Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.145
Peer-review started: July 5, 2015
First decision: July 28, 2015
Revised: August 30, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: December 24, 2015
Processing time: 177 Days and 20 Hours
The reconstruction of the vascular outflow tract of partial liver grafts has received considerable attention in the past, especially in the setting of right liver grafts with undrained segments. Hepatic venous outflow reconstruction is an important factor for successful living donor liver transplantation outcome. However, in presence of undrained anterior sector and presence of multiple short hepatic veins that drain substantial portions of liver, outflow reconstruction without backtable venoplasty may lead to severe graft congestion and subsequent graft dysfunction. Various backtable venoplasty techniques in presence of multiple hepatic veins that can be used in either right- or left-lobe liver transplantation are devised to ensure a single, wide outflow channel. In this overview, various techniques to overcome the hepatic venous variations of liver allograft and outflow reconstruction are discussed.
Core tip: Outflow reconstruction in living donor liver transplantation is crucial for proper graft functioning. The right liver graft is a partial graft and requires backtable venoplasty to restore segmental venous drainage. A right liver graft without the middle hepatic vein along with presence of multiple short hepatic veins makes outflow reconstruction technically complex. To avoid postoperative liver congestion, suitable surgical techniques are applied to form a common outflow channel that provides adequate drainage for all the segments of liver. This article gives a comprehensive viewpoint for the venous outflow reconstruction in living donor liver transplantation.
- Citation: Jeng LB, Thorat A, Yang HR, Li PC. Venous outflow reconstruction in living donor liver transplantation: Dealing with venous anomalies. World J Transplant 2015; 5(4): 145-153
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/145.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.145
In Asia, living donor liver transplantation (LDLT) was adapted and later became the most successful and safe source for liver allografts as the deceased organ donation remains scarce[1]. The living donor liver allograft is a partial graft and graft size discrepancy always remains a concern. Graft-to-recipient-weight ratio > 0.8% is considered an adequate for proper liver graft functioning after the transplantation. To alleviate the problem of graft size disparity, an extended right liver graft, which includes the trunk of the middle hepatic vein (MHV), was devised and later same group concluded the safety of the MHV inclusion without any morbidity in the donors[2,3]. However, inclusion of the MHV in the donor liver graft remains a topic of controversy as the critics think this may increase chance of donor morbidity and right liver allografts without the MHV yield similar results. But, the grafts that are devoid of MHV may cause worrisome congestion of anterior sector that may increase the risk of post-operative liver dysfunction and infection. All the MHV tributaries must be reconstructed during backtable procedure to provide an effective venous drainage, because a balanced portal vein and hepatic artery inflow along with an adequate venous outflow are the crucial factors for successful outcomes after LDLT[4]. In absence of an adequate graft venous drainage, the portal inflow can cause damaging effects on the liver allograft and delay the regenerative capacity that may cause liver dysfunction in post-operative period leading to small-for-size syndrome.
The right liver graft with reconstructed anterior sector venous drainage provides a functioning liver mass comparable to an extended right liver graft[5]. Many technical breakthroughs, modifications in donor hepatic transection and backtable innovative venoplasty procedures that evolved over the last decade have led to a successful long-term outcome after transplantation in most of the LDLT centers. Thus, the venoplasty of MHV tributaries (if MHV not included in graft) has been adapted as the standard procedure in liver transplantation. The venoplasty can be accomplished by using cryopreserved vascular grafts or synthetic polytetrafluoroethylene (PTFE) grafts. In Asia, many centres including us, have resorted expandable PTFE grafts to reconstruct the anterior sector venous drainage and inferior right hepatic veins (IRHVs) if present.
This brief overview presents technical aspects about venous outflow reconstruction in right lobe LDLT and a viewpoint is provided about the techniques and recent progress to overcome the various donor or recipient anatomical variations.
One of challenging aspects of outflow reconstruction in LDLT is the partial liver graft that is harvested. Unlike deceased donor liver, right or left liver allografts need reconstruction of the venous tributaries on the cut surface (if MHV not included in the graft) to restore the venous drainage of the corresponding segments to prevent any post-operative congestion.
Traditionally, the MHV inclusion in the right liver allograft was suggested that required no backtable venoplasty. But, as concerns about donor remnant liver congestion precluded the surgeons from including the MHV in the graft, many transplant centres started using right liver grafts without inclusion of the MHV or modified techniques such as inclusion of the MHV till the V4b drainage to prevent the donor remnant liver congestion[4,6]. But, the liver grafts without the MHV often had congested anterior sector after liver graft implantation. Studies showed increased risk of septic complications and graft dysfunction in right liver grafts with congested anterior sector[7]. Hence, restoration of the graft venous drainage by a backtable venoplasty became a routine standard.
Initial arguments against the venoplasty were the size and the number of venous tributaries that require reconstruction. Venous branches > 4 mm diameter should be reconstructed. The backtable procedure is also influenced by presence of graft venous variations that are found to be present in approximately 40% of donor livers and presence of a single or multiple IRHVs draining to inferior vena cava (IVC) is a common type of short hepatic vein in right liver[8]. These veins drain considerable segmental areas of the liver, and hence, must be reconstructed. But, this makes the outflow reconstruction of the allograft technically complex. However, the MHV tributaries as well as the IRHVs can very well be incorporated into a single lumen using modified venoplasty procedures (described later).
The argument in venoplasty remains about the best method and the type of vascular grafts that can be used to accomplish reconstruction. Various techniques of venoplasty have been described. Hwang et al[9] described a “Quilt venoplasty” by using autologous great saphenous vein to reconstruct multiple short hepatic veins into a single lumen. Unification venoplasty for the venous tributaries during backtable have been successfully used with a good outcome without need of interpositional grafts[10].
But, in certain situations, backtable venoplasty is not feasible without use of interpositional grafts. The various venous grafts used as interpositional material are cryopreserved vascular grafts, donor or recipient’s autologous veins, recipient’s umbilical vein in certain situations and expanded polytetrafluoroethylene (ePTFE) synthetic grafts. Various centres have successfully reported their experience using the cryopreserved grafts[11]. But, as deceased vascular grafts lack in number, ePTFE emerged as an effective alternative for the vascular conduits. Recent experience using ePTFE grafts for the MHV and IRHV reconstruction is encouraging and proved the safety of such grafts in LDLT. The PTFE grafts, either expanded[12,13] or ringed[14], are successfully used for the MHV reconstruction with a good patency rate.
From August 2002 to June 2015, 619 LDLT are performed at our Institute of China Medical University Hospital, Taiwan. Over the years, we have modified and innovated various surgical techniques of graft venoplasty and outflow reconstruction during the implantation of the graft in recipient. As the deceased donation is scarce, we often have shortage of cryopreserved vessels. Autologous veins such as recipient saphenous vein and donor iliac vein can also be harvested as vascular conduits, but this increases the complexity and extent of the surgery. Hence, we prefer ePTFE synthetic graft for venoplasty as their safety is already proven in recent studies[12-14]. ePTFE grafts are easily available, less thrombogenic and requires no additional anticoagulation in postoperative period. However, all the recipients that receive liver allografts with an ePTFE graft are treated with an antiplatelet agent aspirin 100 mg once a day for 2 years post-operatively.
After liver allograft is harvested, it is flushed with 2 L of Histidine-Tryptophan-Ketoglutarate solution. The venous tributaries of the MHV on the cut surface of right liver allograft and any additional accessory right hepatic vein (RHV) or IRHVs are assessed, and appropriate backtable venoplasty is planned. Though arguments remain as to what size of vein should be reconstructed, our dictum is any venous tributaries > 4 mm should be reconstructed.
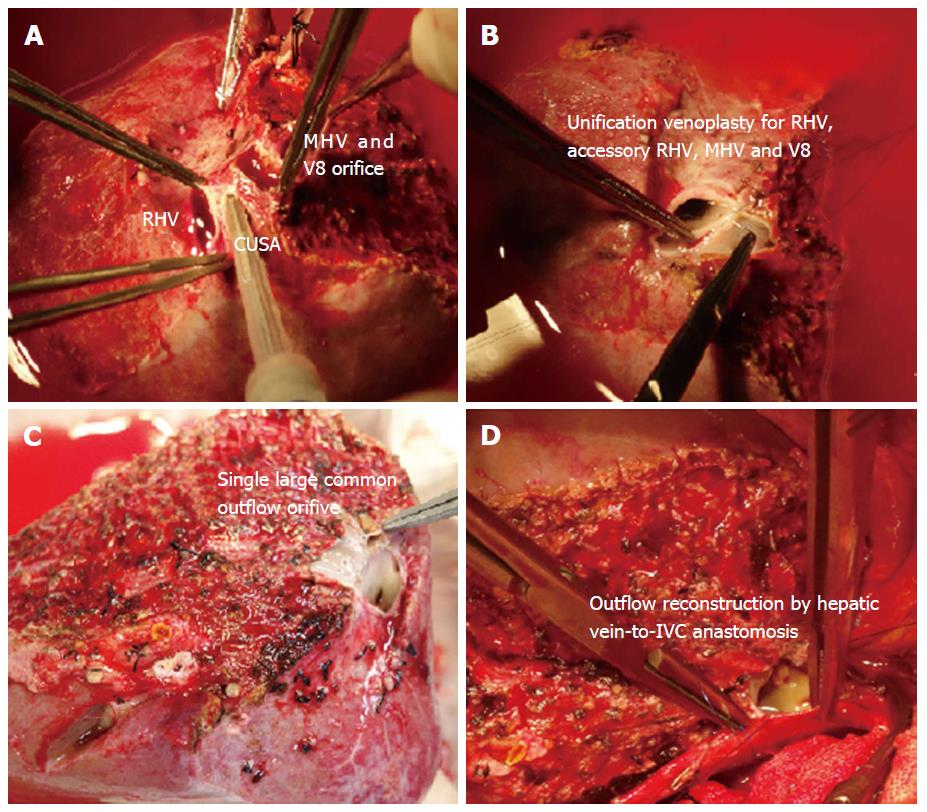
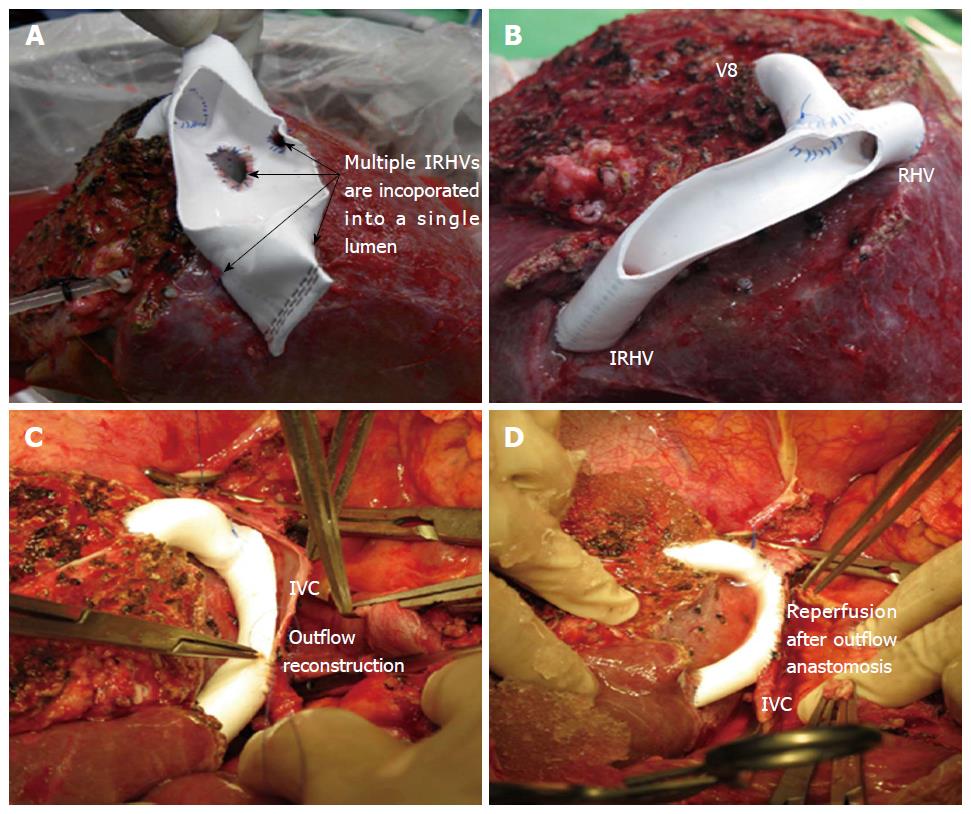
In right lobe grafts, if 2 or more orifices are present, i.e., RHV, accessory RHV, IRHV, or segment 8 vein, venoplasty is performed to fashion a single, wide outflow orifice. Sometimes the intervening liver parenchyma can be transected with the help of cavitron ultrasonic surgical aspirator to facilitate a tension free venoplasty (Figure 1).
If the MHV is included in the graft, the walls of the RHV and the MHV can be sutured to form a common outflow channel. A single large outflow orifice always critical for an adequate outflow. In our center, if we decide to include the MHV, we often use modified technique taking due care of segment 4b venous drainage (V4b) of donor’s remnant liver, and a proximal MHV upto junction of V4b is included in the graft.
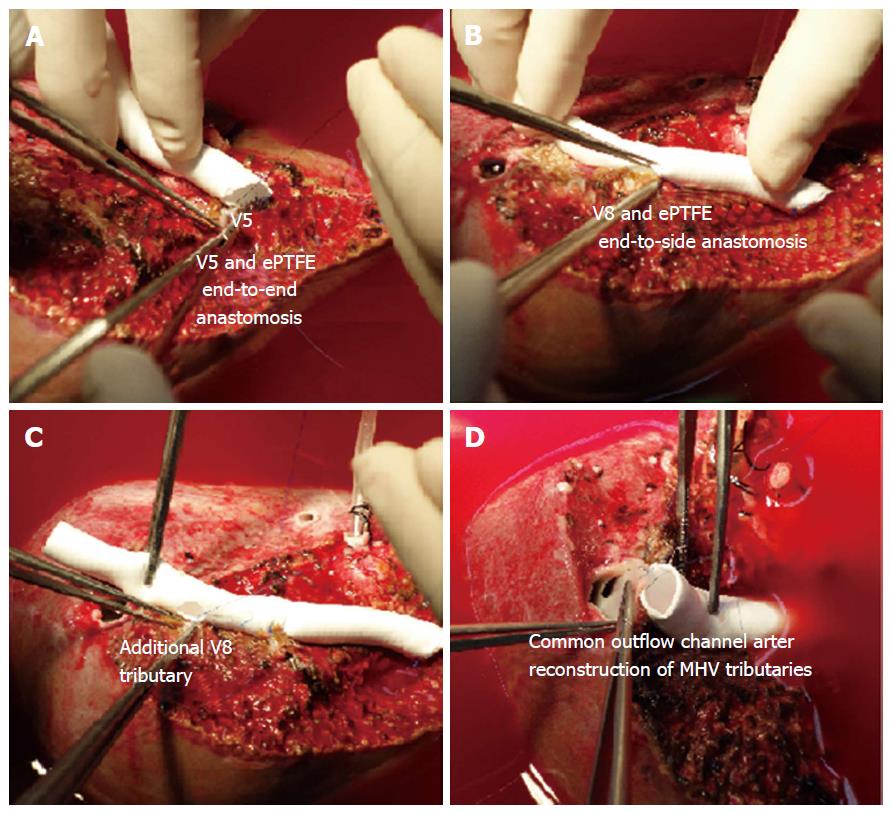
For all the liver allografts that are devoid of the MHV, the venous tributaries of segment 5 (V5) and 8 (V8) that are > 4 mm in diameter should be reconstructed. We often use ePTFE synthetic grafts as an interpositional material to reconstruct the venous branches.
The reconstruction technique is only described briefly: V5 is anastomosed to the ePTFE by an end-to-end technique using 5-0 hemoseal prolene suture in continuous fashion. The posterior wall of the other end of ePTFE is anastomosed to the anterior wall of the RHV to form a common orifice. The remaining V5 and V8 branches are anastomosed to the ePTFE by an end-to-side technique using 5-0 hemoseal prolene (Figure 2). After completion of the reconstruction, we use tissue glue to bridge the gaps between the venous tributaries and the parenchyma as that can be the sites of oozing after reperfusion of the allograft.
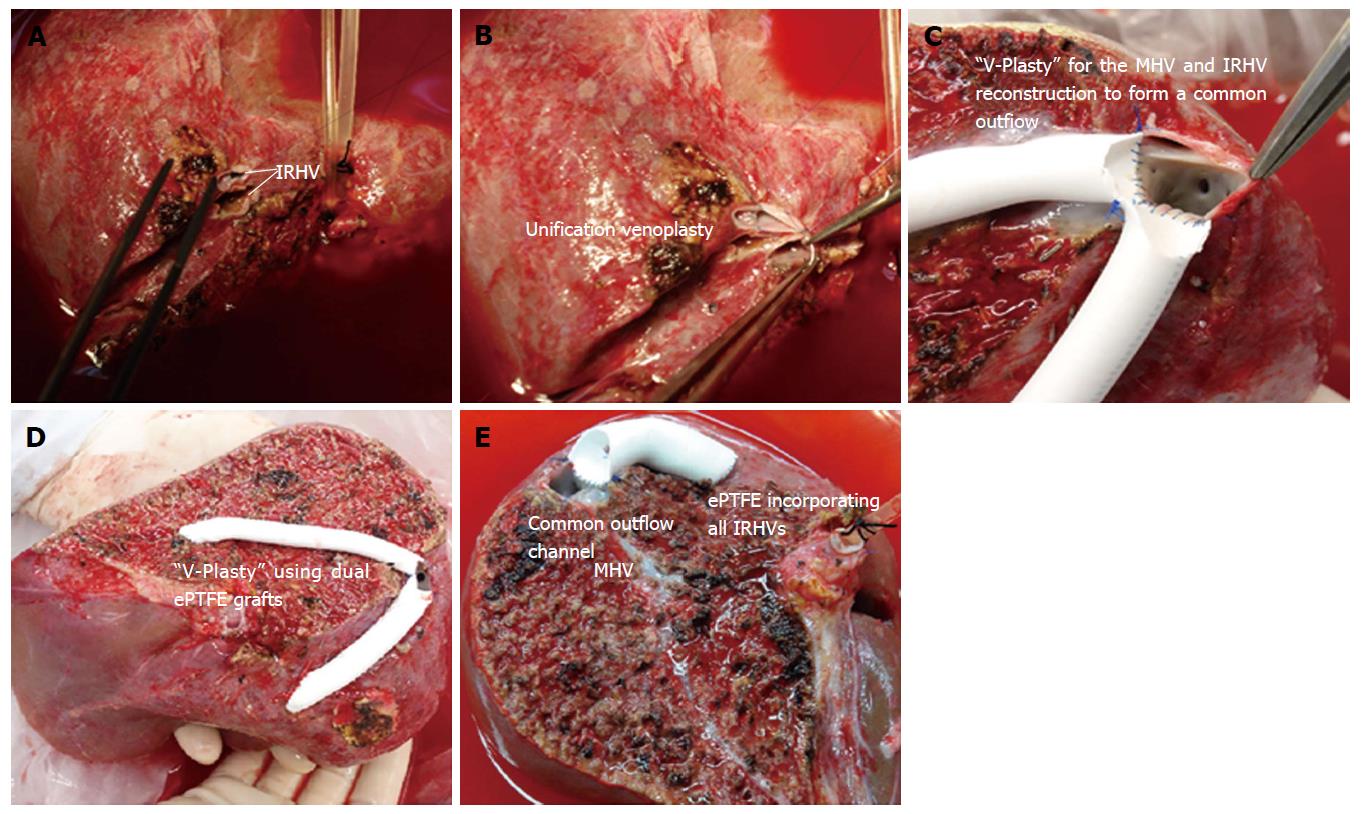
The right liver graft with multiple IRHVs pose technically complex situation as IRHV reconstruction into a single orifice or separate IVC anastomosis still remains unsolved. There are several variations in techniques to reconstruct the IRHVs[15,16]. If IRHVs are present in close proximity, they are included in a single large orifice by an unification venoplasty which can be done by suturing the adjacent walls with 6-0 prolene (Figure 3A and B). If these veins are present in same plane of the RHV, then we perform second direct-to-IVC anastomosis. Although, IRHV can be reconstructed using recipient’s great saphenous vein, reconstruction of the IRHVs with ePTFE synthetic grafts is a relatively new concept.
If more than 2 IRHVs are present, a combined venoplasty including the MHV tributaries can be done using dual artificial grafts to form a single outflow channel (a “V-Plasty” technique) that requires single hepatic vein-to-IVC anastomosis.
“V-Plasty” technique: In presence of undrained anterior sector along with multiple IRHVs, we have developed an unique backtable venoplasty technique also called as “V-Plasty” to form a common outflow orifice[13]. We use the name “V” Plasty because after reconstruction of the MHV and the IRHV tributaries using these dual ePTFE grafts, the venoplasty appears V-shaped with two grafts forming each limb of “V”.
In right liver grafts with undrained anterior sector (without MHV) and with presence of ≥ 2 IRHVs that are located randomly and caudally in relation to the RHV, a “V-Plasty” is performed as shown in Figure 3C and D. Detailed surgical technique for this procedure has been described before. With application of this technique, single hepatic vein-IVC anastomosis is required during graft implantation that decreases the warm ischemia time. As multiple IRHVs drain substantial portion of liver, their reconstruction is important for proper graft function. Second or third hepatic vein-to-IVC anastomosis is impractical in difficult retrohepatic space, in presence of adhesions and collaterals that would only increase the warm ischemia time. In our recent study of 16 patients with V-Plasty, we noticed remarkable decreased warm ischemia time as compared to more than one hepatic vein-to-IVC anastomosis recipients (25.25 ± 8.11 min vs 34.56 ± 5.07 min, P < 0.001)[16]. In this case series, the patency rates of the ePTFE grafts was 100% for first 2 mo. No incidence of venous outflow obstruction was noted in any of the recipients of study cohort[13,16].
In the grafts with inclusion of the MHV, the IRHVs can still be reconstructed using ePTFE graft as shown in Figure 3E.
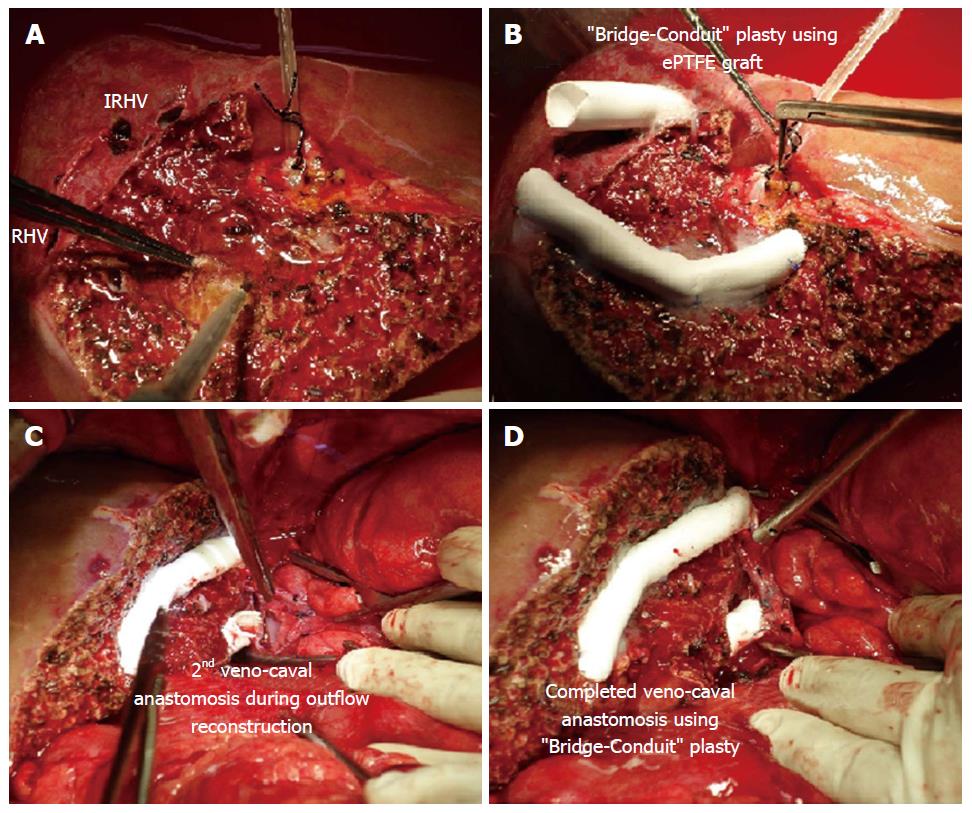
“Bridging-Conduit” Plasty: In presence of single IRHV, we do not use “V-Plasty” as the distance traversed by the blood through such conduit is more and second IVC anastomosis is feasible option. But, if the IRHV is present more ventral and caudal with respect to the RHV, a vascular conduit can be used to bridge the gap between the graft and the IVC. This decreases the stretch effect on the anastomosis and increases the ease of second IVC anastomosis in limited retro-hepatic space.
After the MHV tributaries are reconstructed, the “Bridge-conduit” venoplasty for large IRHV using second ePTFE is performed during backtable procedure using 6-0 prolene in an end-to-end fashion. The other end of the ePTFE graft is then anastomosed to the IVC during graft implantation (Figure 4).
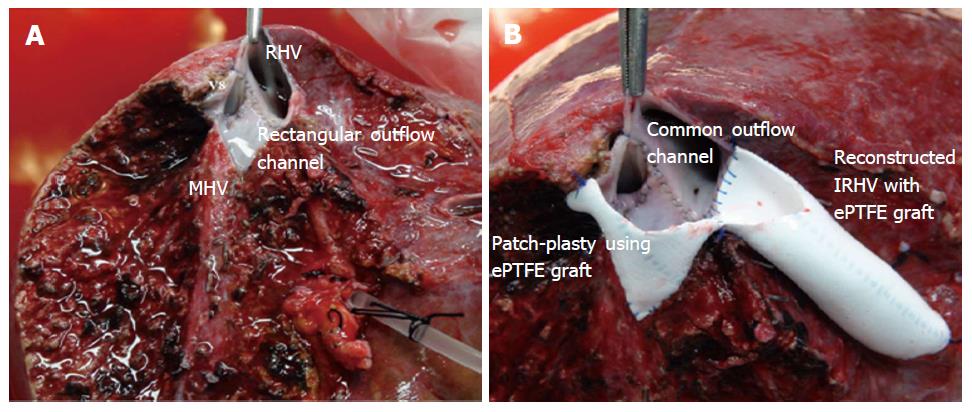
“Single-Oval Ostium” technique using ePTFE grafts: In presence of the multiple hepatic veins that drain the major portion of the posterior sector of right liver allograft, conventional direct-to-IVC anastomosis is not feasible. In such situation, inclusion of all the veins in a common outflow channel by “Single-Oval Ostium” technique ensures proper outflow for all the liver segments (Figure 5). The details of this technique are described before[17]. This novel technique serves a single outflow channel for all the draining veins by a simple backtable venoplasty and also facilitates the ease of the veno-caval anastomosis due to a wide outflow channel.
After the backtable venoplasty of RHV, accessory RHV, distal end of the MHV and any additional venous tributaries in vicinity, occasionally a large rectangular venous outflow orifice is created. In such situation graft implantation is difficult as the anterior edge of graft outflow remains at farther distance and anastomosis comes under great stretch. In such situation, a patch of vascular graft can be used to raise the anterior edge to hasten the anastomosis of the RHV-to-IVC. Such patch can be obtained from recipient umbilical vein, recipient portal vein or synthetic vascular graft. As shown in Figure 6, the ePTFE patch can be used that can be combined with additional venoplasty if the graft demands. A triangular patch of appropriate size is sutured with the anterior wall of the rectangular orifice. This raises the outer edge of the outflow orifice that facilitates a tension free anterior wall anastomosis with the IVC during graft implantation. Although researchers have used patch of either cryopreserved or autologous veins, the “Patch-Venoplasty” using ePTFE graft is safe and feasible.
Graft implantation and outflow anastomosis follows standard guidelines. We cross clamp the IVC at supra-hepatic and infra-hepatic region. A triangular slit is created on the anterior wall of the IVC starting from the junction of the RHV along the IVC border. A triangulation method to create a wide outflow orifice was initially advocated by Emond et al[18]. In presence of the IRHVs, the diameter is > 4 mm, second IVC anastomosis is done whenever feasible by creating s separate venotomy on the IVC. In complex situation we prefer single outflow channel using “V-Plasty” that increases the ease of anastomosis, and also reduces the warm ischemia time. The outflow reconstruction may require modification depending upon the calibre of the recipient IVC.
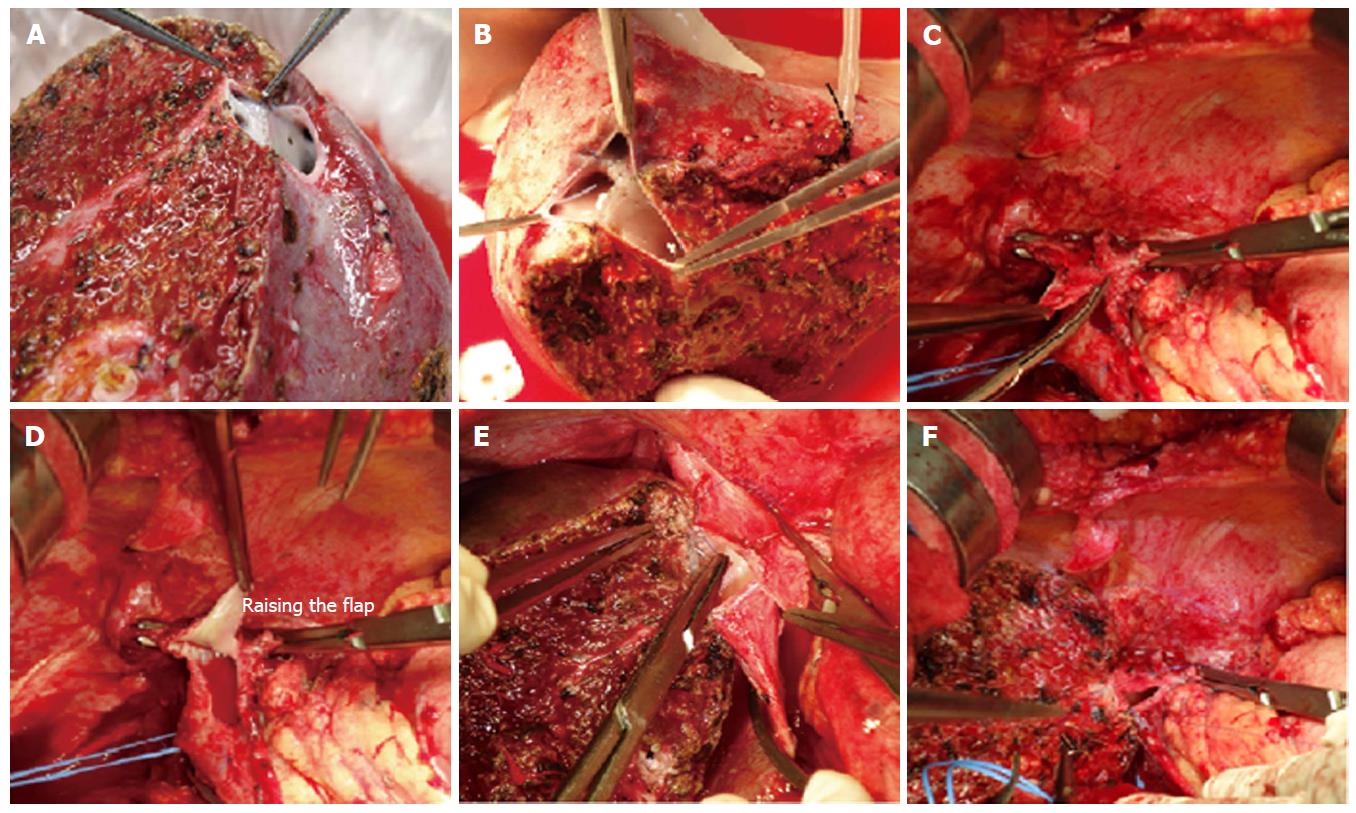
“Raising-flap” technique: In presence of unusually large outflow orifice and small calibre of the recipient IVC, the conventional hepatic vein-to-IVC anastomosis is not feasible as it causes over-riding of the graft onto the IVC that may cause outflow impedance. An unduly small IVC in the recipient that requires a larger opening and can cause a pulling effect on the graft, which may lead to compromised outflow[4]. In such situation we have modified the outflow reconstruction technique by raising a triangular flap on the IVC (Figure 7). The details of techniques are described earlier. This technique not only provides a larger outflow orifice for venous drainage but also avoids undue medial pull on the graft hepatic vein. “Raising-flap technique” allows more caudal extension of the IVC opening and covering of the triangular hepatic venous orifice of the graft with a flap without stretching of the hepatic veins and pull effect on the IVC.
Several variations of graft implantation techniques in challenging situations have been published. Tanaka et al[19] widened the outflow by venoplasty of the recipient middle hepatic vein and left hepatic vein with a right caudal extension in the inferior vena cava. A triple recipient hepatic vein reconstruction with creation of a long venous trunk is appropriate in selected cases[20]. But, whatever is the technique for outflow reconstruction, a wide outflow with adequate venous drainage for all liver segments should be the aim during reperfusion.
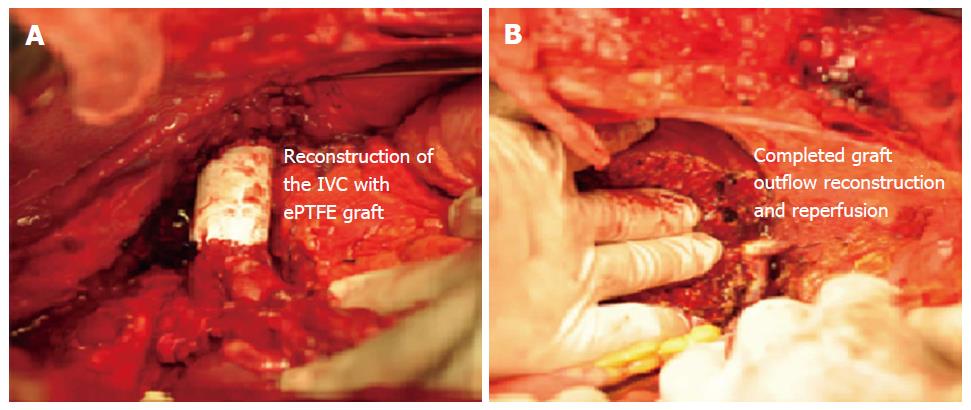
A patient with liver tumor involving the IVC with poor underlying liver functions often has a dismal prognosis and has traditionally been considered to be contraindicated for resection owing to associated high surgical risks and advanced stage of tumor. But, liver transplantation can still be performed if the IVC can be resected along with the HCC in absence of extra-hepatic disease. In LDLT, however, the outflow reconstruction of the graft becomes challenging as the IVC needs to be reconstructed. We have presented feasible technique of resection and reconstruction of the IVC with ePTFE graft with an acceptable outcome[21]. In such cases, after reconstruction of the IVC with ePTFE, graft implantation requires anastomosis of the graft RHV to the ePTFE (Figure 8).
The indication of this technique can also be extended to benign cases where total hepatectomy in the recipient is not possible without formal resection of the IVC due to dense adhesions.
Outflow reconstruction in such scenario is rarely discussed. Our technique shows the safety and feasibility of the LDLT in such cases and IVC can be reconstructed achieving an adequate outflow for the graft. Although graft infection and thrombosis are the major concerns in such cases, none of the patients in our series had abdominal infection or graft dysfunction due to outflow compromise[13,17,21].
Outflow tract reconstruction is critical for proper graft functioning in LDLT as any venous impedance can cause graft congestion that may lead to graft dysfunction and even early graft failure, especially in marginally-sized donor grafts, as venous outflow disturbance adversely affects the regenerative capacity of a partial liver graft. Hence, significantly large venous tributaries must be reconstructed by a backtable venoplasty using vascular grafts. In recent era many centres, including us, have showed the importance of venoplasty and described the innovative venoplasty techniques using various interpositional grafts to overcome the complexity in presence of venous variations.
The initial argument against the need for venoplasty was: (1) the intra-hepatic venous collaterals are present in the right liver grafts that provide adequate segmental drainage; (2) the vascular anastomosis thus established will eventually get occluded; and (3) the synthetic grafts can increase risks of thrombosis and infection. Although intrahepatic collaterals exist, they develop rather slowly over next few weeks and the venous drainage through the intra-hepatic sinusoids appeared to be insufficient to relieve congestion after hepatic vein ligation. The intrahepatic venous collateral are expected to develop by day 7 after transplantation, hence even if the smaller calibre anastomosis are obstructed after few weeks, hepatic dysfunction does not occur. The safety of ePTFE grafts as interpositional vascular conduits have already been proven in many studies. Hence, venoplasty using ePTFE grafts is justified. Besides, its universally recognized that the venous drainage of the graft depends largely on the tributaries of the MHV and the short hepatic veins when present.
Venous outflow reconstruction is thus constitute an important step and with a backtable venoplasty to form a common outflow channel not only prevents congestion of the graft, but also increases the ease graft implantation.
| 1. | Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 2. | Lo CM, Fan ST, Liu CL, Wei WI, Lo RJ, Lai CL, Chan JK, Ng IO, Fung A, Wong J. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261-269; discussion 269-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 420] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Fan ST, Lo CM, Liu CL, Wang WX, Wong J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003;238:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Jeng LB, Thorat A, Li PC, Li ML, Yang HR, Yeh CC, Chen TH, Hsu CH, Hsu SC, Poon KS. Raising-flap technique for outflow reconstruction in living donor liver transplantation. Liver Transpl. 2014;20:490-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Malago M, Molmenti EP, Paul A, Nadalin S, Lang H, Radtke A, Liu C, Frilling A, Biglarnia R, Broelsch CE. Hepatic venous outflow reconstruction in right live donor liver transplantation. Liver Transpl. 2005;11:364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Marcos A, Orloff M, Mieles L, Olzinski AT, Renz JF, Sitzmann JV. Functional venous anatomy for right-lobe grafting and techniques to optimize outflow. Liver Transpl. 2001;7:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Sugawara Y, Makuuchi M, Sano K, Imamura H, Kaneko J, Ohkubo T, Matsui Y, Kokudo N. Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg. 2003;237:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Uchida K, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Ota M, Kamiyama T, Matsushita M, Furukawa H, Todo S. Three-dimensional computed tomography scan analysis of hepatic vasculatures in the donor liver for living donor liver transplantation. Liver Transpl. 2010;16:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY. Quilt venoplasty using recipient saphenous vein graft for reconstruction of multiple short hepatic veins in right liver grafts. Liver Transpl. 2005;11:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Concejero A, Chen CL, Wang CC, Wang SH, Lin CC, Liu YW, Yang CH, Yong CC, Lin TS, Ibrahim S. Donor graft outflow venoplasty in living donor liver transplantation. Liver Transpl. 2006;12:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Hwang S, Lee SG, Ahn CS, Park KM, Kim KH, Moon DB, Ha TY. Cryopreserved iliac artery is indispensable interposition graft material for middle hepatic vein reconstruction of right liver grafts. Liver Transpl. 2005;11:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Yi NJ, Suh KS, Lee HW, Cho EH, Shin WY, Cho JY, Lee KU. An artificial vascular graft is a useful interpositional material for drainage of the right anterior section in living donor liver transplantation. Liver Transpl. 2007;13:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Jeng LB, Thorat A, Li PC, Li ML, Yang HR, Yeh CC, Chen TH, Hsu CH, Hsu SC, Poon KS. "V-Plasty" technique using dual synthetic vascular grafts to reconstruct outflow channel in living donor liver transplantation. Surgery. 2015;158:1272-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Hwang S, Jung DH, Ha TY, Ahn CS, Moon DB, Kim KH, Song GW, Park GC, Jung SW, Yoon SY. Usability of ringed polytetrafluoroethylene grafts for middle hepatic vein reconstruction during living donor liver transplantation. Liver Transpl. 2012;18:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Hwang S, Ha TY, Ahn CS, Moon DB, Kim KH, Song GW, Jung DH, Park GC, Namgoong JM, Jung SW. Reconstruction of inferior right hepatic veins in living donor liver transplantation using right liver grafts. Liver Transpl. 2012;18:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Thorat A, Jeng LB, Yang HR, Li PC, Li ML, Yeh CC, Chen TH, Hsu SC, Poon KS. Outflow Reconstruction for Right Liver Allograft with Multiple Hepatic Veins: “V-Plasty” of hepatic veins to form a Common Outflow Channel vs Two or More Hepatic Vein-to-Inferior Vena Cava Anastomoses in limited retrohepatic space. Liver Transpl. 2015;In Press. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Thorat A, Jeng LB, Li PC, Li ML, Yang HR, Yeh CC, Chen TH, Hsu SC. “Single oval ostium technique” using polytetrafluoroethylene graft for outflow reconstruction in right liver grafts with venous anomalies in living donor liver transplantation. Hepato-Gastroenterology. 2015;62:698-702. [DOI] [Full Text] |
| 18. | Emond JC, Heffron TG, Whitington PF, Broelsch CE. Reconstruction of the hepatic vein in reduced size hepatic transplantation. Surg Gynecol Obstet. 1993;176:11-17. [PubMed] |
| 19. | Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, Nishizawa T, Sawada H, Shirahase I, Kim HJ, Yamaoka Y. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993;217:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 437] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Matsunami H, Makuuchi M, Kawasaki S, Hashikura Y, Ikegami T, Nakazawa Y, Noike T, Urata K, Kawarasaki H, Iwanaka T. Venous reconstruction using three recipient hepatic veins in living related liver transplantation. Transplantation. 1995;59:917-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Hsu SC, Jeng LB, Thorat A, Li PC, Poon KS, Hsu CH, Yeh CC, Chen TH, Yang HR. Management of extensive retrohepatic vena cava defect in recipients of living donor liver transplantation. Transplant Proc. 2014;46:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Grilo I, Khalaf H, Mikulic D, Wan QQ
S- Editor: Gong XM L- Editor: A E- Editor: Li D