Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.199
Peer-review started: July 14, 2015
First decision: September 22, 2015
Revised: November 4, 2015
Accepted: December 17, 2015
Article in press: December 18, 2015
Published online: March 24, 2016
Processing time: 253 Days and 3.1 Hours
Stem cells have their origins in the embryo and during the process of organogenesis, these differentiate into specialized cells which mature to form tissues. In addition, stem cell are characterized by an ability to indefinitely self renew. Stem cells are broadly classified into embryonic stem cells and adult stem cells. Adult stem cells can be genetically reprogrammed to form pluripotent stem cells and exist in an embroyonic like state. In the early phase of embryogenesis, human embryonic stem cells only exist transiently. Adult stem cells are omnipresent in the body and function to regenerate during the process of apoptosis or tissue repair. Hematopoietic stem cells (HSC) are adult stem cells that form blood and immune cells. Autoimmune responses are sustained due to the perennial persistence of tissue self autoantigens and/or auto reactive lymphocytes. Immune reset is a process leading to generation of fresh self-tolerant lymphocytes after chemotherapy induced elimination of self or autoreactive lymphocytes. This forms the basis for autologous HSC transplantation (HSCT). In the beginning HSCT had been limited to refractory autoimmune rheumatic diseases (AIRD) due to concern about transplant related mortality and morbidity. However HSCT for AIRD has come a long way with better understanding of patient selection, conditioning regime and supportive care. In this narrative review we have examined the available literature regarding the HSCT use in AIRD.
Core tip: Hematopoietic stem cell transplantation for the management of autoimmune rheumatic diseases has come a long way. It is being recognized as a viable option in severe autoimmune diseases, in particular for systemic sclerosis.
- Citation: Ramaswamy S, Jain S, Ravindran V. Hematopoietic stem cell transplantation for auto immune rheumatic diseases. World J Transplant 2016; 6(1): 199-205
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/199.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.199
Stem cells have their origins in the embryo and during the process of organogenesis, these differentiate into specialized cells which mature to form tissues. In addition, stem cells are characterized by ability to indefinitely self renew. Stem cells are broadly classified into embryonic stem cells and adult stem cells. Adult stem cells can be genetically reprogrammed to form pluripotent stem cells and exist in an embroyonic like state. In the early phase of embroyogenesis, human embryonic stem cells only exist transiently. Adult stem cells are omnipresent in the body and function to regenerate during the process of apoptosis or tissue repair. Hematopoietic stem cells (HSC) are adult stem cells that form blood and immune cells.
Embryonic stem cells have great promise as they have the capability to replenish every functioning cell in the human body. Uncontrolled replication of embryonic stem cells leads to teratomas. Embryonic stem cell biology is subject to ethical controversy. Currently there are no Food and Drug Administration (FDA) approved embryonic stem cells based therapies available for clinical use. There are several clinical trials ongoing exploring use of human embryonic stem cell based therapies in regenerative medicine. HSC are blood and immune cells that have their origin from adult stem cells. HSC can be isolated from the umbilical cord, peripheral blood or the bone marrow[1].
Manifestations of autoimmune rheumatic diseases (AIRD) are heterogeneous in which the etiology is compounded by genetic risks, racial differences and infection triggered oligoclonal lymphocyte responses. As a result of multitudes of external insult, there is interference in the signal responses that sustain immune tolerance to normal tissues. Breakdown of these signals leads to activation of effecter cellular mechanism and subsequent self-tissue destruction in a self-propagating manner[2]. Autoimmune responses are sustained due to the perennial persistence of tissue auto antigens, which often do not get destroyed. The treatment response is, hence; often generalized and most patients indeed have a relapsing and remitting course. Better understanding of mechanisms involved in immunopathogenesis and of effecter cells have lead to the acceptance of aggressive modalities of treatment namely hematopoietic stem cell transplantation (HSCT) which resets the host immune system[3]. Immune reset is a process leading to generation of fresh self-tolerant lymphocytes after chemotherapy induced elimination of self or auto reactive lymphocytes. This forms the basis for autologous HSCT.
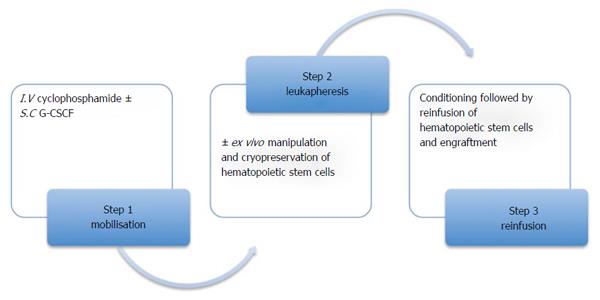
Extensive preclinical animal transplantation experiments lead to HSCT (Figure 1) as a therapeutic option for patients with severe autoimmune diseases began in the late 1990s. In the beginning, the use of HSCT had been limited to refractory diseases due to concern about transplant related mortality and morbidity. Later it became clear that transplant related mortality and morbidity is a function of the disease state[4] and conditioning regimen[5]. The conditioning regimens included either myeloablative or nonmyeloablative. High dose chemotherapy and total body irradiation (myeloablative regimen) together with stem cell support ensures a complete replacement of the entire bone marrow compartment, hence abolishing the entire tumor cell load. Marrow failure is life threatening if HSC are not reinfused. Reduced doses of chemo radiotherapy constitute the nonmyeloblative regimen. This leads to lymph ablation and marrow cells are invariably preserved such that the incidences of a lethal failure is minimized even without HSC reinfusion. However, treatment related marrow suppression could be minimized using autologous stem cell support. The significant reduction in the treatment related mortality and morbidity following the use of non myeloablative regimens over myeloablative regimens, makes it a more viable option for the treatment of autoimmune diseases (natural history is relapsing and remitting) compared to malignant diseases[1].
The major advantage of HSCT for autoimmune diseases is the ability to achieve an “immune reset”, i.e., the ability to eliminate the autoimmune T cell cells clones and alter the natural history of the disease. The major disadvantages of HSCT for autoimmune disease are the added toxicity of the high dose chemotherapy or radiation used as part of conditioning regimen.
The use of HSCT has been reported for various AIRD. Long term data is available from the European Group for Blood and Marrow Transplantation (EBMT) registry[6,7] (Table 1), clinical trials in systemic sclerosis (SSc), systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) with maximum data available in patients with SSc. Isolated reports are available for remission of some other AIRD such as ankylosing spondylitis. In this narrative review we have appraised the available literature on HSCT use in AIRD.
| Disease | Number | Mean age at Tx (yr) | TRM (100 d) | 5 yr progression free survival | 5 yr overall survival | Death due to disease | Deaths due Tx |
| Systemic sclerosis | 175 | 41 | 6% | 55% | 76% | 23 | 12 |
| SLE | 85 | 28 | 11% | 44% | 76% | 5 | 11 |
| Rheumatoid arthritis | 89 | 42 | 1% | 18% | 94% | 0 | 2 |
| JIA | 65 | 11 | 11% | 52% | 82% | 2 | 7 |
For the purpose of present narrative review, the search strategy included screening of primary sources MEDLINE (1990 to date) using the PubMed interface, as well as secondary sources, the Embase, Cochrane Library, Best evidence and Clinical evidence without any time limits. Appropriate combinations of search terms including “autoimmune”, “stem cell transplantation”, “rheumatic diseases”, “hematopoietic” and the names of individual known musculoskeletal disorders were used with limits “(English, human)”. Relevant keyword variations for different databases were used. This was supplemented by a manual search of bibliographies of these articles and of previously published reviews.
SSc is a fibrotic disease characterized by extensive dermal and visceral organ involvement. There is phenotypic difference in the disease subsets, which are classified, as diffuse and limited depending upon the degree of skin involvement, which is semi objectively, measured by the modified Rodnan’s score (mRSS). The extent of skin fibrosis portends the degree of visceral involvement, which has a direct bearing on the long term mortality and morbidity in these patients. The higher the skin score, the presence of cardiac, renal or pulmonary involvement increases the mortality to 40%-50% in the next 5 years[8-12].
HSCT has been explored as a therapeutic option in the treatment of SSc with its first case dating back to 1997. Since then numerous Phase I/II trials have done. The long-term data from the EBMT registry has shown encouraging results with respect to improvement in skin score and stabilization of lung functions and pulmonary hypertension together with improvement in functional status[6,7,13] (Table 1). Three randomized control trials namely - ASTIS[14]: A phase 3 trial (Autologous Stem cell Transplantation International Scleroderma trial); ASSIST[15]: A phase 2 trial (Autologous non-myeloablative hematopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for SSc) and SCOT[16]: A phase 3 (US multicenter Scleroderma: Cyclophosphamide or Transplantation) exists which have evaluated the efficacy of HSCT in Scleroderma (Table 2). SCOT completed the recruitment of patients in May 2011 and some of the results are expected soon.
| Trial name | Patients | Controls | Number | Outcome | TRM | Comments |
| ASTIS[14] | mRSS 15 for disease duration 4 yr, mRSS 20 if disease duration is 2 yr; and major organ involvement | IV CYC | 156 (79 HSCT, 77 CYC) | 5 yr survival: 52% (40 patients) in CYC; 70% (55 patients) in HSCT | 10.01% | At 2 yr: significantly better event free survival, mRSS, EuroQol. HAQ; decline in creatinine clearance and increase in FVC/VC |
| Median follow up 5.8 yr | ||||||
| ASSIST[15] | mRSS 14 with internal organ involvement or coexistent pulmonary Involvement if mRSS was < 14 | IV CYC | 19 (10 HSCT, 9 CYC) | HSCT: all improved; CYC: 8 progressed | None | Small study, 7/8 that progressed in CYC group switched to HSCT. All HSCT patients (including switches) had significant improvement in mRSS and FVC and TLC |
| Follow up 2 yr | ||||||
| SCOT[16] | mRSS > 16, significant visceral organ involvement, disease duration < 4 yr | IV CYC | 75 | Not reported | - | Recruitment competed, yet to be published. Identical regimen to ASTIS except total body irradiation in HSCT |
Most of the data available for HSCT in SSc has shown a significant improvement in skin scores in patients and moderate improvement in FVC and DLCO. In the ASSIST trial[15], 19 patients with SSc and organ involvement were randomized to HSCT (n = 10) or monthly cyclophosphamide for 6 mo (n = 9). Eight/nine patients on monthly cyclophosphamide progressed vs none for HSCT group within the first year after randomization. Seven patients underwent HSCT after evidence of progression on monthly cyclophosphamide. For 11 patients who underwent HSCT and had follow up for at least 2 years there was significant improvements in mRSS (P < 0.0001) and FVC (P < 0.03) compared to baseline. This trial was closed early and there were no deaths reported in either arm.
In ASTIS trial 156 patients with SSc and heart, lung or kidney involvement were randomized to HSCT (n = 79) vs monthly cyclophosphamide (n = 77) for 12 mo. During the first year there were more events (death and irreversible organ failure) in the HSCT group, 13 (16.5%) vs 8 (10.4%) in the cyclophosphamide group. However during the second the cumulative events were similar in two groups 14 (17.7%) vs 14 (18.2%). By 4 year the cumulative events in HSCT group 15 (19%) were less than cyclophosphamide group 20 (26%).
SLE is a prototype autoimmune disease characterized by a wide array of autoantibodies with myriad clinical presentations. Major organ involvement and persistent disease activities are predictors of poor outcome[17]. Treatment response varies in population subsets owing to the genetic composition and racial differences[18]. Hormonal influences in the adult and pediatric patients of SLE further add to the heterogeneity of the disease manifestations. Immunosuppressive therapy is often protracted for adequate disease control and to minimize organ damage in patients with very high disease activity. These are however, associated with significant treatment-related morbidities. Prolonged uses of corticosteroids and repeated flares requiring higher doses of immunosuppressant, inadequate responses have resulted in unfavorable long-term disease free outcomes or drug free intervals[19].
In a trial by Burt et al[20], non-myeloablative HSCT in refractory SLE showed significant advantages of HSCT in terms of progression free survival and alleviation of nephritic symptoms in patients with SLE. HSCT in SLE showed promising results with respect to the SLEDAI score and the serological markers with increasing 5-year progression free survival. There was a stabilization of the nephritic disease with disappearance of APLA titers in a majority[20]. A follow up study using third generation “rituximab sandwich” conditioning regimen (cyclophosphamide, rabbit ATG and CD20 monoclonal antibody rituximab) is ongoing[21]. In EBMT too, positive trends in progression free and overall survival were noted (Table 1)[6].
RA is characterized by progressive joint destruction due to the formation of an inflammatory pannus, which erodes the synovial cartilage and the surrounding bone. The manifestations include articular symptoms like pain and morning stiffness and as the disease progresses extra-articular manifestations like pulmonary fibrosis, vasculitis and eye disease may occur.
With the advent of biologics and early aggressive DMARD therapy, adequate control and a possibility of remission has been possible in early disease. Despite aggressive modalities, some patients are resistant to therapy. Functional disabilities as assessed by Health Assessment Questionnaire (HAQ) and persistence of inflammation in multiple joints are prognostic indicators for a poor survival.
HSCT in RA dates back to 1997. Pilot studies have shown that sustained remission responses were short lived for up to 6-12 mo which was followed by reintroduction of DMARD’s/anti TNF therapy. This was due to the failure to completely obliterate the synovial T cell repertoire following a HSCT. However, following HSCT there was a better response to biologic and non-biologic DMARDs supporting the immunomodulating effect of HSCT. There has been variable success of HSCT in RA but the results have not been encouraging as compared to diseases like SSc[22-24] (Table 1).
The success of HSCT is measured in terms of progression free survival and disease free survival both being the highest in-patient with SSc and RA as compared to other AIRD. Though the results for RA in terms of overall survival rates have been approximately 98%[6], the ability to maintain a sustained ACR 70 response was low with only 28% achieving a progression free survival at the end of 3 years for such an expensive therapy.
Juvenile idiopathic arthritis (JIA) is a deforming joint disease in children a majority of them have a protracted clinical course as with a failure to respond to conventional DMARD’s and biologicals[25,26] and this causes severe morbidity with significantly impaired quality of life. Increased mortality is often due to disease, and from drug toxicities, especially in patients with systemic JIA[27,28]. Published data from the EBMT registry showed transplant related mortality in 7 out of 65 patients of JIA and 52% and 85% of the patients having 3 year progression free and overall survival rates respectively[6] (Table 1).
The experience with HSCT in patients with severe primary systemic vasculitis (PSV) as published in case reports and from EULAR and EBMT-databases gives some evidence that HSCT might be an effective treatment option in refractory cases of PSV and related diseases[29]. In 15 transplanted patients of different forms of vasculitis with an overall response rate of 93% (46% complete and 46%) partial responses were observed[29].
HSCT has been tried in other AIRD such as polymyositis/dermatomyositis, Sjogrens syndrome, psoriatic arthritis[30] and ankylosing arthritis[31]. However, the experience is limited to only few patients to allow any generalisable conclusions.
Several factors determine the sustained clinical remissions or even cure in the treatment of AIRD namely: (1) type and stage of the autoimmune disease; (2) type of transplant allogenic vs autologous[32]; and (3) conditioning regimen (non-myeloablative vs myeloablative)[33]. The EBMT data suggests that in addition to the influence of original diagnosis; age less than 35 years and HSCT performed after December 2000 were associated with a higher progression-free survival[6]. The original diagnosis was a strong determinant of overall survival (highest in RA and lowest in SSc); other factors associated with a better overall survival were the centers’ experience, the use of peripheral blood stem cells, and a disease duration longer than the median before HSCT[6].
The best results with HSCT have been reported for patients with SSc and SLE, whereas for RA it was associated with a higher rate of relapses. Restricted synovial T cell repertoire[34] and T cell responses to a variety of microbial antigens and self-antigens such as type II collagen epitopes are probably the reasons for higher rate of RA relapses in patients who have undergone HSCT. With the advent of biologicals, over the years the use of SCT for RA has become almost obsolete due to the failure of suppression of the synovial T cells.
In SSc, overall there has been a statistically significant improvement in the mRSS and the pulmonary function tests whereas in SLE, the results have been encouraging with higher rates of renal remission.
Treatment of AIRD has been revolutionized over the last two decades with increasing use of biological agents and HSCT in refractory diseases. Careful selection of patients, especially in those with SSc and SLE for HSCT offers long-term progression free and overall survival. Though, till date no one therapy has offered complete remission from these diseases due to multifactorial etiology of this disease along with various external factors also play a role in the progression of these diseases.
| 1. | Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Ikehara S, Kawamura M, Takao F, Inaba M, Yasumizu R, Than S, Hisha H, Sugiura K, Koide Y, Yoshida TO. Organ-specific and systemic autoimmune diseases originate from defects in hematopoietic stem cells. Proc Natl Acad Sci USA. 1990;87:8341-8344. [PubMed] |
| 3. | McColl G, Kohsaka H, Szer J, Wicks I. High-dose chemotherapy and syngeneic hemopoietic stem-cell transplantation for severe, seronegative rheumatoid arthritis. Ann Intern Med. 1999;131:507-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Blaise D, Castagna L. Do different conditioning regimens really make a difference? Hematology Am Soc Hematol Educ Program. 2012;2012:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 5. | Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, Ritz J, Sorror ML, Lee SJ, Deeg HJ. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 6. | Gratwohl A, Passweg J, Bocelli-Tyndall C, Fassas A, van Laar JM, Farge D, Andolina M, Arnold R, Carreras E, Finke J. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 2005;35:869-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (2)] |
| 7. | Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, Ouyang J, Kozak T, Moore J, Kötter I. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Altman RD, Medsger TA, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma). Arthritis Rheum. 1991;34:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Jacobsen S, Halberg P, Ullman S. Mortality and causes of death of 344 Danish patients with systemic sclerosis (scleroderma). Br J Rheumatol. 1998;37:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Bryan C, Howard Y, Brennan P, Black C, Silman A. Survival following the onset of scleroderma: results from a retrospective inception cohort study of the UK patient population. Br J Rheumatol. 1996;35:1122-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Abu-Shakra M, Lee P. Mortality in systemic sclerosis: a comparison with the general population. J Rheumatol. 1995;22:2100-2102. [PubMed] |
| 12. | Dumoitier N, Lofek S, Mouthon L. Pathophysiology of systemic sclerosis: state of the art in 2014. Presse Med. 2014;43:e267-e278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Farge D, Passweg J, van Laar JM, Marjanovic Z, Besenthal C, Finke J, Peter HH, Breedveld FC, Fibbe WE, Black C. Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis. 2004;63:974-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, Craig R, Hirano I, Marshall K, Ruderman E. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 402] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, Schuerwegh AJ, Marijt EW, Vonk MC, Schattenberg AV. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311:2490-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (6)] |
| 16. | Naraghi K, van Laar JM. Update on stem cell transplantation for systemic sclerosis: recent trial results. Curr Rheumatol Rep. 2013;15:326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Ippolito A, Petri M. An update on mortality in systemic lupus erythematosus. Clin Exp Rheumatol. 2008;26:S72-S79. [PubMed] |
| 18. | Mirabelli G, Cannarile F, Bruni C, Vagelli R, De Luca R, Carli L. One year in review 2015: systemic lupus erythematosus. Clin Exp Rheumatol. 2015;33:414-425. [PubMed] |
| 19. | Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, Steinberg AD. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001;135:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 315] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, Verda L, Krosnjar N, Quigley K, Yaung K. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Cyclophosphamide and rATG/Rituximab in Patients With Systemic Lupus Erythematosus. Clinicaltrials.gov, NCT00278538. Available from: https://clinicaltrials.gov/ct2/show/NCT00278538. |
| 22. | Moore J, Brooks P, Milliken S, Biggs J, Ma D, Handel M, Cannell P, Will R, Rule S, Joske D. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum. 2002;46:2301-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Burt RK, Georganas C, Schroeder J, Traynor A, Stefka J, Schuening F, Graziano F, Mineishi S, Brush M, Fishman M. Autologous hematopoietic stem cell transplantation in refractory rheumatoid arthritis: sustained response in two of four patients. Arthritis Rheum. 1999;42:2281-2285. [PubMed] |
| 24. | Verburg RJ, Kruize AA, van den Hoogen FH, Fibbe WE, Petersen EJ, Preijers F, Sont JK, Barge RM, Bijlsma JW, van de Putte LB. High-dose chemotherapy and autologous hematopoietic stem cell transplantation in patients with rheumatoid arthritis: results of an open study to assess feasibility, safety, and efficacy. Arthritis Rheum. 2001;44:754-760. [PubMed] |
| 25. | Wallace CA, Sherry DD. Trial of intravenous pulse cyclophosphamide and methylprednisolone in the treatment of severe systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 1997;40:1852-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S, LeBideau M. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003;48:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 248] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Petty RE. Prognosis in children with rheumatic diseases: justification for consideration of new therapies. Rheumatology (Oxford). 1999;38:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Spiegel LR, Schneider R, Lang BA, Birdi N, Silverman ED, Laxer RM, Stephens D, Feldman BM. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 2000;43:2402-2409. [PubMed] |
| 29. | Daikeler T, Kötter I, Bocelli Tyndall C, Apperley J, Attarbaschi A, Guardiola P, Gratwohl A, Jantunen E, Marmont A, Porretto F. Haematopoietic stem cell transplantation for vasculitis including Behcet’s disease and polychondritis: a retrospective analysis of patients recorded in the European Bone Marrow Transplantation and European League Against Rheumatism databases and a review of the literature. Ann Rheum Dis. 2007;66:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Hügle T, van Laar JM. Stem cell transplantation for rheumatic autoimmune diseases. Arthritis Res Ther. 2008;10:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Jantunen E, Myllykangas-Luosujärvi R, Kaipiainen-Seppänen O, Nousiainen T. Autologous stem cell transplantation in a lymphoma patient with a long history of ankylosing spondylitis. Rheumatology (Oxford). 2000;39:563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Khorshid O, Hosing C, Bibawi S, Ueno N, Reveille J, Mayes MD, Champlin RE. Nonmyeloablative stem cell transplant in a patient with advanced systemic sclerosis and systemic lupus erythematosus. J Rheumatol. 2004;31:2513-2516. [PubMed] |
| 33. | Loh Y, Oyama Y, Statkute L, Verda L, Quigley K, Yaung K, Barr W, Jovanovic B, Burt RK. Non-myeloablative allogeneic hematopoietic stem cell transplantation for severe systemic sclerosis: graft-versus-autoimmunity without graft-versus-host disease? Bone Marrow Transplant. 2007;39:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Goronzy JJ, Zettl A, Weyand CM. T cell receptor repertoire in rheumatoid arthritis. Int Rev Immunol. 1998;17:339-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kiselev SL, Liu J S- Editor: Ji FF L- Editor: A E- Editor: Wang CH