Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.196
Peer-review started: July 5, 2015
First decision: July 31, 2015
Revised: September 2, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: December 24, 2015
Processing time: 175 Days and 16.1 Hours
Induction of tolerance remains a major goal in transplantation. Indeed, despite potent immunosuppression, chronic rejection is still a real problem in transplantation. The humoral response is an important mediator of chronic rejection, and numerous strategies have been developed to target either B cells or plasma cells. However, the use of anti-CD20 therapy has highlighted the beneficial role of subpopulation of B cells, termed regulatory B cells. These cells have been characterized mainly in mice models of auto-immune diseases but emerging literature suggests their role in graft tolerance in transplantation. Regulatory B cells seem to be induced following inflammation to restrain excessive response. Different phenotypes of regulatory B cells have been described and are functional at various differentiation steps from immature to plasma cells. These cells act by multiple mechanisms such as secretion of immuno-suppressive cytokines interleukin-10 (IL-10) or IL-35, cytotoxicity, expression of inhibitory receptors or by secretion of non-inflammatory antibodies. Better characterization of the development, phenotype and mode of action of these cells seems urgent to develop novel approaches to manipulate the different B cell subsets and the response to the graft in a clinical setting.
Core tip: Regulatory B cells have been characterized mainly in auto-immune diseases but emerging literature suggests their role in graft tolerance in transplantation. Regulatory B cells exhibit different phenotypes and act by multiple mechanisms such as secretion of immuno-suppressive cytokines, cytotoxicity, expression of inhibitory receptors or secretion of non-inflammatory antibodies. Better characterization of the development, phenotype and mode of action of these cells seems urgent to develop novel approaches to manipulate the different B cell subsets and the response to the graft in a clinical setting.
- Citation: Durand J, Chiffoleau E. B cells with regulatory properties in transplantation tolerance. World J Transplant 2015; 5(4): 196-208
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/196.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.196
The major goal in the field of transplantation is to prevent allograft rejection due to the response of recipient’s immune system against alloantigen. Despite strong advances in immuno-suppression regimens that allow the control of acute rejection, chronic rejection subsists and the lack of antigen specificity leads to increased risks for infectious diseases and malignancies[1,2]. Achievement of long-term immunologic tolerance, defined at long-term graft function in the absence of immunosuppression is difficult to achieve in humans. Nevertheless, operational tolerance has been reported in some liver and in more rare cases of kidney transplantations[3,4]. Therefore, understanding the mechanisms of tolerance in these patients and in animal models is of great importance for subsequent breakthroughs in the transplantation field. Last decades, research in transplantation has focused mostly on T cell-directed therapy. Nevertheless, the role of B cells in transplantation and especially in chronic rejection with their production of deleterious antibodies has recently pushed the immunologist to develop more B cell-targeted therapies. However, recent literature demonstrates that B cells can also be beneficial for the grafted tissue by the secretion of anti-inflammatory cytokines or by the production of protective antibodies. Among these populations, different subsets of regulatory B cells have been described. These findings have generated great interest and urge immunologists to modulate B cell-directed therapies to target specifically effector B cells while sparing regulatory B cells.
B cells play an important role in graft rejection by stimulating directly CD4+ T lymphocytes to produce cytokines including interferon-γ (IFNγ) interleukin-4 (IL-4) and IL-6[5]. B cells infiltrate allografts and locally stimulate effector T cells. Indeed, it has been demonstrated the presence of ectopic germinal centers in the transplanted tissue, called tertiary lymphoid tissues[6,7].
The most deleterious role of B cells in transplantation is due to their differentiation in plasma cells producing high level of alloantibodies[8,9]. The mode of action of these alloantibodies depends mainly of two mechanisms. The first is the activation of the complement proteolytic cascade and the second, the antibody dependent cellular cytotoxicity. These cytotoxic mechanisms are triggered by the fixation of alloantibodies on donor class I and II MHC molecules expressed especially by endothelial cells of the graft.
Classical pathway of complement activation (antibody-dependent) is induced by the fixation of the C1 component to the Fc fragment of antibodies bound to their antigen. The enzymatic complement cascade leads to the formation of an attack membrane complex (C5b, 6, 7, 8, 9) which forms a channel in the cell membrane and damages the endothelium[10]. Activated endothelial cells produce then pro-inflammatory cytokines such as IL-1, IL-8 and MCP-1 that attract neutrophils and monocytes in the graft, promote vascular permeability and the secretion of procoagulant factors. This cascading event results in bleeding, vascular thrombosis and causes ischemia and graft rejection[11]. The C4d resulting from the hydrolysis of C4b deposits on the graft cells and is a marker for activation of the humoral response[12]. Therefore, similarly to the presence of donor-specific antibodies, the C4d deposit detection on cells of the graft is usually a bad prognostic. It can provide an indication of graft outcome and the mechanisms involved in rejection. The deleterious effect of donor-specific antibodies can vary according to their concentration, affinity, isotype and their glycans groups at their Fc fragment[13,14].
For cellular cytotoxicity mechanism, the Fc fragments of alloantibodies attached on the target cells are thus recognized by Fc fragment receptors on natural killer cells and macrophages. This recognition will lyse target cells via granzyme/perforin pathway and induces the production of pro-inflammatory mediators such as NO, ROS and TNF.
Different strategies have been developed to reduce the level of donor-specific antibodies in transplanted patients. One approach is to induce the depletion of B cells using depleting antibodies such as anti-CD20 (Rituximab) or anti-CD22. Rituximab is a glycosylated immunoglobulin G (IgG) chimeric mouse/human antibody. Rituximab binds to the CD20 antigen present at the cell-surface of the pre-B cells to terminally differentiated plasma cells. However, pro–B cells or mature plasma cells that produce about 90% of circulating IgG do not express CD20. Therefore, Rituximab is not able to prevent the regeneration of B cells from precursors, and does not directly prevent immunoglobulin productions[15]. Rituximab is efficient to treat auto-immune diseases and lymphoma[16], however, in clinic, no convincing benefit was found so far as induction therapy in renal transplantation. However, in conjunction with other treatment it has been reported to have a beneficial effect on antibody production in chronic antibody-mediated rejection[17]. CD22 corresponds to an Ig superfamily glycoprotein that acts as an inhibitory receptor. In mice, anti-CD22 treatment, has been shown to deplete B cells in spleen, bone marrow, lymph nodes and peripheral blood and since CD22 is also expressed on CD138+ plasma cells, it decreases antibody production[18]. Thus, this antibody has been reported to reduce the anti-donor immune response in some mouse models of islet transplantation[19]. In Human, Epratuzumab, a humanized anti-CD22 antibody, has been shown to induce depletion of both naive and transitional B cells, to inhibit B cell activation and proliferation leading to a beneficial effect for treatment of systemic lupus erythematosus[20]. Other strategical approach has been to modulate the B cell response by targeting B-cell survival, proliferation and maturation. In this regard, to modulate the B-cell-activating factor (BAFF) pathway is promising[21]. BAFF belongs to the tumor necrosis factor family and is produced by monocytes, macrophages and dendritic cells. The three BAFF receptors, BAFF-R, transmembrane activator and calcium modulator and cyclophyllin ligand interactor and B-cell-maturation antigen (BCMA) are expressed on B cells (follicular, germinal centre and memory), with BCMA preferentially expressed on plasma cells[22]. In vivo BAFF neutralization has been shown to be efficient in experimental models of auto-immune diseases such as diabete[23]. In transplantation, BAFF-deficient recipients exhibit prolongation of allograft survival in a murine cardiac model[24]. In addition, in an islet allograft model, BAFF blockade in conjunction with immunosuppression allowed long-term allograft survival[25]. In Human, BAFF-blockade has been used as strategy in the treatment of autoimmune diseases[26] such as systemic lupus erythematous (SLE)[27], and must now be tested in combination with immunosuppressive agents. Other strategies, such as plasmapheresis or injection of polyclonal intravenous immunoglobulins (IVIGs) allow a more rapid elimination of circulating donor-specific antibodies. The IVIGs treatment consists in injection of high doses of human purified IgG from many healthy donors. It is suggested that the immunosuppressive effect of these Ig involves their attachment to the donor-specific antibodies hindering their function but also through regulatory mechanisms induced by the fixation of their Fc fragment on Fc receptors present on many cells, such as B cells, dendritic cells and macrophages[28]. Bortezomib, a proteasome inhibitor blocking the production of antibodies and inducing apoptosis of plasma cells[29,30], in combination with dexamethasone, is commonly used in multiple myeloma patients and represents a promising strategy. A humanized monoclonal antibody targeting the C5 complement compound (Eculizumab) and donor-specific antibodies function is also under study and provides encouraging results. It inhibits the formation of attack membrane complex, thus preventing the full complement activation[31].
As mentioned, B cells play a crucial role in graft rejection and auto-immune diseases by their ability to induce inflammatory immune response through their role of antigen-presenting cells and their unique ability to produce and secrete deleterious antibodies. Therefore, numerous strategies have been developed to target these B cells or the produced antibodies.
However the last years, numerous studies have also reported regulatory properties of B cells[32,33]. Existence of B cells with suppressive properties has originally been highlighted in the 60 s. Authors observed that transfer into naive syngeneic mice of antibody-secreting cells from spleen of mice immunized with sheep red blood cells suppressed the antibody production against these sheep cells[34]. Then, concept of suppressive B cells was confirmed in 1974 in a model of guinea pig delayed hypersensitivity[35,36]. First report that described precisely the existence of regulatory B cells was in a model of experimental autoimmune encephalomyelitis (EAE) in mice. They showed that (μMT) B-cell deficient mice, developing EAE following myelin oligodendrocyte glycoprotein immunization, were not able to spontaneously enter in remission compared to wild-type mice[37]. Then, the regulatory properties of B cells have been described in mice in other models of autoimmune diseases, such as rheumatoid arthritis[38], SLE[39], diabetes[40], colitis[41], as well as more recently in infectious diseases and cancer[42-44].
Since then, numerous studies in humans and rodents demonstrated common features of suppression by these cells in these different models. However, a single phenotype of regulatory B cells common to the different species is at present not yet identified.
In transplantation, implication of B cells as inductors of tolerance has been demonstrated in several experimental models. In mouse pancreatic islet and cardiac MHC mismatched allograft models, administration of allogenic donor B cells together with CD40 ligand blockade prior transplantation induced prolongation of allograft survival[45,46]. In rat, B cells from donor administrated at the time of transplantation induce long-term kidney graft acceptance[47]. By CD45 immunosuppressive targeted therapy, that modulated T cell development and activation, it has been shown that tolerance was lost in (μMT) B cell-deficient mice and was restored by B cell transfer, demonstrating that tolerogenic effect requires host B cells[48]. These host B cells require the costimulatory molecules CD80, CD86 and CD40 to exert their suppression suggesting cooperation with T cells.
Ding et al[49] demonstrated in mice that T-cell immunoglobulin and mucin domain 1 (Tim1) represents a cell-surface phenotypic marker of IL-10+ enriched regulatory B cells and that enhancement of this population by anti-Tim1 antibody treatment prolong islet and cardiac allograft survival. In this model, depletion of CD4+CD25+ regulatory T cells before transplantation leads to allograft rejection demonstrating that tolerance induction is dependent on interaction between regulatory B and regulatory T cells[50].
We previously demonstrated a model of cardiac allograft tolerance in rat induced by a short-term treatment with the immuno-suppressor LF15-0195, a deoxyspergualin analog[51,52]. In this model, we observed after treatment cessation an accumulation of B cells in the blood over-expressing inhibitory molecules and B cells from spleen were able to transfer allograft tolerance to new recipients demonstrating the presence of regulatory B cells[53]. In the graft, we observed cluster of mature B cells that in contrast to the ones from chronically rejected recipients do not express IgG suggesting B cells blocked at the switch recombination process[53].
Interestingly, several research groups have demonstrated a B cell gene signature in blood of patients that spontaneously developed operational tolerance to kidney transplant after immuno-suppressive treatment cessation[54-56]. These patients exhibit higher mRNA expression of immunoglobulin light chains, CD20 and proliferation and cell cycle genes[55]. Moreover, B cells from tolerant patients expressed more of the inhibitory receptors Fcgr2b and of the CD40 signaling modulator BANK-1 (B-Cell Protein Scaffold With Ankyrin Repeats)[54]. This signature is associated with increased or at least preserved pool of CD19+ CD24high CD38high IL10+ B cells[54-56]. The precise mechanisms of this suppression mediated by B cells remains elusive but it has been suggested that that transforming growth factor (TGF) could play a function since a third of the modulated genes in the blood are target of TGF[57]. More recently, the same team shows that B cell from operationally tolerant patients cells have a defect in their in vitro differentiation into CD38+ CD138+ plasma cell and a more important susceptibility to apoptosis at late differentiation step[58]. In addition, these B cells secrete more IL-10 following in vitro stimulation. Interestingly, during pregnancy that corresponds to a particular state of tolerance to alloantigens, a population of CD19+ CD27+ CD24high regulatory B cells is induced to maintain tolerance to the fetus[59]. These B cells present in the mother produce high amounts of IL-10 and suppress in co-culture the production of TNFα by effector T cells. Consistent with a regulatory function for B cells in human transplantation, a clinical trial has shown an increased risk for acute cellular rejection following depletion of B cells prior to transplantation that could be due to a loss of regulatory B cells[60]. Therefore, all these results suggest a role of regulatory B cells in the induction or maintenance of tolerance in these operationally tolerant patients.
Regulatory B cells cannot be defined based on a phenotype composed of conventional B cell-surface markers. Therefore, characterization has relied exclusively on assessing their suppressive activity. Although several regulatory B cell subsets have been described in Humans and mice, most of them share the ability to express the anti-inflammatory cytokine IL-10. IL-10 producing cells have been identified in the immature, naive, CD27+ memory as well as the plasmablast/plasma B cell subpopulations[61-64]. In mice, regulatory B cells are described in the CD19+ CD1dhigh CD5+ subset in the spleen and may present as CD21high IgMhigh either with or without expression of CD23 (Cf Table 1 representing different phenotype of regulatory B cells in different compartment in mice). In humans, regulatory B cells were identified in CD19+ CD24high subsets of both CD27- CD38high immature and CD27+ memory B cell compartments highlighting the diversity of these cells[65-68].
| Peritoneal cavity | Periarteriolar lymphoid sheaths | FO | MZ |
| Mature B1a cells: CD19+CD11b+CD5+IgMhighCD23-CD21- | T1 B cells: | T2 FO B cells: | T2 MZP B cells: |
| CD19+CD24+IgMhigh | CD19+CD24+IgMhighIgD+CD23+CD21- | CD19+CD24+ | |
| IgD-CD23-CD21- | IgMhighIgD- CD23intCD21+CD1d+ | ||
| Pro-B10 cells: | FO B cells: | B10 B cells: | |
| CD19+CD1d+CD5+ | CD19+CD24+IgMintIgD+CD23+CD21int | CD19+ CD1d+CD5+IL-10+ | |
| Plasma cells: | MZ B cells: | ||
| CD19+CD138high | CD19+CD24+ | ||
| IgMhighIgDlowCD1dintCD43hi | IgMhighIgD-CD23-CD21+CD1d+CD5+ | ||
| CD44hi |
The most recurrent phenotype for identifying murine regulatory B cells is probably the secretion of IL-10. These regulators B lymphocytes are then called B-10. Apart from this particular property, many studies have described subpopulations of murine regulatory B cells with different phenotypes. The B1a cells, present in the peritoneal cavity were one of the first sub-population identified as IL-10-secreting B cells[69]. B cells of the marginal zone of the spleen and having a CD19+ CD21+ CD23- CD24+ CD1d+ IgM+ phenotype secrete IL-10 following CpG (TLR9 agonist) stimulation and are able to regulate the immune response in a model of lupus[39]. Mauri et al[38] describe precursors of these cells, called transitional 2-marginal zone precursor (T2-MZP), in a mouse model of collagen-induced arthritis. These B cells produce IL-10, have a CD19+ CD21high CD23+ CD93+ CD24high phenotype and their adoptive transfer in immunized mice prevents the development of the disease by IL-10 dependent mechanisms, since B cells deficient for IL-10 are inefficient[38,61].
The regulatory role of B10 cells (CD19+ CD5+ CD1d+) was identified in various autoimmune models such as EAE, inflammatory bowel disease, collagen-induced arthritis and lupus[70]. B10 cells, share phenotypic markers with B1a cells, T2-MZP and marginal zone B cells and correspond to less than 2% of B cells from the spleen of naive mice. The Tedder team demonstrates the existence of rare B10 enriched in the subpopulation of CD1dhigh CD5+ B cells in the spleen of naive mice and secreting large quantities of IL-10 in response to strong stimulation[71]. In addition, another study demonstrated that this subpopulation of CD5+ CD1d+ IL-10+ B cells could, in vitro, be substantially increased following stimulation with the B cell activating factor BAFF and help to reduce following transfer the development of collagen-induced arthritis[72]. Indeed, BAFF is required for transition from T1 immature to T2 transitional stage of B cells and is essential for marginal zone B cell development[73].
Interestingly, the TIM-1 protein has been identified as expressed by a large part of IL10+ regulatory B cells in mice. Transfer of these TIM-1+IL-10+cells, obtained from any B cell subpopulation of the spleen can directly induce tolerance to islet allograft[49]. However, some studies described regulatory B cells exerting their suppressive role through IL-10 independent mechanisms[74].
The existence of regulatory B cells in humans has been suggested by different teams. As for the mouse, the phenotype of these cells varies depending on the study. Duddy et al[75] observed a decrease in IL-10 secreting B cells in multiple sclerosis patients. The team of Mauri highlighted in patients with SLE, a decreased in the subpopulation of immature CD19+ CD24high CD38high cells defined as secreting large amounts of IL-10 and suppressing TNF and IFN secretion by autologous T cells[65]. Subpopulations of regulatory B cells equivalent to murine B10 were also identified in humans. Indeed, although most publications described regulatory B cells with a transitional/naive phenotype, some teams identify them in humans among the pool of memory B cells with the CD27 or CD148 markers[66,68]. In addition, CD19+ CD24high CD38high CD5high IgD+ B cells with suppressive properties and inducing an expansion of regulatory T cells in vitro were also identified[76].
Taken together, all these studies suggest that the development of B cells with regulatory functions described at different B-cell differentiation step and in several compartments may depend on the microenvironmental factors and may not derived from a single lineage population.
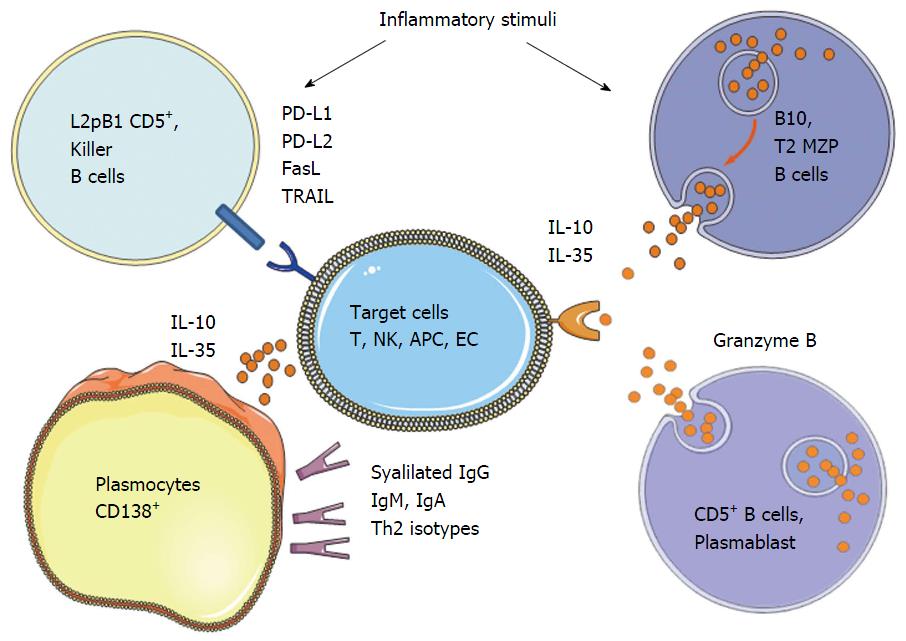
Suppressive mechanisms appear to be diverse and can act by the production of anti-inflammatory antibodies, the secretion of immunosuppressive agents, or by the cell surface expression of inhibitory receptors (Figure 1).
Natural antibodies: It has been shown in Humans and mice that B cells from neonates expressed a basal level of IgM named natural antibodies or non-immune antibodies generated by the B-1 cells, derived from a small portion of precursors capable of self-renewing[77]. IgM produced have restricted repertoire, low affinity and respond to T-independent signals by cross-reactivity. They play a role in the removal of apoptotic cells by inducing the recruitment of complement component that will following complex phagocytosis prevents excessive immune activation[78] and induces Th2-deviation[79].
The secretion of suppressive cytokines: The most described and studied mechanism of suppression employed by the regulatory B cells is the secretion of IL-10, which is one of the most potent anti-inflammatory cytokine to control inflammation. The importance of IL-10 in suppression by B cells has been demonstrated only in the last decade with the identification of a subpopulation of B cells producing high level of IL-10 and over-expressing CD1d molecule in the gut-associated lymphoid tissues in a chronic intestinal inflammation model[80]. Moreover, remission of multiple sclerosis in mice correlates with the presence in the spleen of B cells producing IL-10 following stimulation[81]. Interestingly, they found that IL-10 producing B cells can reduce the severity of the disease following adoptive transfer, while B cells from mice deficient for IL-10 could not. In the following years, other studies have shown that the IL-10 secreting B cells regulate autoimmunity in different mouse models including models of arthritis[38], diabetes[40] and systemic lupus erythematosus[82]. Recently, the TIM-1 protein (T-cell Ig and mucin domain domain protein 1) has been described to identify the two-thirds of IL-10 producing B cells, making it the most specific cell-surface marker identified to date[49]. The treatment of mice with anti-TIM-1 antibody induced an increase of the TIM-1 B cell pool producing IL-10 and improved the tolerance to an allograft, suggesting that TIM-1 is also involved in the function of these cells and not only a cell-surface marker. These results suggest that targeting TIM-1 could be a new therapeutical approach for enhancing the expansion of these regulatory B cells in transplantation or auto-immune disease fields. It is suggested that the IL-10+ expressing B cells (called B10 cells) are not T2-MZP B cells[83]. It is also possible that the production of IL-10 appears indifferently in the various subpopulations of B cells, depending on the activation or differentiation state. The action of IL-10 appears to be closely linked to the one of another cytokine less described, IL-35. Indeed, mice deficient in p35 or EBI3, the two subunits of IL-35 and specifically in B cells exhibit an exacerbation of EAE compared to control mice[63]. In addition, culture of B cells in the presence of IL-35 induced an increase in the B cell subpopulation expressing IL-35, called IL-35+ regulatory B cells, and half of them expressed IL-10. Similarly, in in vivo experiments, although IL-35 inhibits the proliferation of conventional B cells, it selectively induces the expansion of CD19+ CD5+ B220low B10 regulatory B cells. These B10 cells are capable upon transfer to limit established uveitis in mice, and 60% of B10 found in the spleen also express IL-35. This urges the importance to better knowledge these mechanisms, which could then represent new therapeutic targets for autoimmune disorders and infectious diseases.
Interestingly, studies in genetically modified mice expressing eGFP linked to IL-10 gene, so as IL10 reporter, have shown that the cells that express the most IL10 have the plasma cell marker CD138, suggesting that the most potent regulatory B cells are plasma cells[67]. Similarly, a 2014 study showed following infection with Salmonella, the emergence of IL-10 and IL-35 producing B cells enriched in the pool of IgM+ CD138++ BLIMP1 plasma cells[84]. Furthermore, after PCR analysis, transcripts for Ebi3 and p35 were co-expressed by the CD138high B cells, also expressing high levels of Blimp-1 and IRF4 transcripts and corresponding to the most efficient antibodies secreting cells. EBI3 and p35 proteins were found as expressed by CD138+ plasma cells and not by CD19+ CD138-B cells in mice following infection with Salmonella or during EAE. B cells depend on IRF4 and BLIMP-1, which are required for plasma cell differentiation, to provide regulatory functions in vivo. These data, although referenced in 2014 by Dang et al[64] and Ries et al[85], suggest that plasma cells have roles other than the one of antibodies producers, such as the secretion of immunosuppressive cytokines able to modulate many immune responses. Indeed, B1 cells were demonstrated to be able to differentiate into CD19+ CD138+ IgM+ plasma cells producing GM-CSF in a mouse model of septic shock[86]. Similarly, plasma cells expressing iNOS and TNF were found in the lamina propria of the intestine in mice[87]. In normoglycemic NOD mice, which do not develop diabetes, islet-infiltrating B cells were identified as more antigen-experienced IL-10+ cells with more diverse B-cell receptor repertoires compared to those of hyperglycemic mice. In addition, healthy individuals showed increased numbers of IL-10+ B cells compared to type 1 diabetic patients[88]. Therefore, cytokine production is also an important aspect of B cell biology. Further work is therefore required to identify the additional signals that specify the differentiation of B cells into “regulatory plasma cells” producing anti-inflammatory cytokines.
The expression of cytotoxic mediators: Expression of the cytotoxic component Granzyme B by B cells was first described in chronic leukemia patients whose B cells undergo apoptosis following stimulation with TLR agonist and IL-21[89]. Such cells were then identified following Epstein-Barr virus transformation, but also in patients developing psoriasis, rheumatoid arthritis or lupus (SLE)[89-91]. Although the existence of B cells expressing Granzyme B was confirmed in humans, there is nothing in the mouse. Indeed, in different mouse strains, B cells are not capable of expressing Granzyme B even with strong stimuli (IL-21, anti-BCR, LPS, CpG-ODN) or after viral infection, unlike cytotoxic T cells for which the level of expression is significantly increased[92]. Recently, it has been shown that untreated human immunodeficiency virus (HIV) patients display CD4+ T cells with enhanced IL-21 expression and high in vivo frequencies of regulatory B cells over-expressing the Granzyme B[93]. These cells may contribute significantly to immune dysfunction in HIV patients, and may also explain ineffective antibody responses after vaccination. In transplantation, kidney-transplanted tolerant recipients exhibited a higher number of Granzyme B expressing B cells with a plasma cell phenotype and that required IL-21 production[94]. Granzyme B - expressing CD19+ IgA+ CD27+ CD138high CD20- plasma cells with cytotoxic properties have also been described in the normal intestinal mucosa, and were significantly more frequent in both Crohn’s disease and ulcerative colitis[95]. Granzyme B expression by B cells has been shown to act by limiting T-cell proliferation by degradation of the T-cell receptor ζ-chain[96].
FasL (CD178) expression by human B cells has been observed first following strong in vitro stimulation[97]. Since then, different teams demonstrated the expression of FasL by human and murine B cells[98,99], and was suggested in the cases of malignancy, to be a way to increase the virulence of the tumor by promoting apoptosis of the T cells[100]. B cells expressing FasL were also found in various types of viral infections including Epstein-Barr virus[101], HIV[102] and virus murine leukemia virus[103], and also by leading to T cell apoptosis lead to persistent infections. B cells expressing FasL were demonstrated to suppress the induction of autoimmune diabetes in NOD mice[104] and were found at high levels in a mouse model of lupus[105]. In a minor mismatch transplantation model in mice, injection of splenic purified B cells is sufficient to prevent graft rejection, whereas the one from FasL deficient mice does not[106].
The role of tumor necrosis factor-related apoptosis inducing-ligand (TRAIL) or CD253 in B cell mediated immunosuppression is less characterized. Expression of TRAIL by B cells was described in human lines and murine B lymphoma[107,108]. TRAIL has also been detected in cases of leukemia and myeloma and in non-transformed human and murine B cells[107-109].
The programmed death receptor 1 (PD-1), and its two ligands, PD-L1 and PD-L2 are important regulator of tolerance[110]. PD-L1 is expressed by numerous resting immune cells and regulates Th1 responses[111]. In contrast, PD-L2 is more restricted to activated antigen-presenting cells[112], and regulates Th2 responses, such as asthma[113]. It has been demonstrated PD-L2 expression on half of a subpopulation of peritoneum CD5+, the L2pB1 cells in mice[114]. Recently, it has been shown the presence of regulatory B cells in a model of human-therapy-resistant prostate cancer. The crucial immunosuppressive B cells that infiltrate the tumors are plasma cells that express IgA, IL-10 and PD-L1. Their appearance depends on TGFβ receptor signaling and there elimination allows CTL-dependent eradication of oxaliplatin-treated tumours[115].
Immunosuppressive IgG antibodies and deviation of the response: During an effective immune response, high-affinity IgG antibodies are produced to recognize epitopes from pathogens and their Fc fragment binds to the Fc receptors expressed on immune cells, thus altering their activation and phagocytosis property[116]. Particular glycoforms of IgG have been identified to alter binding of IgG to Fc receptors[117]. In addition, IgG glycoforms having a sialic acid group at terminal position showed an anti-inflammatory activity[28,118]. These glycoforms suppress inflammation by binding to specific intracellular adhesion molecule 3 grabbing nonintegrin homolog-related 1 (SIGN-R1)[119], leading to induction of an immunosuppressive Th2 response[120]. The events involved in sialylation of IgG are currently unknown and surprisingly, pro-inflammatory stimuli induced in vitro, rather than a decrease, an increase in sialylation[121].
Inhibition or deviation of the Th T cell response and induction of regulatory T cells by B cells have been demonstrated in numerous in vitro and in vivo studies and may implied direct interactions or act through the mechanisms described early. Antigen specific immunosuppressive T cells can be expanded in vitro by co-cultures with regulatory B cells isolated in a transplantation tolerance model in mice[122]. In vivo, following adoptive transfer, regulatory B cells induced the expansion of regulatory T cells via IL-10 and were able to regulate autoimmune[123] and infectious diseases[124]. In addition, various studies have demonstrated that allogeneic T cells with suppressive properties could be induced in vitro with the only presence of naive B cells[125,126].
Other interesting aspect of the properties of antibody in transplantation is the phenomenon called accommodation. Indeed, in some models of allo-and xenotransplantation, it is possible to observe the presence of donor-specific antibodies without functional deterioration of the tissue or the graft[127]. Accommodation is associated with the expression of cytoprotective molecules such as HO-1, IDO, NO, Bcl-2 and Bcl-XL that protect graft endothelial cells by regulating immune response, inflammation and apoptosis[128-134]. It is suggested that these antibodies with particular isotype would not be harmful but rather protective toward the graft and could be the source of the expression of protective molecules.
Interestingly, beekeepers who have a long-term tolerance to bee venom allergens have a subpopulation of CD25high CD71high regulatory B cells which produces the specific antibody isotype IgG4[135]. Indeed, the bee venom-based vaccines induce the production of IgG4 antibodies specific to allergen and capable to inhibit the interaction IgE/allergen and to promote the expansion of regulatory T cells[136]. It is necessary to identify the conditions responsible for the production of protective antibodies to the graft to adapt immunosuppressive treatment and therapy protocols targeting B cells or antibodies.
Although largely described as involved in the prevention of auto-immune diseases, the importance of CD19+ CD24high CD38high immature B cells in kidney transplantation in a clinical setting, has been highlighted by their increased frequencies in operationally tolerant patients after immunosuppressive treatment cessation[55,56]. The proof as to their direct role in this phenomenon is still lacking but these studies suggest the relevance of these cells as biomarkers of tolerance. In this sense, a recent longitudinal prospective study aiming to track the relationship between these cells and clinical events demonstrates that transitional B cell frequencies (but not “regulatory” T cells) were associated with protection from acute rejection[137]. Another study demonstrates in the cases of chronic antibody-mediated rejection, a reduced ratio of activated to memory B cells and an impaired immunosuppressive activity[138]. Therefore, these clinical studies highlighted the potential utility of these cells as biomarkers of predictive graft outcome, to adapt immunosuppressive treatment.
According to immuno-suppression protocols, there constitution of the B cell compartment in the presence of alloantigens could create a favorable environment for the development and maintenance of tolerance towards antigens of the graft. Indeed, Parsons et al[139] demonstrated in mice that depletion of the B cell compartment at the time of transplantation induces tolerance by depleting allo-reactive B cell clones and reshaping the B cell repertoire. Following some immunosuppressive treatments, the B cell compartment is recolonized by B cell populations exhibiting a phenotype of regulatory B cells. For example, following an induction treatment with Alemtuzumab (anti-CD52), the authors observed a temporary increase in the proportion of transitional B cells, described as regulatory B cells[140]. It has also been sown that that Alemtuzumab in contrast to Basiliximab (anti-CD25) induced the expansion of a novel peripheral lymphocyte phenotype, although clinical outcomes were similar. This appearance of naive, transitional and regulatory B-cell subtypes was associated with more stable graft function and is due to homeostatic repopulation following lymphocyte depletion[141]. Furthermore, similar results were obtained in non-human primates following depletion of B cells with Rituximab (anti-CD20). Indeed, the reconstitution of the compartment by immature and transitional B cells was associated with long-term graft survival of pancreatic islets[142]. In a model of diabetes in mice, anti-CD22 treatment also demonstrated the generation of a pool of immature reemerging B220+ CD93+ CD23+ IgMlow B cells, unable to present efficiently antigens, and that can regulate at long-term the autoimmune response by establishing tolerance toward autoantigens[18].
Moreover, a novel role of CD24high CD27+ and IL-10+ plasmablast B cells has been suggested in the regulation of human chronic graft-versus-host disease[143]. Therefore, depletion of B cells as the central strategy for preventing rejection is a paradigm. Depleting strategy at the induction phase may help to reshape the immune B cell repertoire and the re-emergence of regulatory immature B cells but at a latter phase or for the treatment of antibody-mediated rejection, although prevent the donor-specific antibody formation, may be deleterious for the pre-existing regulatory B cell population. The exact therapeutical narrow of depletion and the beneficial effect of combined immunosuppressive regimens are now urgent to evaluate in the setting of transplantation. Another aspect to consider is the potential adverse side effects of B-cell modulation in the development of infections. Indeed, as such for immunosuppressive regimens, B cell depletion and emergence of regulatory B cells could lead to infectious complications and reactivation of some virus notably following B cell transfer[144].
This review highlighted the recent literature suggesting that B cells can also act as beneficial players in organ transplantation by controlling inflammation and promoting long-term regulatory mechanisms leading to operational tolerance. They exhibit various phenotypes and mode of action that may depend on their localization and their induction. They seem to expand following inflammation to restrain immune response and are therefore involved in the maintenance of the fine-tune balance equilibrium between effector and regulatory cells. Mechanisms exert by these cells are diverse and have mostly been described in auto-immune diseases. However, recent literature data suggests similar mechanisms in transplantation. They can act through the production of anti-inflammatory cytokines, protective antibodies or by depleting effectors or inducing other types of regulatory cells. The depletion of B cells as the central strategy for preventing antibody-mediated rejection should be reconsidered since this therapy deplete also B cells displaying regulatory activity and consequently could impact badly the graft outcome.
Therefore, it is crucial to better characterize the temporal expansion of these cells, the stimuli that activate them, their precise phenotype and mode of action to develop new strategies in a clinical setting.
| 1. | Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 543] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 2. | Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benítez C, Pons JA, Parrilla P, Ramírez P. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Roussey-Kesler G, Giral M, Moreau A, Subra JF, Legendre C, Noël C, Pillebout E, Brouard S, Soulillou JP. Clinical operational tolerance after kidney transplantation. Am J Transplant. 2006;6:736-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Ron Y, Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987;138:2848-2856. [PubMed] |
| 6. | Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, Mandet C, Belair MF, Bruneval P, Meilhac O. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102:14723-14728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Thaunat O, Patey N, Morelon E, Michel JB, Nicoletti A. Lymphoid neogenesis in chronic rejection: the murderer is in the house. Curr Opin Immunol. 2006;18:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Haririan A, Kiangkitiwan B, Kukuruga D, Cooper M, Hurley H, Drachenberg C, Klassen D. The impact of c4d pattern and donor-specific antibody on graft survival in recipients requiring indication renal allograft biopsy. Am J Transplant. 2009;9:2758-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Piazza A, Poggi E, Borrelli L, Servetti S, Monaco PI, Buonomo O, Valeri M, Torlone N, Adorno D, Casciani CU. Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: posttransplant analysis using flow cytometric techniques. Transplantation. 2001;71:1106-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM. Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002;169:4620-4627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Rocha PN, Plumb TJ, Crowley SD, Coffman TM. Effector mechanisms in transplant rejection. Immunol Rev. 2003;196:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 13. | Chang AT, Platt JL. The role of antibodies in transplantation. Transplant Rev (Orlando). 2009;23:191-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678-1685. [PubMed] |
| 16. | Fiorina P, Sayegh MH. B cell-targeted therapies in autoimmunity: rationale and progress. F1000 Biol Rep. 2009;1:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Fehr T, Rüsi B, Fischer A, Hopfer H, Wüthrich RP, Gaspert A. Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation. 2009;87:1837-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, Wu E, Tian Z, Abdi R, Guleria I. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57:3013-3024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Carvello M, Petrelli A, Vergani A, Lee KM, Tezza S, Chin M, Orsenigo E, Staudacher C, Secchi A, Dunussi-Joannopoulos K. Inotuzumab ozogamicin murine analog-mediated B-cell depletion reduces anti-islet allo- and autoimmune responses. Diabetes. 2012;61:155-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Dörner T, Kaufmann J, Wegener WA, Teoh N, Goldenberg DM, Burmester GR. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Vugmeyster Y, Seshasayee D, Chang W, Storn A, Howell K, Sa S, Nelson T, Martin F, Grewal I, Gilkerson E. A soluble BAFF antagonist, BR3-Fc, decreases peripheral blood B cells and lymphoid tissue marginal zone and follicular B cells in cynomolgus monkeys. Am J Pathol. 2006;168:476-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, Goenka R, Miller JP, Cho YH, Long V. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci USA. 2008;105:15517-15522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Zekavat G, Rostami SY, Badkerhanian A, Parsons RF, Koeberlein B, Yu M, Ward CD, Migone TS, Yu L, Eisenbarth GS. In vivo BLyS/BAFF neutralization ameliorates islet-directed autoimmunity in nonobese diabetic mice. J Immunol. 2008;181:8133-8144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Ye Q, Wang L, Wells AD, Tao R, Han R, Davidson A, Scott ML, Hancock WW. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol. 2004;34:2750-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Parsons RF, Yu M, Vivek K, Zekavat G, Rostami SY, Ziaie AS, Luo Y, Koeberlein B, Redfield RR, Ward CD. Murine islet allograft tolerance upon blockade of the B-lymphocyte stimulator, BLyS/BAFF. Transplantation. 2012;93:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, Singer NG. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14:R33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918-3930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1235] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 28. | Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 689] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
| 29. | Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, Stegall MD. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | Walsh RC, Alloway RR, Girnita AL, Woodle ES. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int. 2012;81:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 32. | Vitale G, Mion F, Pucillo C. Regulatory B cells: evidence, developmental origin and population diversity. Mol Immunol. 2010;48:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 946] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 34. | Morris A, Möller G. Regulation of cellular antibody synthesis effect of adoptively transferred antibody-producing spleen cells on cellular antibody synthesis. J Immunol. 1968;101:439-445. [PubMed] |
| 35. | Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251:550-551. [PubMed] |
| 36. | Katz SI, Parker D, Sommer G, Turk JL. Suppressor cells in normal immunisation as a basic homeostatic phenomenon. Nature. 1974;248:612-614. [PubMed] |
| 37. | Wolf SD, Dittel BN, Hardardottir F, Janeway CA. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271-2278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 533] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 38. | Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 640] [Cited by in RCA: 680] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 39. | Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol. 2007;179:7225-7232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997;186:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 273] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, Heise MT, Tedder TF. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol. 2013;190:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Siewe B, Stapleton JT, Martinson J, Keshavarzian A, Kazmi N, Demarais PM, French AL, Landay A. Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8+ T cell function in vitro. J Leukoc Biol. 2013;93:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121:4268-4280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci USA. 1995;92:9560-9564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 330] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Niimi M, Pearson TC, Larsen CP, Alexander DZ, Hollenbaugh D, Aruffo A, Linsley PS, Thomas E, Campbell K, Fanslow WC. The role of the CD40 pathway in alloantigen-induced hyporesponsiveness in vivo. J Immunol. 1998;161:5331-5337. [PubMed] |
| 47. | Yan Y, van der Putten K, Bowen DG, Painter DM, Kohar J, Sharland AF, McCaughan GW, Bishop GA. Postoperative administration of donor B cells induces rat kidney allograft acceptance: lack of association with Th2 cytokine expression in long-term accepted grafts. Transplantation. 2002;73:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, Lee MK, Sonawane S, Kim J, Wang J. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178:6028-6032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645-3656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 391] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 50. | Lee KM, Kim JI, Stott R, Soohoo J, O’Connor MR, Yeh H, Zhao G, Eliades P, Fox C, Cheng N. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Chiffoleau E, Bériou G, Dutartre P, Usal C, Soulillou JP, Cuturi MC. Induction of donor-specific allograft tolerance by short-term treatment with LF15-0195 after transplantation. Evidence for a direct effect on T-cell differentiation. Am J Transplant. 2002;2:745-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Chiffoleau E, Bériou G, Dutartre P, Usal C, Soulillou JP, Cuturi MC. Role for thymic and splenic regulatory CD4+ T cells induced by donor dendritic cells in allograft tolerance by LF15-0195 treatment. J Immunol. 2002;168:5058-5069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Le Texier L, Thebault P, Lavault A, Usal C, Merieau E, Quillard T, Charreau B, Soulillou JP, Cuturi MC, Brouard S. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2011;11:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Pallier A, Hillion S, Danger R, Giral M, Racapé M, Degauque N, Dugast E, Ashton-Chess J, Pettré S, Lozano JJ. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 55. | Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 572] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 56. | Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 442] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 57. | Brouard S, Mansfield E, Braud C, Li L, Giral M, Hsieh SC, Baeten D, Zhang M, Ashton-Chess J, Braudeau C. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci USA. 2007;104:15448-15453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 294] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 58. | Chesneau M, Pallier A, Braza F, Lacombe G, Le Gallou S, Baron D, Giral M, Danger R, Guerif P, Aubert-Wastiaux H. Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant. 2014;14:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Rolle L, Memarzadeh Tehran M, Morell-García A, Raeva Y, Schumacher A, Hartig R, Costa SD, Jensen F, Zenclussen AC. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol. 2013;70:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 60. | Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, Smith KG. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 61. | Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868-7878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 62. | Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459-7472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 63. | Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 605] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 64. | Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr Opin Immunol. 2014;28:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 65. | Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1272] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 66. | Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 907] [Cited by in RCA: 893] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 67. | Lin W, Cerny D, Chua E, Duan K, Yi JT, Shadan NB, Lum J, Maho-Vaillant M, Zolezzi F, Wong SC. Human regulatory B cells combine phenotypic and genetic hallmarks with a distinct differentiation fate. J Immunol. 2014;193:2258-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N, Mielke S, de Lavallade H, Muftuoglu M, Fernandez Curbelo I. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124:2034-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 69. | O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 405] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 70. | Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15 Suppl 1:S1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 71. | Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1030] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 72. | Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, Cao X, Lu L. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184:3321-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 73. | Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, Cachero TG, Finke D, Beermann F, Tschopp J. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 74. | Montandon R, Korniotis S, Layseca-Espinosa E, Gras C, Mégret J, Ezine S, Dy M, Zavala F. Innate pro-B-cell progenitors protect against type 1 diabetes by regulating autoimmune effector T cells. Proc Natl Acad Sci USA. 2013;110:E2199-E2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092-6099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 515] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 76. | Lemoine S, Morva A, Youinou P, Jamin C. Human T cells induce their own regulation through activation of B cells. J Autoimmun. 2011;36:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Herzenberg LA. B-1 cells: the lineage question revisited. Immunol Rev. 2000;175:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 173] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 520] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 79. | Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 80. | Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 749] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 81. | Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1294] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 82. | Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789-4800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 83. | DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, Matsushita T, Matta KM, Chen Y, Venturi GM. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 2013;27:170-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 84. | Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 847] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 85. | Ries S, Hilgenberg E, Lampropoulou V, Shen P, Dang VD, Wilantri S, Sakwa I, Fillatreau S. B-type suppression: a role played by “regulatory B cells” or “regulatory plasma cells”? Eur J Immunol. 2014;44:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 87. | Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, Larijani M, Gosselin J, Ivanov II. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2012;481:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 88. | Kleffel S, Vergani A, Tezza S, Ben Nasr M, Niewczas MA, Wong S, Bassi R, D’Addio F, Schatton T, Abdi R. Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes. 2015;64:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 89. | Jahrsdörfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, Taylor CM, Weiner GJ. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108:2712-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 90. | Hagn M, Schwesinger E, Ebel V, Sontheimer K, Maier J, Beyer T, Syrovets T, Laumonnier Y, Fabricius D, Simmet T. Human B cells secrete granzyme B when recognizing viral antigens in the context of the acute phase cytokine IL-21. J Immunol. 2009;183:1838-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Hagn M, Ebel V, Sontheimer K, Schwesinger E, Lunov O, Beyer T, Fabricius D, Barth TF, Viardot A, Stilgenbauer S. CD5+ B cells from individuals with systemic lupus erythematosus express granzyme B. Eur J Immunol. 2010;40:2060-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Hagn M, Belz GT, Kallies A, Sutton VR, Thia KY, Tarlinton DM, Hawkins ED, Trapani JA. Activated mouse B cells lack expression of granzyme B. J Immunol. 2012;188:3886-3892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Kaltenmeier C, Gawanbacht A, Beyer T, Lindner S, Trzaska T, van der Merwe JA, Härter G, Grüner B, Fabricius D, Lotfi R. CD4+ T cell-derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. J Immunol. 2015;194:3768-3777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Chesneau M, Michel L, Dugast E3 Chenouard A, Baron D, Pallier A, Durand J, Braza F, Guerif P, Laplaud DA, Soulillou JP. Tolerant Kidney Transplant Patients Produce B Cells with Regulatory Properties. J Am Soc Nephrol. 2015;2:pii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 95. | Cupi ML, Sarra M, Marafini I, Monteleone I, Franzè E, Ortenzi A, Colantoni A, Sica G, Sileri P, Rosado MM. Plasma cells in the mucosa of patients with inflammatory bowel disease produce granzyme B and possess cytotoxic activities. J Immunol. 2014;192:6083-6091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 96. | Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, Beyer T, Reister F, Fabricius D, Lotfi R. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013;73:2468-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 97. | Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornard T, MacDonald HR, Tschopp J. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 98. | Lundy SK, Fox DA. Reduced Fas ligand-expressing splenic CD5+ B lymphocytes in severe collagen-induced arthritis. Arthritis Res Ther. 2009;11:R128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Lundy SK. Killer B lymphocytes: the evidence and the potential. Inflamm Res. 2009;58:345-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Villunger A, Egle A, Marschitz I, Kos M, Böck G, Ludwig H, Geley S, Kofler R, Greil R. Constitutive expression of Fas (Apo-1/CD95) ligand on multiple myeloma cells: a potential mechanism of tumor-induced suppression of immune surveillance. Blood. 1997;90:12-20. [PubMed] |
| 101. | Tanner JE, Alfieri C. Epstein-Barr virus induces Fas (CD95) in T cells and Fas ligand in B cells leading to T-cell apoptosis. Blood. 1999;94:3439-3447. [PubMed] |
| 102. | Samuelsson A, Sönnerborg A, Heuts N, Cöster J, Chiodi F. Progressive B cell apoptosis and expression of Fas ligand during human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 1997;13:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Rich RF, Cook WJ, Green WR. Spontaneous in vivo retrovirus-infected T and B cells, but not dendritic cells, mediate antigen-specific Fas ligand/Fas-dependent apoptosis of anti-retroviral CTL. Virology. 2006;346:287-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 320] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 105. | Bonardelle D, Benihoud K, Kiger N, Bobé P. B lymphocytes mediate Fas-dependent cytotoxicity in MRL/lpr mice. J Leukoc Biol. 2005;78:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 106. | Minagawa R, Okano S, Tomita Y, Kishihara K, Yamada H, Nomoto K, Shimada M, Maehara Y, Sugimachi K, Yoshikai Y. The critical role of Fas-Fas ligand interaction in donor-specific transfusion-induced tolerance to H-Y antigen. Transplantation. 2004;78:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Mariani SM, Krammer PH. Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur J Immunol. 1998;28:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 108. | Mariani SM, Krammer PH. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur J Immunol. 1998;28:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 109. | Secchiero P, Tiribelli M, Barbarotto E, Celeghini C, Michelutti A, Masolini P, Fanin R, Zauli G. Aberrant expression of TRAIL in B chronic lymphocytic leukemia (B-CLL) cells. J Cell Physiol. 2005;205:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 4263] [Article Influence: 236.8] [Reference Citation Analysis (0)] |
| 111. | Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 764] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 112. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2292] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 113. | Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy. 2011;66:155-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 114. | Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 115. | Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 476] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 116. | Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1948] [Cited by in RCA: 2329] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 117. | Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 935] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 118. | Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1413] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 119. | Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571-19578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 437] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 120. | Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 509] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 121. | Wang J, Balog CI, Stavenhagen K, Koeleman CA, Scherer HU, Selman MH, Deelder AM, Huizinga TW, Toes RE, Wuhrer M. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol Cell Proteomics. 2011;10:M110.004655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 122. | Ashour HM, Niederkorn JY. Peripheral tolerance via the anterior chamber of the eye: role of B cells in MHC class I and II antigen presentation. J Immunol. 2006;176:5950-5957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569-5579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 124. | Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114-1124.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 125. | Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam KT, Lewis DB, Lau YL, Tu W. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J Immunol. 2009;183:3742-3750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Zheng J, Liu Y, Lau YL, Tu W. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4(+) regulatory T cells. Cell Mol Immunol. 2010;7:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J Immunol. 2004;172:5143-5148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 128. | Bach FH, Hancock WW, Ferran C. Protective genes expressed in endothelial cells: a regulatory response to injury. Immunol Today. 1997;18:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 129. | Bach FH, Ferran C, Hechenleitner P, Mark W, Koyamada N, Miyatake T, Winkler H, Badrichani A, Candinas D, Hancock WW. Accommodation of vascularized xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 338] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 130. | Heslan JM, Renaudin K, Thebault P, Josien R, Cuturi MC, Chiffoleau E. New evidence for a role of allograft accommodation in long-term tolerance. Transplantation. 2006;82:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 132. | Soares MP, Lin Y, Sato K, Stuhlmeier KM, Bach FH. Accommodation. Immunol Today. 1999;20:434-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 133. | Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 510] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 134. | Soares MP, Brouard S, Smith RN, Bach FH. Heme oxygenase-1, a protective gene that prevents the rejection of transplanted organs. Immunol Rev. 2001;184:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 135. | van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, Rückert B, Akdis CA, Akdis M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 502] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 136. | Meiler F, Zumkehr J, Klunker S, Rückert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887-2898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 367] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 137. | Shabir S, Girdlestone J, Briggs D, Kaul B, Smith H, Daga S, Chand S, Jham S, Navarrete C, Harper L. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant. 2015;15:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 138. | Nouël A, Ségalen I, Jamin C, Doucet L, Caillard S, Renaudineau Y, Pers JO, Le Meur Y, Hillion S. B cells display an abnormal distribution and an impaired suppressive function in patients with chronic antibody-mediated rejection. Kidney Int. 2014;85:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 139. | Parsons RF, Vivek K, Rostami SY, Zekavat G, Ziaie SM, Luo Y, Koeberlein B, Redfield RR, Cancro MP, Naji A. Acquisition of humoral transplantation tolerance upon de novo emergence of B lymphocytes. J Immunol. 2011;186:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Heidt S, Hester J, Shankar S, Friend PJ, Wood KJ. B cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naive B cells. Am J Transplant. 2012;12:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 141. | Cherukuri A, Salama AD, Carter C, Smalle N, McCurtin R, Hewitt EW, Hernandez-Fuentes M, Clark B, Baker RJ. An analysis of lymphocyte phenotype after steroid avoidance with either alemtuzumab or basiliximab induction in renal transplantation. Am J Transplant. 2012;12:919-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 142. | Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, Green T, Rickels MR, Tomaszewski JE, Koeberlein B. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 143. | de Masson A, Bouaziz JD, Le Buanec H, Robin M, O’Meara A, Parquet N, Rybojad M, Hau E, Monfort JB, Branchtein M. CD24(hi)CD27+ and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood. 2015;125:1830-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 144. | Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. Induction of IL-10-producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS One. 2012;7:e46553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fiorina P S- Editor: Qiu S
L- Editor: A E- Editor: Li D