindower J, Glicklich D, de Boccardo G, Greenstein S, Chapochnick-Friedmann J, Kayler L. Pretransplant anti-HLA-Cw and anti-HLA-DP antibodies in sensitized patients. Hum Immunol. 2012;73:879-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Bryan CF, Luger AM, Smith JL, Warady BA, Wakefield M, Schadde E, Murillo D, Nelson PW. Sharing kidneys across donor-service area boundaries with sensitized candidates can be influenced by HLA C. Clin Transplant. 2010;24:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Gilbert M, Paul S, Perrat G, Giannoli C, Pouteil Noble C, Morelon E, Rigal D, Dubois V. Impact of pretransplant human leukocyte antigen-C and -DP antibodies on kidney graft outcome. Transplant Proc. 2011;43:3412-3414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Bachelet T, Couzi L, Guidicelli G, Moreau K, Morel D, Merville P, Taupin JL. Anti-Cw donor-specific alloantibodies can lead to positive flow cytometry crossmatch and irreversible acute antibody-mediated rejection. Am J Transplant. 2011;11:1543-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Aubert O, Bories MC, Suberbielle C, Snanoudj R, Anglicheau D, Rabant M, Martinez F, Scemla A, Legendre C, Sberro-Soussan R. Risk of antibody-mediated rejection in kidney transplant recipients with anti-HLA-C donor-specific antibodies. Am J Transplant. 2014;14:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Bachelet T, Martinez C, Del Bello A, Couzi L, Kejji S, Guidicelli G, Lepreux S, Visentin J, Congy-Jolivet N, Rostaing L. Deleterious Impact of Donor-Specific Anti-HLA Antibodies Toward HLA-Cw and HLA-DP in Kidney Transplantation. Transplantation. 2016;100:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Neisig A, Melief CJ, Neefjes J. Reduced cell surface expression of HLA-C molecules correlates with restricted peptide binding and stable TAP interaction. J Immunol. 1998;160:171-179. [PubMed] |
| 12. | Lopes D, Barra T, Malheiro J, Tafulo S, Martins L, Almeida M, Pedroso S, Dias L, Castro Henriques A, Cabrita A. Effect of Different Sensitization Events on HLA Alloimmunization in Kidney Transplantation Candidates. Transplant Proc. 2015;47:894-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Chapman JR, Taylor C, Ting A, Morris PJ. Hyperacute rejection of a renal allograft in the presence of anti-HLA-Cw5 antibody. Transplantation. 1986;42:91-93. [PubMed] |
| 14. | Rogers NM, Bennett GD, Toby Coates P. Transplant glomerulopathy and rapid allograft loss in the presence of HLA-Cw7 antibodies. Transpl Int. 2012;25:e38-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Tambur AR, Ramon DS, Kaufman DB, Friedewald J, Luo X, Ho B, Skaro A, Caicedo J, Ladner D, Baker T. Perception versus reality?: Virtual crossmatch--how to overcome some of the technical and logistic limitations. Am J Transplant. 2009;9:1886-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Higgins R, Lowe D, Hathaway M, Williams C, Lam FT, Kashi H, Tan LC, Imray C, Fletcher S, Chen K. Human leukocyte antigen antibody-incompatible renal transplantation: excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. 2011;92:900-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Mujtaba MA, Goggins W, Lobashevsky A, Sharfuddin AA, Yaqub MS, Mishler DP, Brahmi Z, Higgins N, Milgrom MM, Diez A. The strength of donor-specific antibody is a more reliable predictor of antibody-mediated rejection than flow cytometry crossmatch analysis in desensitized kidney recipients. Clin Transplant. 2011;25:E96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Otten HG, Verhaar MC, Borst HP, Hené RJ, van Zuilen AD. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant. 2012;12:1618-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Malheiro J, Tafulo S, Dias L, Martins LS, Fonseca I, Beirão I, Castro-Henriques A, Cabrita A. Analysis of preformed donor-specific anti-HLA antibodies characteristics for prediction of antibody-mediated rejection in kidney transplantation. Transpl Immunol. 2015;32:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, Rioux-Leclercq N, Comoz F, Cordonnier C, François A. Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transplant. 2011;11:2153-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Heilman RL, Devarapalli Y, Chakkera HA, Mekeel KL, Moss AA, Mulligan DC, Mazur MJ, Hamawi K, Williams JW, Reddy KS. Impact of subclinical inflammation on the development of interstitial fibrosis and tubular atrophy in kidney transplant recipients. Am J Transplant. 2010;10:563-570. [PubMed] |
| 23. | Zachary AA, Leffell MS. Detecting and monitoring human leukocyte antigen-specific antibodies. Hum Immunol. 2008;69:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cantarovich F, Markic D, Marino IP S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ