INTRODUCTION
Liver transplantation (LT) was historically associated with massive blood loss. Over the last two decades mean transfusion requirements have dramatically reduced. It is increasingly common for patients to undergo the procedure without transfusion of allogeneic blood[1]. Nevertheless, a significant proportion of patients (10%-20%) will still require large volume transfusion of red blood cells (RBC) and blood products[2]. The understanding of the causes of and the consequences of bleeding in these patients continues to evolve, and has contributed to the steady fall in transfusion requirements. However, substantial variability in transfusion practice remains[3]. Patients undergoing LT represent a heterogenous group, yet such variation also suggests a lack of clear guidance and consensus regarding transfusion practice. Evidence for the negative impact of transfusion upon outcomes has driven the reduction in transfusion, alongside refinement of surgical and anaesthetic techniques, and use of point of care coagulation monitoring with goal directed haemostatic interventions. In this review we aim to provide an overview of the current understanding of the causes and predictors of excess bleeding, and methods available to minimise transfusion requirements in patients undergoing LT.
HISTORICAL CONTEXT AND PROGRESS
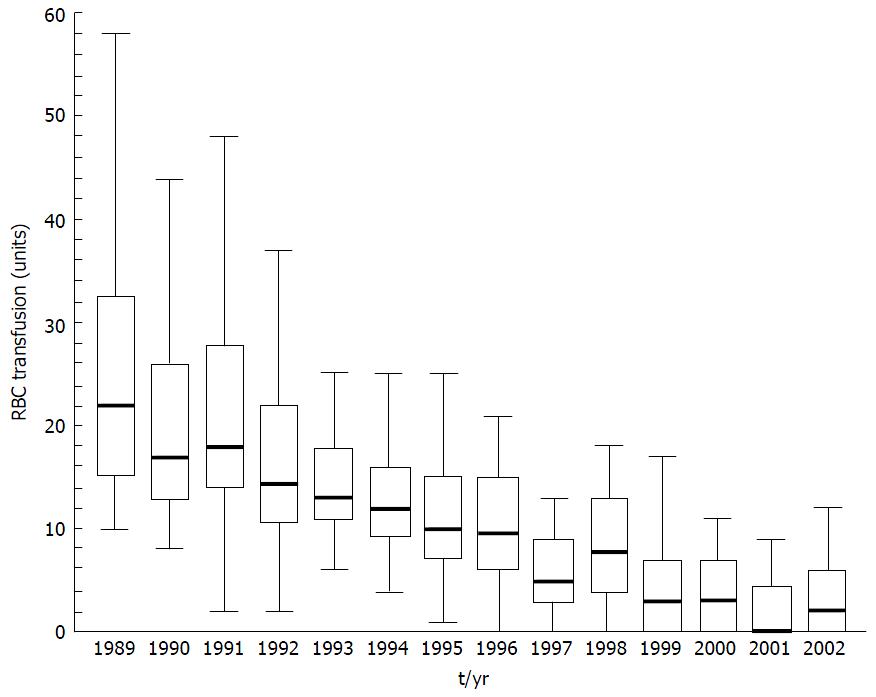
The early years of transplantation in the 1980’s were associated with high perioperative mortality, often related to bleeding complications[4]. Massive transfusion was commonplace with typical mean RBC transfusions of 20 units per patient[2] and blood products accounting for up to 10% of transplant costs[5] (Figure 1).
Figure 1 Red blood cell transfusion requirement in patients undergoing primary liver transplantation at the University Medical Centre, Groningen from 1989 to 2002.
Data presented as box and whisker plots representing median, interquartile range and 5%-95% range. Both variation in transfusion and median number of RBC transfused has declined over time. From Porte et al[6] 2004. RBC: Red blood cell.
Blood conservation, transfusion requirement, patient outcomes and survival have all improved in the subsequent years with evolving experience, techniques and therapeutic options. In some centres, transfusion free transplants are now a common occurrence[7]. Massicotte et al[8,9] reported a mean RBC transfusion per patient of 0.5 (± 1.4) units and a 77.4% transfusion-free transplantion rate in over 700 LTs.
WHY IS REDUCING TRANSFUSION OF ALLOGENIC BLOOD AND PRODUCTS DESIRABLE?
Drivers for transfusion-free transplantation
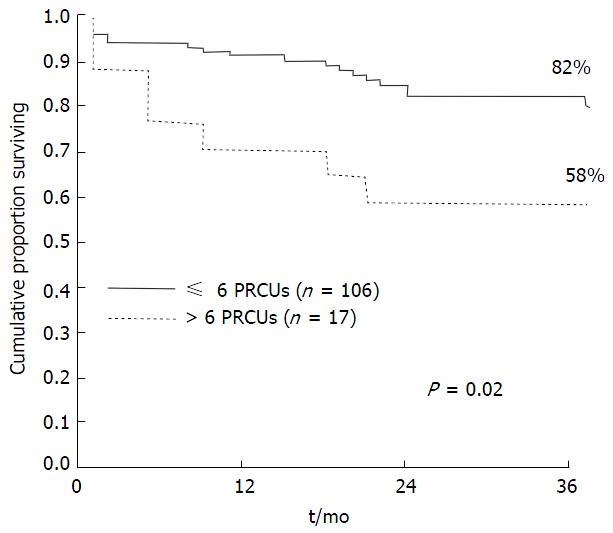
The negative implications of transfusion are increasingly recognized despite improvements in donor screening, leucocyte and pathogen depletion[10]. Adverse outcomes include immediate and delayed immune and non-immune reactions. A growing body of evidence suggests that allogenic blood and products are associated with excess short and longer-term morbidity (reduced graft function, infection, renal injury, re-operation) and mortality in LT[11,12]. It is difficult to exclude the possibility that RBC transfusion might be a surrogate marker for other underlying causes of poor outcome. Ramos et al[13] noted that RBC transfusion was the most significant factor adversely affecting length of stay (LOS) and survival (Figure 2). de Boer et al[14] also found that patient survival was significantly associated with number of RBCs transfused. Transfusion remained the strongest independent predictor of survival when disease severity was excluded as a potential confounder. A transfusion-free perioperative period was associated with improved early outcomes, fewer infections, reduced dialysis requirement, shorter hospital LOS and a reduction in mortality compared with a transfused group with similar recipient, graft and donor quality variables[15]. Benson et al[16] reported a significant dose dependent association between transfusion of RBC, fresh frozen plasma (FFP) and platelets and post-operative infections. Number of RBC units transfused was predictive for re-operation post LT in one centre[17], an event associated with high financial burden and excess mortality[18].
Figure 2 Kaplan meier survival curve demonstrating reduced survival in patients who received > 6 units red blood cell up to 36 mo post liver transplantation.
From Ramos et al[13]. PRCUs: Packed red cell units.
Mechanisms for poor outcomes and increased post-operative infection associated with RBC transfusion are not fully understood. One theory is transfusion-related immunomodulation (TRIM). A retrospective analysis by Boyd et al[19] noted reduced survival post LT in patients bearing anti-RBC alloantibodies (suggestive of previous transfusion). These findings raise the possibility that transfusion may alter the immune system and impact negatively on susceptibility to infection and survival.
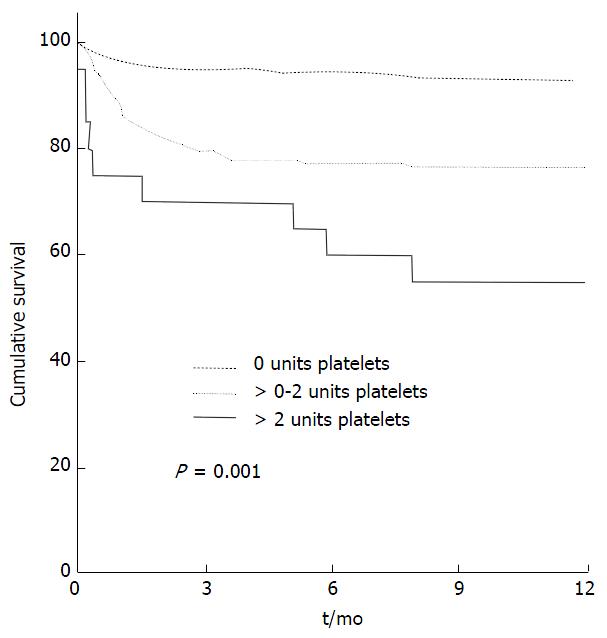
Platelet transfusions have been identified as an independent predictor of adverse outcomes post LT, in keeping with findings in cardiac surgery[20]. de Boer et al[14] demonstrated that even a 1 unit platelet transfusion was an important prognostic factor for post LT survival with a hazard of death ratio (HR) 1.37/unit platelets (Figure 3).
Figure 3 Kaplan Meier curve representing cumulative survival with transfusion of no, 0-2 units and > 2 units of platelets.
From de Boer et al[14] 2008.
Pereboom et al[21] attributed the reduced survival in patients who received platelets to the significantly higher rate of transfusion related acute lung injury (TRALI) and associated early mortality. The biologically active mediators in plasma rich products (for example FFP and particularly platelets) are thought to underlie the risk of TRALI. Interestingly the incidence of TRALI appears to be lower in patients undergoing LT (1.3%) compared with other critically ill patients with liver disease (29.3%), possibly due to modulation of the inflammatory response by the high dose steroids administered intra-operatively or effects of the grafted liver itself[16]. The development of TRALI post LT carries a poor prognosis with a tenfold increase in mortality. Platelets have been implicated in worsening ischaemia-reperfusion injury, which accounts for 10% of early graft dysfunction and predisposes to acute and chronic rejection. The suggested mechanism is via platelet sequestration in the hepatic microvasculature, where up regulation of proinflammatory endothelial injury and necrotic apoptosis could be exacerbated by platelet transfusion[22]. The vasoactive effects of platelets may cause other negative systemic consequences such as pulmonary hypertension and haemodynamic disturbance. In one study platelet infusion was associated with reduced re-operation rates, a reminder that balancing risks of bleeding and side effects is a real clinical challenge[18,23].
While it is difficult to isolate the detrimental effects of platelet transfusion from the association with sicker thrombocytopenic patients and higher intraoperative blood loss, Pereboom et al[21] attempted to minimize confounders using a propensity score adjusted analysis. An independent negative effect of platelets on survival was suggested. Patients with low preoperative platelet counts and high intraoperative blood loss who did not receive platelets had improved survival compared with those that did, and in fact had similar survival to a reference population of patients with a normal preoperative platelet count and minimal blood loss[21]. The decision to transfuse platelets should be therapeutic rather than prophylactic to avoid excess morbidity when the actual bleeding risk is unknown[24].
FFP used for volume replacement or pre-emptive non-specific correction of coagulopathy in the dissection phase of LT may exacerbate splanchnic hyperaemia and portal hypertension[25]. Bleeding, increased RBC requirement and a cycle of dilutional coagulopathy may ensue[26]. In a retrospective analysis of 206 LTs, transfusion of plasma was associated with an increase in mortality at one year. The negative impact of FFP on survival was greater than that associated with RBC transfusion[27].
The current evidence suggests transfusion of blood or blood products conveys negative consequences yet it remains a life saving intervention in certain clinical situations. Without clear evidence-based triggers for transfusion in LT however, the risk-benefit decision-making process remains subjective and results in variation in clinical practice.
PATIENT BLOOD MANAGEMENT
Concerns over patient safety and cost have fuelled interest in improving bleeding and transfusion management. Research into bloodless and transfusion free surgery in Jehovah’s Witness patients has pushed the boundaries of best practice for general application[28]. Patient blood management (PBM) refers to the implementation of evidence-based practices to minimize transfusion of blood and products and improve patient outcomes. PBM principles are based on three pillars: Recognition of anaemia and optimization of RBC mass, minimization of blood loss, and improved tolerance to anaemia with implementation of restrictive transfusion triggers and alternatives. These principles have been endorsed by the World Health Organisation and are now considered standards of care[29]. The principles should be rigorously applied to patients awaiting and undergoing LT in order to reduce and rationalise unnecessary transfusion and improve outcomes[30].
Preoperative anaemia is a major predictor for perioperative blood transfusion and poor outcome[9,31]. Preoperative management with erythropoiesis stimulating agents and intravenous iron has been shown to improve haemoglobin levels in anaemic patients with absolute or functional iron deficiency[32,33]. Further research is needed to define the role of intravenous iron in LT. Surgical and anaesthetic strategies to minimize haemoglobin drop (including autologous transfusion) should be implemented and will be discussed further in this article. Clear transfusion triggers reflecting the emerging evidence for the safety and benefits of restrictive policies, even in high-risk patients, should be applied[34]. In a transplant centre with exceptionally low transfusion rates, a trigger Hb of 60 g/L has been used successfully[35]. The principles of PBM should continue into the postoperative period.
WHY DO PATIENTS UNDERGOING LT BLEED AND HOW CAN BLEEDING RISK BE MITIGATED?
Underlying coagulation incompetence
Patients with acute and chronic end stage liver disease are a heterogenous group with complex alterations of coagulation. Certain aetiologies convey increased thrombotic tendency, such as the cholestatic conditions and non-alcoholic steatohepatitis[36]. Liver disease leads to a fragile state of rebalanced haemostasis affecting multiple cellular and humeral components of clot formation and stability[37]. Production of both pro and anti-haemostatic factors is impaired. Diminished prothrombotic factors V, VII, IX, X, XII, prothrombin (II) and fibrinogen (I) are offset by increased factor VIII activity due to endothelial injury and reduced levels of the anticoagulants antithrombin, protein C, co-factor S and tissue factor pathway inhibitor. Reduced platelet number or function due to bone marrow suppression, the hypersplenism of portal hypertension or uraemia is counteracted by enhanced platelet-endothelial adhesion mediated by von Willebrand factor (vWF). vWF concentrations are increased and breakdown reduced in the context of impaired production of liver derived ADAMTS-13, a vWF-cleaving protease[38]. Thrombin generation is well preserved[39] and may even be enhanced in patients with liver disease compared with healthy controls[40]. Fibrinolytic pathways are altered. Increased tissue plasminogen activator (tPA) and reduced thrombin activateable fibrinolysis inhibitors plasminogen activator inhibitor (PAI) drive lysis whilst reduced plasminogen levels limit clot breakdown. In acute liver failure (ALF) and cholestatic disease, elevated levels of PAI, further reduce fibrinolysis.
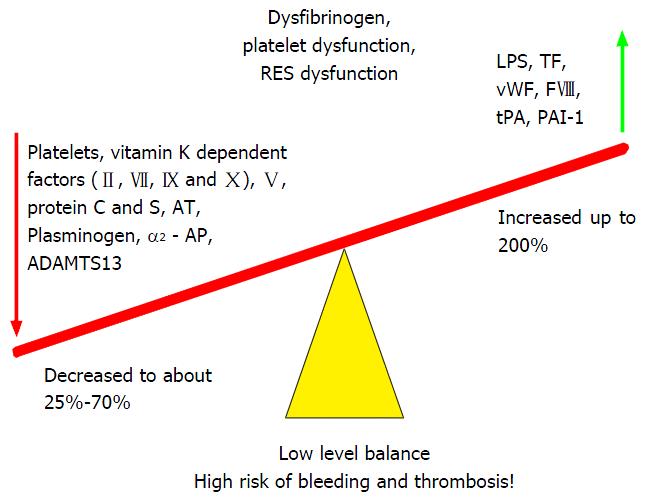
Conventional laboratory tests fail to capture the subtlety and complexity of this rebalanced state and over-emphasise the potential for bleeding. The prothrombin time, international normalized ratio (INR) or platelet count offer information about underlying hepatic synthetic function and clinical status, but provide an incomplete and misleading description of the coagulation profile in vivo and are of limited use in predicting bleeding risk or guiding haemostatic management[41]. Whole blood viscoelastic tests (VETs) assess clot initiation, formation, strength and stability and are more representative of the in vivo process. The rebalanced haemostasis in liver disease is precarious and patients with limited haemostatic reserve can be readily tipped towards either bleeding or thrombotic tendency[42] (Figure 4).
Figure 4 Fragile rebalancing of pro and anti-haemostatic factors with limited reserves leads to increased thrombotic and bleeding tendency.
From Saner et al[43] 2013. TF: Tissue factor; vWF: Von Willebrand Factor; FVIII: Factor VIII; tPA: Tissue plasminogen activator; PAI: Plasminogen activator inhibitor; ADAMTS13: A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; AT: Antithrombin; LPS: Lipopolysaccharide.
IMPACT OF TRANSPLANTATION ON COAGULATION
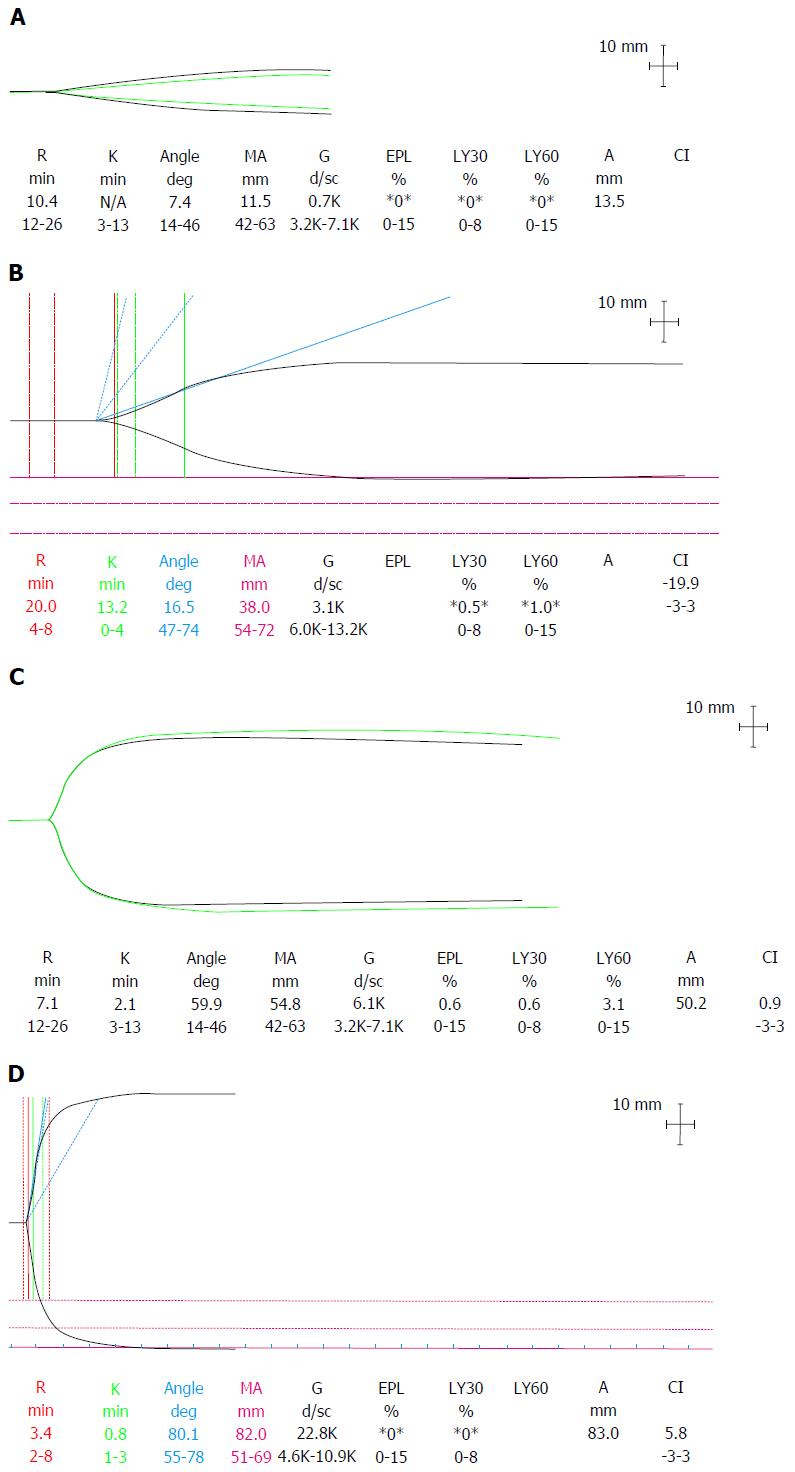
From this complex and variable baseline, the dynamic stresses during transplantation add to the challenge of maintaining optimal haemostasis. Surgical dissection can precipitate acute haemorrhage, particularly in patients with portal hypertension and collateral vessels. Volume resuscitation, especially with colloids, can lead to a dilutional coagulopathy. Clot disturbance due to haemodilution is exaggerated in patients with liver disease due to a diminished haemostatic reserve[44]. Concentrations of prothrombotic factors rapidly reach critical levels and there is a marked impairment in thrombin generation[45]. The loss of thrombin potential can be detected on thromboelastography (TEG) as a characteristic “arrowhead” trace (Figure 5).
Figure 5 Thromboelastography traces depicting.
A: Dilutional coagulopathy with loss of endogenous thrombin generating potential, low fibrinogen and platelets seen as a typical “arrowhead” trace; B: Hypocoagulable state with prolonged R time but retention of thrombin generation; C: Normal coagulation profile; D: Hypercoagulability with a short R time, steep rate of clot formation (α angle) and increased clot MA. R: Reaction time; K: Kinetics time taken to achieve a certain level of clot strength (20 mm); MA: Maximum amplitude; G: Log derivation of the MA; EPL: Estimated percent lysis; Ly30: Lysis at 30 min; Ly60: Lysis at 60 min; CI: Coagulation index.
During the anhepatic period, reduced coagulation factor production including fibrinogen and reduced tPA clearance can lead to a hypocoagulable state with reduced clot quality and hyperfibrinolysis.
At reperfusion several factors contribute to increased bleeding tendency. Platelet entrapment and sinusoidal sequestration in the new liver depletes circulating numbers. Coagulation factors are globally reduced. Release of endogenous heparinoids from the vascular endothelial glycocalyx of the donor liver causes a heparin like effect (HLE) that can be detected on VETs with heparin inhibitors. This may be more pronounced with marginal grafts from DCD donors and with significant hepatic-ischaemic-reperfusion insult[46]. The clinical significance of the HLE is not fully understood but it usually resolves spontaneously by the end of the case in the context of a good functioning graft[47]. Hyperfibrinolysis is common due to an increase in tPA from the new liver and decreased production of antifibrinolytic factors and may be associated with increased bleeding[48].
It has been demonstrated that patients frequently become pro-thrombotic during the perioperative period[49]. This typifies the challenges of haemostatic management during LT since inappropriate correction of a transient coagulopathy could lead to excess thrombotic complications, including hepatic artery thrombosis[50].
THE ROLE OF GRAFT FUNCTION
The quality of the graft liver plays a significant role in bleeding post reperfusion. Delayed or primary non-function of the graft liver can cause the sustained deterioration of coagulation status with a global reduction of coagulation factors and fibrinogen, hyperfibrinolysis and HLE[51]. Predisposing factors for graft failure include: Marginal grafts, poor preservation and prolonged cold and warm ischaemic times. In one retrospective analysis the use of extended donor criteria grafts was an independent risk factor for re-operation for bleeding[52].
SURGICAL BLEEDING AND MANAGEMENT STRATEGIES
Surgical skill and experience play an important role in limiting blood loss but are difficult to quantify. Patient factors including previous abdominal surgery with adhesions, portal hypertension with collateral vessels and portal vein thrombosis all increase technical difficulty, surgical duration and risk of bleeding[53]. During surgery poor placement of retractors can increase venous pressure and exacerbate bleeding.
Different surgical techniques have been introduced and refined with the aim of improving patient outcomes. Veno-venous bypass decompresses the splanchnic system during the anhepatic phase and may reduce venous pressure and bleeding, whilst improving venous return to the heart. However increased blood loss due to hyperfibrinolysis, haemolysis and platelet consumption within the extracorporeal circuit has been reported. A Cochrane review found no evidence for reduced transfusion requirement with use of veno-venous bypass in LT (MD 1.13 units; 95%CI: -0.06 to 2.31; P = 0.062)[54].
In the piggyback technique part of the native retrohepatic inferior vena cava is left in situ, preserving venous return to the heart throughout the anhepatic stage[55]. Extensive retroperitoneal dissection is avoided and the single anastomosis between native and donor cavae is quicker and reduces warm ischaemic time. A favourable cardiovascular status may facilitate anaesthetic strategies to maintain low central venous pressure (CVP) and indirectly contribute to reduced blood loss[56]. There are inconsistent reports of the blood conserving efficacy of the technique[57,58]. A Cochrane review found no difference in transfusion requirement between piggyback and classical groups (SMD -0.09; 95%CI: -0.47 to 0.29; P = 0.65)[59].
Preoperative portal decompression with splenic artery trunk embolization (SATE) is described by Li et al[60]. Portal pressures and hepatic artery flows were improved, as was hepatic functional reserve with more favourable INR, platelet count and plasma albumin a month post SATE. Surgery was shorter with less bleeding.
Live-related LT was associated with significantly lower allogenic blood and product usage compared with cadaveric LT despite the fact that the split graft has a raw surface from which bleeding could occur[61]. The authors attribute this to the better graft quality and clinical status in live related recipients compared to those receiving cadaveric grafts on the waiting list.
PERIOPERATIVE ANAESTHETIC STRATEGIES
The evolution of modern anaesthetic practice means there are more options for monitoring, modifying and minimizing surgical and non-surgical bleeding during the perioperative period[62].
CENTRAL VENOUS PRESSURE REDUCTION AND BLOOD VOLUME MANAGEMENT
Liberal volume loading in cirrhotic patients may have several detrimental consequences. Acute volume loading tends to pool in the splanchnic circulation leading to bleeding and hepatic congestion with minimal improvement in cardiac preload or output[63]. Dilution of clotting factors and interruption of clot formation (particularly with colloid administration) can lead to significant clot disturbance[64,65] in this susceptible patient group[44]. Reduction of CVP and therefore portal pressures with reduced engorgement of collateral vessels can help minimize surgical venous bleeding[66]. Methods to lower CVP include volume restriction, phlebotomy, use of diuretics such as mannitol, low tidal volume ventilation and avoidance of high positive end expiratory pressure (PEEP)[2].
Volume restriction and early use of compensatory vasopressors is common practice in liver resection surgery, although a Cochrane review did not find that this strategy was associated with reduced transfusion (RR = 0.72; 95%CI: 0.45 to 1.14)[67]. Massicotte et al[9,68] reported excellent transfusion rates and outcomes when stable LT patients with preoperative Hb > 85 g/L received protocolised care with a 40% reduction in CVP by phlebotomy according to body weight (mean CVP 6.4 mmHg SD ± 3.9). Volume was not replaced until after reperfusion when the collected blood was returned, thus maintaining a low CVP and higher coagulation factor concentrations.
Normovolaemic haemodilution involves phlebotomy plus volume replacement with an acellular fluid leading to a reduced haematocrit and limited loss of RBCs during subsequent bleeding[69]. Evidence from an animal model of haemodilution indicated that the kidney is at risk of ischaemic insult with this technique[70]. Schroeder et al[71] compared outcomes at two centres with contrasting protocols where CVP was maintained at either < 5 mmHg or 7-10 mmHg. Though transfusions were reduced in the low CVP centre, postoperative renal impairment, dialysis requirement and 30 d mortality were increased.
Any reduction in CVP must be a decision in which the potential benefits outweigh the risks of organ hypoperfusion and renal injury in these physiologically complex patients[72]. Anaesthetic management ultimately seeks to maintain tissue oxygen delivery in the perioperative period, guided only by surrogate markers of this endpoint (e.g., mixed venous saturations and lactate). The ability to directly measure tissue oxygenation could lead to individualized haemodynamic management based upon this ultimate physiological goal.
MAINTENANCE OF HOMEOSTATIC CONDITIONS FOR CLOTTING
Maintenance of core body temperature > 35 °C, pH > 7.2 and plasma calcium levels > 1 mmol/L optimizes conditions for clot formation[42]. Acidosis reduces thrombin generation and increases clot lysis, while hypothermia reduces fibrin and clotting factor synthesis and impairs platelet function. Active patient warming with forced air blankets, heated mattresses and efficient fluid delivery systems with the ability to heat rapid infusions to > 39 °C should be standard practice.
VISCOELASTIC TESTS FOR MONITORING AND GUIDING COAGULATION MANAGEMENT
Laboratory assays offer limited and potentially misleading information in the context of liver disease. They have long turnaround times for results (30-90 min). VETs rapidly describe the cellular and humeral contribution to clot initiation, formation, strength and stability, enabling near patient thera-nostics. Though VETs have been around since the 1940’s there has been a recent explosion of interest in their utility. The European Society of Anaesthesiology (ESA) guidelines recommend VETs in the management of severe bleeding in liver transplant (grade 1C evidence)[26].
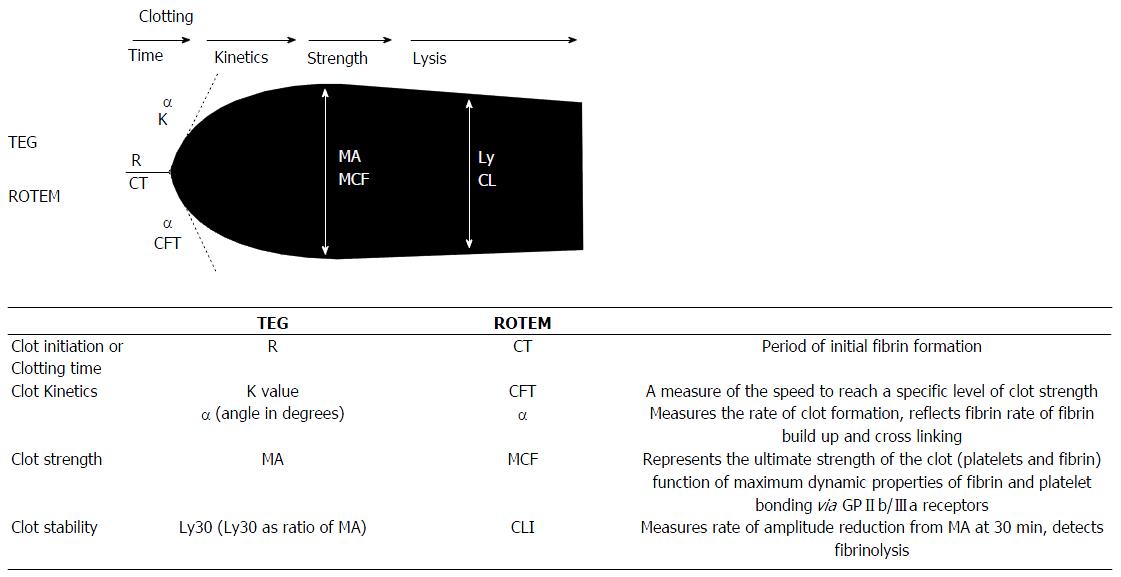
Two main devices exist on the market: TEG and rotational thromboelastometry (ROTEM). These are based on similar principles measuring change in resistance to free rotation of a pin and cup as clotting occurs over time. Use of different reagents and activators results in measurements of clot dynamics that are comparable but not interchangeable, between devices[73] (Figure 6).
Figure 6 Schematic of thromboelastography/rotational thromboelastometry parameters.
TEG: Thromboelastography; K time: Coagulation time (20-mm clot); Ly30: Lysis at 30 min; MA: Maximum amplitude; R time: Reaction time (2-mm clot); ROTEM: Rotational thromboelastometry; CFT: Clot formation time (20-mm clot); CLI: Clot lysis index; CT: Clotting time (2-mm clot); MCF: Maximum clot firmness; ML: Maximal lysis. From Mallett[37] 2015.
Empirical management of coagulation leads to excessive administration of allogenic products. VETs can improve perioperative care in LT through rapid diagnosis of coagulation defects, enabling individualised goal directed therapy and reducing unrationalised product usage. Both TEG and ROTEM employ activators and reagents to broaden their diagnostic scope[74]. Results can be obtained more quickly by adding kaolin (K-TEG) or thromboplastin/ellagic acid (INTEM-ROTEM) to activate the intrinsic pathway or tissue factor (Rapid TEG and EXTEM-ROTEM) to activate the extrinsic pathway[75].
Measurement of TEG functional fibrinogen (TEG FF) or FIBTEM-ROTEM to quantify the fibrin contribution to clot strength can guide fibrinogen replacement. Without this additional information there may be a tendency to treat a low maximum amplitude (MA) or maximum clot firmness (MCF) due to hypofibrinogenaemia inappropriately with platelets. Normal clot strength should negate need for fibrinogen or platelet supplementation since a MA/MCF greater than 40 mm has a high negative predictive accuracy for bleeding (> 95%). The decision to supplement fibrinogen or platelets should not solely be based on low MA/MCF as the positive predictive value is low (< 50%) and would risk overtreatment[76].
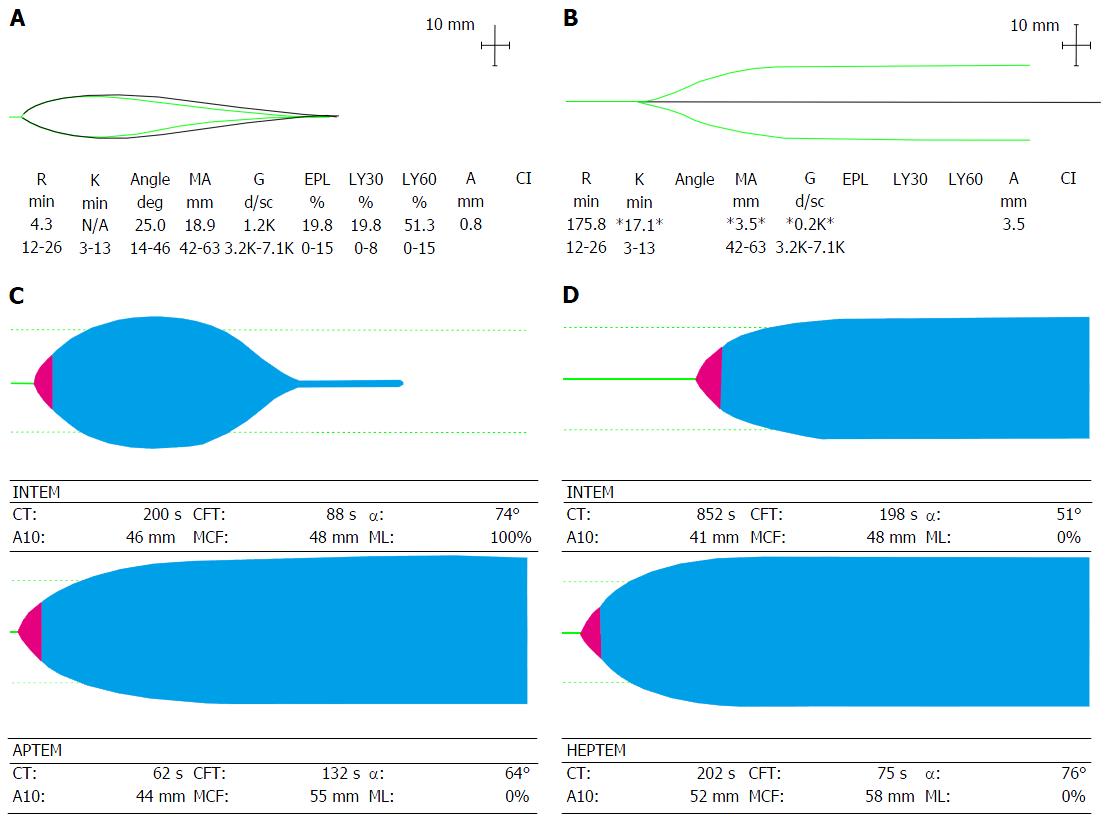
Hyperfibrinolysis can be detected by a reduction in MA/MCF of greater than 15% in an hour (TEG Ly30 7.5% or Ly60 15% and ROTEM Clot lysis index CLI60 < 85%). Platelet induced clot retraction is seen as a modest reduction in MA/MCF, which could be misinterpreted as fibrinolysis. An additional test, the APTEM-ROTEM, involves adding the antifibrinolyitic aprotinin to whole blood in order to inhibit fibrinolysis. Comparison with the uninhibited curve enables early detection of accelerated clot breakdown (Figure 7A and C).
Figure 7 Viscoelastic traces depicting hyperfibrinolysis (A) on thromboelastography and (C) on rotational thromboelastometry with aprotinin thromboelastometry test (reversal of fibrinolysis with aprotinin); Viscoelastic traces depicting heparin-like effect (B) on thromboelastography and (D) on Rotational thromboelastometry with reversal of heparin-like effect on heparinase modified thromboelastometry.
APTEM: Aprotinin thromboelastometry; HEPTEM: Heparinase modified thromboelastometry; INTEM: Intrinsic thromboelastometry; CT: Clotting time; CFT: Clot formation time; A10: Clot firmness (amplitude) at 10 min; MCF: Maximum clot firmness; ML: Maximum lysis.
Use of a heparin inhibitor (TEG heparinase and HEPTEM-ROTEM) enables demonstration of an endogenous HLE common in ALF and transiently post reperfusion (Figure 7B and D).
Several studies have demonstrated the close correlation and predictive value of clot firmness at 5 min (A5) with maximum mature clot strength[77], reinforcing VETs usefulness in real-time coagulation management[78].
Görlinger[79] developed and implemented a ROTEM based point of care (POC) algorithm for LT to guide response to clearly defined abnormal ROTEM parameters. A POC algorithm with first line use of factor concentrates applied to a variety of clinical contexts (trauma, cardiac, transplant and critical care) in three major teaching hospitals in Germany and Austria was associated with an impressive 90% reduction in FFP use, 72% reduction in platelet use, 62% reduction in RBC use and 50% reduction in incidence of massive transfusion and increased use of factor concentrates[80]. It must be remembered that comparisons were made with a historical cohort introducing many confounding factors. Small single centre observational and randomised studies have demonstrated reductions in RBC and FFP usage with VET guided haemostasis, with varying significance[81,82], and improved postoperative outcomes with reduced rates of reoperation and kidney injury[83].
TEG was one of only three interventions found on Cochrane review to have potential for RBC and FFP transfusion reduction in LT (SMD -0.73; 95%CI: -1.25 to -0.2 and SMD -0.82; 95%CI: -1.6 to -0.05 respectively)[84].
LIMITATIONS OF VET
Large robust randomized controlled trials are lacking and as yet there is no evidence that use of VETs has a positive impact on morbidity or mortality[85]. Introduction of VET requires infrastructure to establish and maintain quality controls and training. The moderate complexity of performing and interpreting the tests can lead to inter-operator variability and limits widespread use. It should be acknowledged that VETs do not incorporate the endothelial, vascular or flow related contribution to clot formation in vivo. Nor do they readily detect platelet inhibition, which require specialized tests (TEG platelet mapping or impedance aggregometry)[86]. New generation devices with cartridge technology will address some of these issues by simplifying testing and incorporating platelet inhibition assays.
PHARMACOLOGICAL STRATEGIES
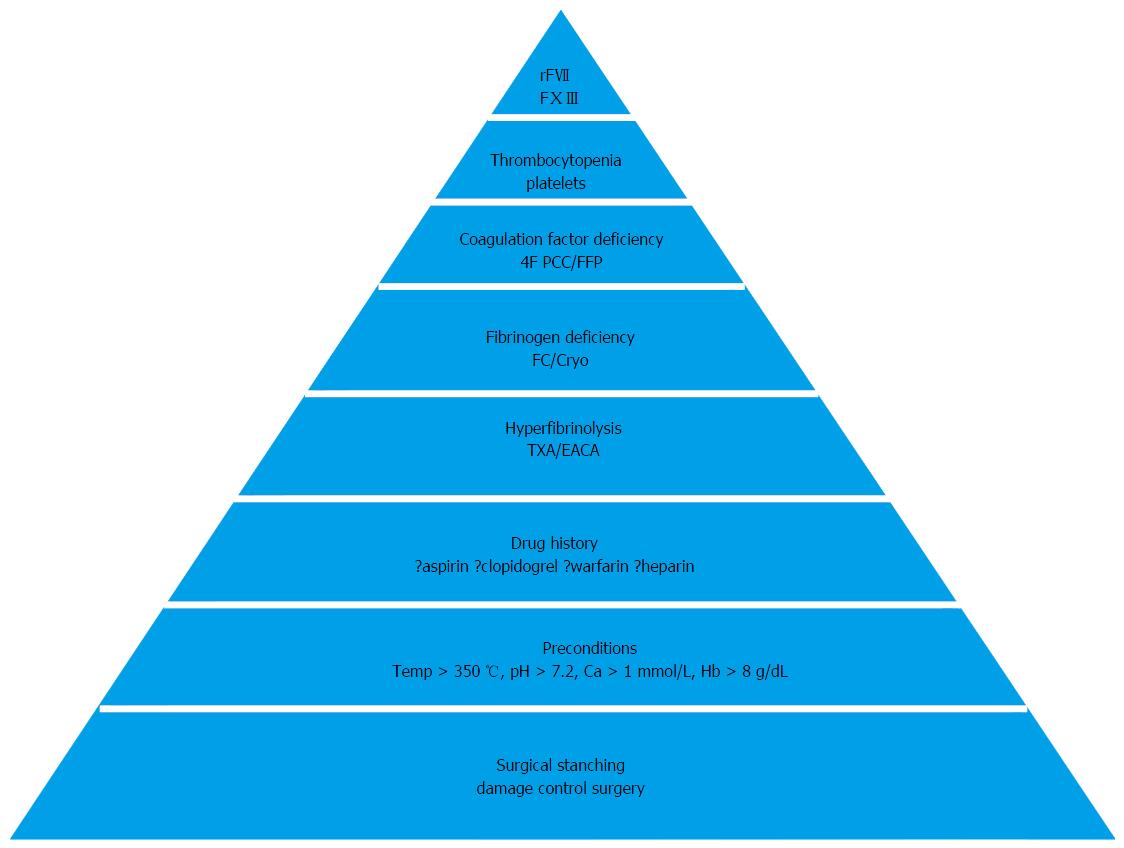
POC results enable a timely and accurate pharmacological response. A number of drugs and concentrates have been assessed for their beneficial haemostatic potential in LT and can be administered according to a “pyramid of therapy” (Figure 8).
Figure 8 Pyramid of therapy in coagulopathy with sequence of hemostatic therapeutic interventions (from base to tip).
Ca: Calcium; Hb: Haemoglobin; TXA: Tranexamic acid; EACA: Epsilon aminocaproic acid; FC: Fibrinogen concentrate; Cryo: Cryoprecipitate; rFVII: Recombinant activated factor VII; FXIII: Factor XIII. From Görlinger et al[79] 2006.
ANTIFIBRINOLYTICS
Hyperfibrinolysis can contribute significantly to non-surgical bleeding during transplantion. Antifibrinolytic drugs can target such bleeding and reduce transfusion requirement. Empirical prophylactic use of antifibrinolytics is no longer recommended in LT because the balance of risk has shifted with declining massive haemorrhage[26]. The significance of detected fibrinolysis depends on clinical context and timing. Fibrinolysis is relatively common in the initial post reperfusion stages and may not require treatment in the absence of clinical clot lysis and in the presence of a good quality graft where spontaneous resolution is expected. A retrospective review of practice in a single centre found that only 60% of patients with TEG evidence of fibrinolysis received antifibrinolytics[87] and similar practice has been reported elsewhere[79]. Significant pre-reperfusion fibrinolysis is concerning and may warrant treatment in anticipation of further deterioration of clot stability.
Aprotinin is a serine protease inhibitor inhibiting plasmin and kallikrein. It was used widely to reduce hyperfibrinolysis and bleeding in cardiac patients on cardiopulmonary bypass and in high-risk liver transplant patients. It had been accredited with reducing LT transfusion requirements dramatically[88,89]. Aprotinin was withdrawn from the market amid concerns over excess mortality in 2007 but it is available once more, following a revoked suspension in 2012 by the European Medicines Agency[90,91]. A meta-analysis of 7 studies in LT demonstrated significantly lower RBC and FFP requirements patients who received aprotinin compared with controls but no differences in terms of reoperation rates, thrombotic events or mortality[92].
Tranexamic acid and epsilon aminocaproic acid (EACA) are lysine analogues that adhere to lysine binding sites on plasminogen preventing its conversion to plasmin. Tranexamic acid has superior efficacy compared with EACA[93]. Use of tranexamic acid at a dose of 25 mg/kg is recommended in the ESA guidelines for the treatment of surgical ooze with viscoelastic evidence of fibrinolysis during LT (grade 1C evidence)[26].
There is variation in the literature regarding the relative efficacy of aprotinin and tranexamic acid. Some authors found equivalent blood conservation, transfusion requirements and outcomes in cohorts receiving aprotinin and tranexamic acid[35,87] whilst in two Cochrane reviews aprotinin appeared to have superior efficacy[84], but with an increased risk of death[94].
FIBRINOGEN CONCENTRATES
Fibrinogen is the first factor to reach critically low levels in the context of bleeding or dilution. Hypofibrinogenaemia is an important early occurrence which compromises clot quality and haemostasis.
Several studies in a variety of clinical settings have demonstrated reduced blood loss, transfusion requirements[95,96] and increased transfusion-free transplantation[97] with use of fibrinogen concentrate (FC). A Cochrane review reported efficacy of FC without increased thromboembolic risk[98], though a lack of large robust trials was noted. Fibrinogen is the vital substrate of the clot and supplementation may be beneficial in the context of dilutional coagulopathy and platelet impairment[44,99]. In both in vitro and in vitro studies of dilutional coagulopathy, FC improved clot strength but did not increase thrombin generation[100,101]. Adequate fibrinogen levels may compensate for a degree of platelet dysfunction and FC may represent a platelet concentrate sparing therapy (Figure 9)[99,102].
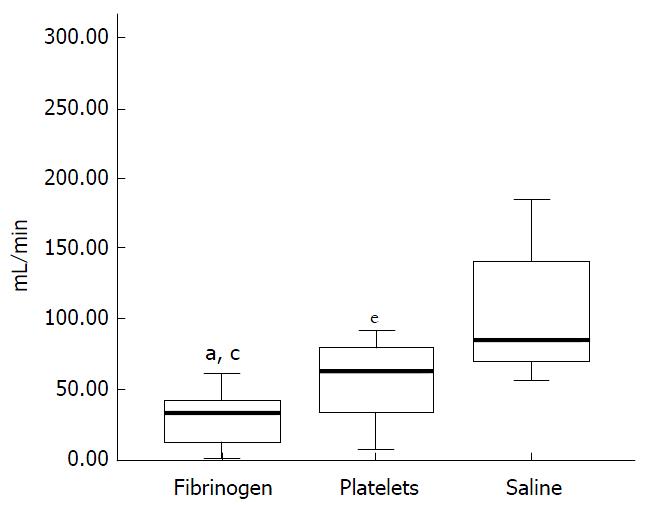
Figure 9 Rate of blood loss (mL/min) in thrombocytopenic pigs with an induced haemorrhagic liver injury infused with fibrinogen concentrate, platelet concentrate or saline.
Lowest rate of blood loss seen in pigs infused with fibrinogen concentrate. aP < 0.05 fibrinogen vs platelet group, cP < 0.05 fibrinogen vs saline group, eP < 0.05 platelet vs saline group. From Velik-Salchner et al[103] 2007.
There is no consensus on the appropriate trigger for fibrinogen supplementation in LT but it should be considered at a plasma level of 1.5-2 g/L or with evidence of hypofibrinogenaemia on TEG FF/FIBTEM (MCFFIB < 8 mm) in the context of bleeding. Prophylactic use is not recommended since not all patients with low fibrinogen will bleed[76].
Fibrinogen concentrate is available as a lyophilized powder for rapid reconstitution to deliver a precise dose. The dose can be calculated according to the measured and target fibrinogen levels or according to desired MCFFIB increase[80].
Fibrinogen dose = Target level (g/L) - Measured level (g/L)/0.017 (g/L per mg/kg body weight)
Fibrinogen dose = Target increase in MCFFIB (mm) × body weight (kg)/140
Typical dosing is 2-4 g (approximately 25-50 mg/kg) with assessment of clinical and viscoelastic response to guide subsequent titrated doses.
Fibrinogen can be also be supplemented with cryoprecipitate (200-250 mg fibrinogen/unit) or FFP (1-2.5 g fibrinogen/L). The low fibrinogen content of FFP means large volumes of up to 30 mL/kg are required to increase plasma fibrinogen levels risking dilutional coagulopathy and other adverse events[104].
PROTHROMBIN COMPLEX CONCENTRATES
Prothrombin complex concentrate (PCC) comprises either 3 or 4 vitamin K dependent procoagulant factors (II, ± VII, IX, X) and the anticoagulants protein C and S, extracted from pooled plasma. PCCs can improve haemostasis where loss or dilution of prothombotic factors is contributing to bleeding[45]. In LT a dose of 25 iu/kg is advocated if there is severe bleeding associated with prolonged clotting time on VETs (TEG R time or EXTEM Clotting time > 80 s) after excluding a HLE. PCC may be the ideal therapy to restore thrombin generation in dilutional coagulopathy (Figure 10).
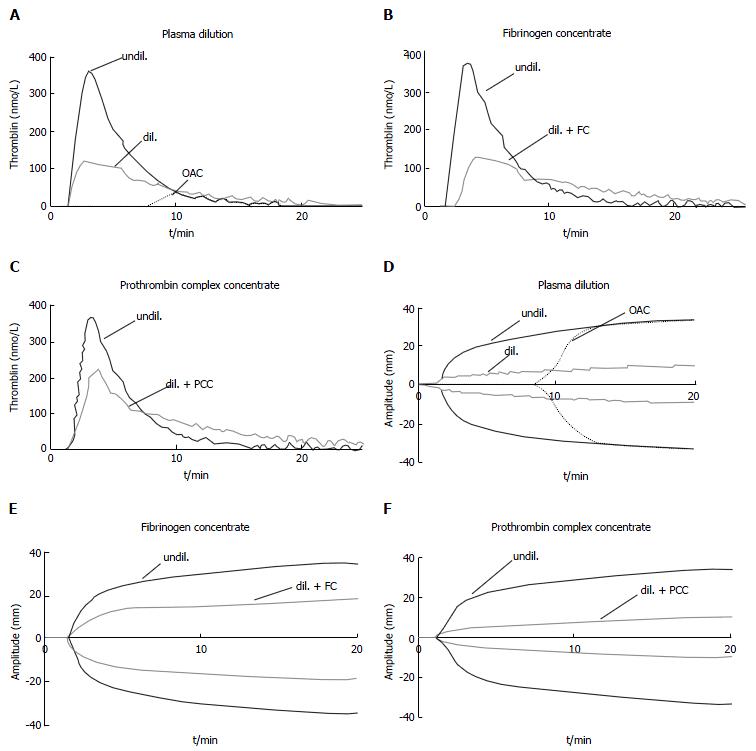
Figure 10 Effect of plasma dilution and factor deficiency on thrombin generation and fibrin clot formation.
Representative curves of tissue factor - induced thrombin generation (A-C) and fibrin clot formation (D-F) of undiluted normal plasma (undil.), 5 × diluted plasma (dil.), and plasma from patient taking oral anticoagulants (OAC, INR = 4). Effects of supplementing diluted plasma with fibrinogen concentrate (+ FC): Increase in clot strength with no effect on thrombin generation (B, E). Effects of supplementing diluted plasma with prothrombin complex concentrate (+ PCC): Increase in thrombin generation but no effect on clot strength (C, F). From Schols[105] 2010. OAC: Oral anticoagulatnts; INR: International normalized ratio.
There is currently a lack of evidence to inform and guide PCC use in LT. A large multicentre double blinded RCT “PROTON-trial” will test the hypothesis that preoperative normalization of INR with PCC will reduce perioperative blood loss and transfusion[106]. Several European centres have incorporated PCC into POC guided algorithms in view of the potential benefits of low volume delivery of a potent thrombin generator without excess thromboembolic events[79,95]. In trauma patients, concerns remain about the sustained prothrombotic potential for up to 4 d post PCC administration[107]. In light of evidence for perioperative hypercoagulability in some patients with liver disease the prothrombotic potential of PCC should not be dismissed[108-110].
RECOMBINANT FACTOR VIIA
Recombinant factor VIIa (rFVIIa) binds with tissue factor at the site of injury to activate factor X and generate a thrombin burst. Concerns over thromboembolic risk exist[111]. rFVIIIa was associated with increased transfusion-free LT[112] and tentatively identified as a method for reducing blood loss and transfusion in LT, without excess thrombotic events, in a Cochrane review[84].
ESA guidelines advocate the use of rFVIIa as a rescue therapy at a dose of 40 mcg/kg in the context of intractable bleeding following correction of coagulation factors, fibrinogen, platelets and calcium (grade 1A evidence). Where POC guided algorithms have been implemented use of rFVIIIa has declined[113].
PROTAMINE
Protamine is a positively charged polypeptide that neutralizes heparin. Reversal of endogenous heparins with protamine in LT is rarely necessary. A small empirical dose of 50 mg protamine can be considered if there is profuse bleeding and a HLE on VET. At high doses protamine can exert a paradoxical anticoagulant effect by inhibiting Factor V activation and impairing endogenous thrombin potential[114].
FACTOR XIII
Factor XIII (FXIII) contributes to clot stability by crosslinking the fibrin mesh and rendering fibrin chains insoluble. Levels can become depleted in the context of massive blood loss, reaching clinical significance when < 60%. FXIII deficiency may be difficult to detect with standard assays and an index of suspicion is needed. Mild reduction in MA or MCF may be seen on VETs that persists despite anti-fibrinolytic therapy or reverses with the addition of FXIII to whole blood. FXIII (e.g., Fibrogammin) can be supplemented at a dose of 15-30 mL/kg to help support clot durability. There is no clear evidence for its use in LT.
AUTOLOGOUS CELL SALVAGE
Autologous cell salvage (ACS) enables collection of blood from the operative field, which is then anticoagulated, centrifuged, filtered, washed and suspended in saline with a haematocrit of up to 80%. The volume of RBC suspension will be in proportion to blood losses and the patient’s haematocrit. The suspension lacks factors and platelets and supplementation should be considered in large autologous transfusions to avoid dilutional coagulopathy. A Cochrane review found that use of cell salvage in elective patients was associated with a 38% reduction in rate of exposure to allogenic blood, though included trials were subject to bias given the impossibility of blinding the intervention[115]. No safety concerns were raised. Attempts to avoid aspiration of ascitic fluid and bile should be made. The role for ACS in the context of LT with malignancy (hepatocellular carcinoma) remains controversial. The ESA suggest pragmatism when balancing relative risks of haemorrhage and allogenic transfusion (implicated in tumour recurrence[116]) with the theoretical potential for reinfusion of tumour cells. Leucocyte depletion filters may reduce tumour cell load at the expense of reinfusion rate. There is a paucity of evidence appraising the cost-effectiveness of ACS in the setting of LT and its economic impact will vary according to the transfusion rates and practice in different institutions[117]. Obviating the need for at least 2 units of allogenic RBC delivers a cost-benefit and therefore ACS is likely to be financially viable in major blood loss surgery where transfusion is expected.
PREDICTING BLEEDING AND TRANSFUSION
Several recipient, donor and surgical related variables predicting intra and postoperative bleeding and transfusion have been identified in different patient populations and transplant centres. There are many similarities and discrepancies: Preoperative haemoglobin, haematocrit, preoperative platelet count, fibrinogen levels[118], coagulation assays, bilirubin, creatinine, recipient age, disease severity scores, previous surgery, surgical technique and graft function have all been found to be positive predictors by some studies but not others[12,13,57,119-121]. McCluskey derived and internally validated a predictive risk index for > 6 unit RBC transfusion based on 7 preoperative variables: Baseline haemoglobin < 10 g/dL, INR > 2, platelet count < 70 × 109, elevated creatinine, albumin < 24 g/L and redo procedure[122]. Such indices are unlikely to be applicable in other clinical settings with different patient populations, practices and transfusion protocols. The main value of identifying predictors is in highlighting those factors that can be modified and intervening pre-emptively.
Low preoperative haemoglobin is perhaps the most relevant modifiable predictor of non-massive perioperative transfusion in transplant and other patient groups[9,123,124]. As transfusion declines generally, optimising preoperative haemoglobin may have an increasingly significant impact on allogenic blood use. Identifying and managing preoperative anaemia in patients awaiting transplantation could impact dramatically on transfusion if implemented alongside other PBM interventions such as restrictive transfusion triggers[35].
Our inability to definitively predict or exclude risk of massive haemorrhage in LT despite best practice means that the infrastructure to deliver a massive transfusion should always be available perioperatively.
THE SECRET INGREDIENT
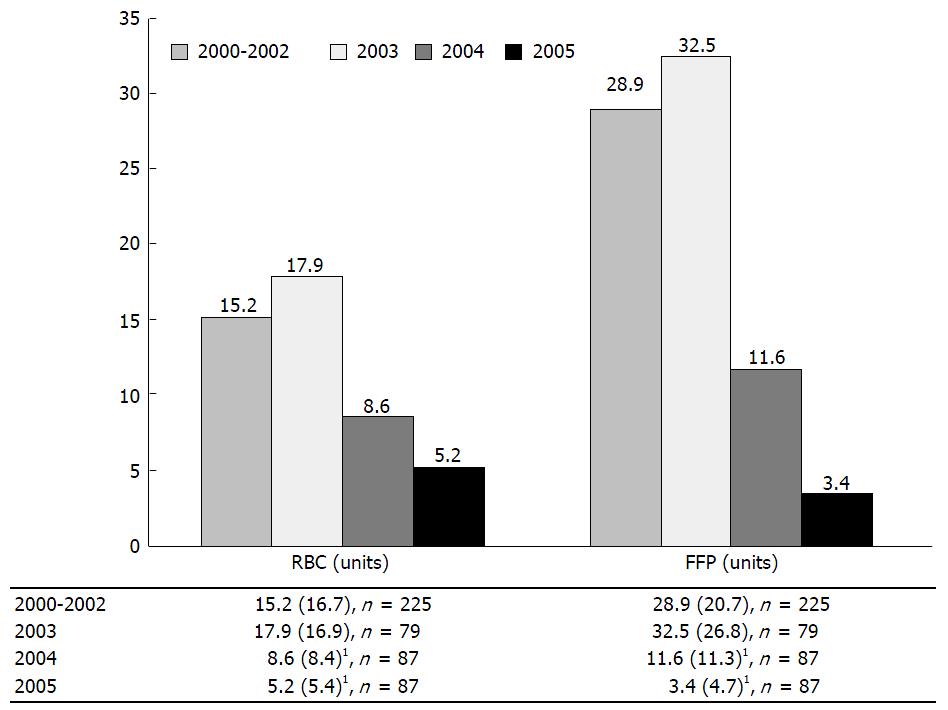
Organisations with different aspirations, expectations and experiences undoubtedly account for a great deal of the inter-institutional variation in patient management. By addressing various aspects of the LT service, fostering strong multidisciplinary relationships and implementing evidence based protocols, Hevesi et al[125] were able to deliver improvements in patient outcomes. Transfusion rates, ventilator days and LOS fell dramatically following the introduction of their quality improvement measures (Figure 11).
Figure 11 Blood transfusion during liver transplantation per case: Bar plots of red blood cells and fresh frozen plasma by year.
1Indicates a significant difference from the 2000-2002 value at a significance level of P = 0.05 based on the Wald t-test for the respective regression coefficient with adjustments for age, gender, baseline weight and height. FFP: Fresh frozen plasma; n: Samples size; RBC: Red blood cells. From Hevesi et al[125] 2009.
CONCLUSION
The multifactorial reduction in bleeding and allogenic transfusion over the past decades reflects progress and the changing landscape of LT. Rates of massive transfusion are generally declining but in an era increasingly reliant on marginal grafts, profound coagulopathy and bleeding remain pertinent issues. With the decline in median RBC transfusion per LT, the impact of baseline haemoglobin gains significance. Widespread preoptimisation and adoption of restrictive transfusion thresholds may increase transfusion-free LT in future. As our understanding of the interplay between bleeding, transfusion and outcome improves, there is a responsibility to uphold best evidence based practice and reduce the current inter-institutional variability.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ohkoshi S, Xia VW S- Editor: Ji FF
L- Editor: A E- Editor: Li D