Published online Mar 24, 2015. doi: 10.5500/wjt.v5.i1.19
Peer-review started: January 6, 2014
First decision: January 23, 2014
Revised: September 22, 2014
Accepted: October 28, 2014
Article in press: October 29, 2014
Published online: March 24, 2015
Processing time: 442 Days and 11.3 Hours
AIM: To investigate if conversion to the mammalian target of rapamycin inhibitors (mTORi) improves renal function in diabetic and/or hypertensive liver transplant patients immunosuppressed with tacrolimus or cyclosporine.
METHODS: The study included 86 liver graft recipients immunosuppressed with mTORi treatment after orthotopic liver transplantation (OLT), including all liver recipients with worsening renal function before conversion to mTORi (n = 55 patients) and recipients with normal renal function who converted to mTORi for other reasons (n = 31 patients). We identified patients with diabetes mellitus (n = 28), arterial hypertension (n = 27), proteinuria (n = 27) and all three factors (n = 8) (some patients have hypertension and diabetes and no proteinuria). The primary endpoint was evolution in renal function defined as the development in plasma creatinine as a function of diabetes mellitus (DM), hypertension (HT) or proteinuria. We required elevated serum creatinine for at least two weeks to define renal dysfunction.
RESULTS: Only patients that converted because of renal failure with plasma creatinine levels > 1.5 mg/dL showed an improvement of renal function (2.14 to 1.77 mg/dL) (P = 0.02). Patients with DM showed no improvement of serum creatinine levels (1.31 mg/dL to 1.37 mg/dL) compared with non DM patients (1.31 mg/dL to 1.15 mg/dL) (P = 0.01), HT patients (1.48 mg/dL to 1.5 mg/dL) with non HT patients (1.21mg/dL to 1.08 mg/dL) and patients with proteinuria (1.44 mg/dL to 1.41 mg/dL) and no proteinuria (1.31 mg/dL to 1.11 mg/dL).
CONCLUSION: In OLT recipients with diabetes or hypertensive nephropathy, conversion to mTORi does not improve renal function but stabilizes plasma levels of creatinine. Proteinuria is not a contraindication to conversion to mTORi; it also stabilizes renal function. Conversion to mTORi should only be avoided in patients with diabetes, hypertension and proteinuria.
Core tip: These results could be useful in choosing an immunosuppressant regimen in liver transplant recipients, especially in patients with diabetes mellitus and/or arterial hypertension with proteinuria and possibly renal dysfunction.
- Citation: Álamo JM, Olivares C, Barrera L, Marín LM, Suarez G, Bernal C, Serrano J, Muntané J, Padillo FJ, Gómez MA. Conversion from calcineurin inhibitors to mTOR inhibitors stabilizes diabetic and hypertensive nephropathy after liver transplant. World J Transplant 2015; 5(1): 19-25
- URL: https://www.wjgnet.com/2220-3230/full/v5/i1/19.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i1.19
Survival after orthotopic liver transplantation (OLT) is getting better because of improvement in surgical techniques and better management in immunosuppressant therapy. This important survival leads to more side effects from immunosuppression agents so it is very important to identify the best drug regimen for each patient to reduce toxicity[1]. Calcineurin inhibitors (CNI) tacrolimus and cyclosporine have a common (18%-25%) side effect of chronic renal dysfunction[2] and some of these patients will need hemodialysis with the possibility that this renal failure could be the cause of death[3]. Immunosuppressive therapies that reduce or eliminate CNI based treatment should preserve renal function after OLT.
mTOR inhibitors, sirolimus and everolimus (mTORi), block cell proliferation based on interleukin-2 pathway interacting kinases called the mammalian target of rapamycin[4]. CNI inhibit production of cytokines as interleukin-2 in the first phases of the lymphocyte cell cycle[5]. These days, mTORi is being studied more in renal transplant patients and less in liver transplant patients. There are some studies that show that elimination or reduction of CNI and inclusion of mTORi preserve renal function[6-12]. However, there are no controlled studies of the effect of mTORi exposure in liver transplant patients with well-known chronic renal insufficiency because of diabetes and/or hypertension associated with worsening urinary protein excretion and renal function. It is probable that improvement in renal function is reduced in patients with diabetes mellitus (DM), hypertension (HT) and/or proteinuria.
The potential side effects of mTORi, such as hyperlipidemia, hepatic artery thrombosis and a bad wound cicatrization, have been investigated in these patients[13]. No controlled studies have examined these potential effects in the OLT population.
This study attempts to compare outcomes of renal function in cohorts treated with mTORi with diabetes mellitus, hypertension or/and proteinuria.
We studied 86 liver recipients immunosuppressed with mTORi treatment after OLT at our center from March 2007 to June 2013. Renal dysfunction was defined as serum creatinine ≥ 1.2 mg/dL for at least two weeks (whenever it occurred at least two months after OLT). We included all liver recipients who were diagnosed with renal dysfunction before conversion to mTORi (n = 55 patients) as well as patients with normal renal function who converted to mTORi for other reasons (n = 31 patients). We identified patients with diabetes mellitus (n = 28), arterial hypertension (n = 27), proteinuria (n = 27), and all three factors (n = 8) (some patients had hypertension and diabetes and no proteinuria).
Baseline creatinine was determined as plasma creatinine level at the moment of switching to mTORi, then at 6, 12 and 18 mo, and actual creatinine (last drawn serum creatinine) when collected.
DM patients were defined by the American Diabetes Association criteria [Diabetes Care 2005; 28 (suppl 19): 37-42]. HT patients were catalogued as patients with systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg. Proteinuria was defined as the appearance of proteins in urine. There was no difference in severity.
The main endpoint was evolution of renal function, determined by serum creatinine and according to the presence of DM, HT and/or proteinuria. Renal dysfunction was defined by elevated serum creatinine for at least two weeks.
The immunosuppressant regimen after OLT was administered inside a wide protocol at our center. Induction drugs at time of OLT were given in cases of well-known renal dysfunction before transplant. Post OLT, tacrolimus was given to obtain serum levels between 7 and 10 ng/mL for 180 d after OLT and levels between 5 and 8 ng/mL for the next 180 d. Cyclosporine was only used in cases of neurotoxicity because of tacrolimus. If cyclosporine was used, the serum level was between 250-350 lg/L after OLT with a maintenance cyclosporine level of 50-100 lg/L. Prednisone was administered after OLT and generally stopped within the first two months, except in autoimmune, primary biliary and primary sclerosing cholangitis cirrhosis. Mycophenolate mofetil was used in all patients, one gram/day, except in CMV infection.
mTORi has been used in liver grafts recipients with renal dysfunction, patients with tacrolimus and cyclosporine neurotoxicity, in high risk hepatocellular carcinoma (HCC) liver transplant patients to avoid its recurrence, and in patients with “de novo” neoplasia after OLT, adjusting the dosage to obtain levels between 5 and 8 ng/mL. After two to four weeks of double immunosuppressant treatment with tacrolimus, this is usually discontinued. mTORi use is stopped for an elective surgical procedure.
After hospital discharge, patients are visited and blood samples taken every week and after three/four months, patients are visited monthly for laboratory testing. One hundred and eighty days after OLT, visits were every 60 d.
Elevation in serum creatinine, blood pressure and blood sugar or the appearance of proteinuria were registered.
Patient information is prospectively registered in an SPSS electronic register on all OLT patients at our center. The database is available only for clinical studies. For this study, data were extracted on mTORi treated patients from this clinical register. Clinical and demographic information contained sex, age, donor age, cause of liver cirrhosis, graft quality, existence of HCC, OLT date, complications, cause of CNI treatment being converted to mTORi, retransplantation and presence of diabetes mellitus and/or hypertension before OLT. Biochemistry and hematological data included baseline plasma creatinine levels (just to conversion to mTORi), at 6, 12, 18 mo, and the last serum creatinine level while taking mTORi treatment. One independent investigator audited 10% of the results and found > 99% data congruity.
This analysis used means for parametric data and medians for non-parametric data. We used Fisher’s exact tests for comparisons of categorical variables. We analyzed non-normally distributed variables with Mann-Whitney U-tests and two-sided t-tests were used to compare normally distributed variables.
Linear regression was applied to examine the effect of mTORi exposure on the last serum creatinine at the end of follow-up. MTORi exposure was examined as a continuous and a dichotomous variable.
We applied only confounders which influenced the point estimate by ≥ 10% for adjusted models (19). We considered P value < 0.05 as significant; two-sided tests were used.
mTORi was started at a median 48 mo (DT: 56.8, range = 0-241) after OLT. Recipients were followed on mTORi for a median of 40.6 mo (DT: 18.0, range = 18-76). Reasons for switching to mTORi were avoiding HCC recurrence (n = 27), neurotoxicity because of tacrolimus (limb tremors, headaches, paresthesia) (n = 3), prevention of renal insufficiency (n = 28), acute rejection with tacrolimus/cyclosporine (n = 6), and “de novo” neoplasia (n = 22).
No mTORi patient developed serious adverse effects and there was no hepatic artery thrombosis. The clinical characteristics of the patients converted to mTORi are described in Table 1.
| Variable | DM (28) | HT (27) | Prot (27) | DM + HT + prot (8) | P-value |
| Age (yr) | 54.3 | 55.1 | 54.8 | 55.2 | 0.61 |
| Male | 21 | 23 | 22 | 7 | 0.43 |
| DM prior to OLT | 28 | 8 | 8 | 8 | 0.52 |
| Hypertension prior to OLT | 5 | 19 | 5 | 5 | 0.34 |
| Proteinuria prior to OLT | 7 | 6 | 19 | 6 | 0.42 |
| Etiology of liver disease | |||||
| Hepatitis C | 11 | 11 | 10 | 3 | 0.32 |
| Alcohol | 14 | 13 | 13 | 4 | 0.67 |
| Other | 3 | 3 | 4 | 1 | 0.56 |
| Hepatocellular carcinoma | 6 | 5 | 5 | 1 | 0.48 |
| Initial creatinine | 1.31 | 1.48 | 1.44 | 1.35 | 0.23 |
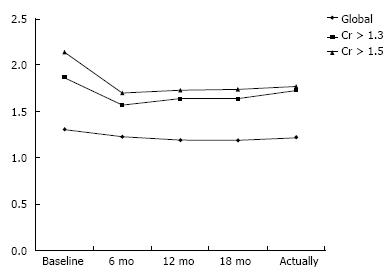
Initial plasma creatinine levels of patients at the moment of initiating mTORi treatment (median 48 mo after OLT) were 1.31 mg/dL. Creatinine was (mg/dL) 1.19, 1.19, 1.22 at 6, 12 and 18 mo and 1.23 mg/dL at the follow-up after the mTORi switch. There was an improvement between the initial and final creatinine levels while taking mTORi, but without statistical significance: 1.31 mg/dL and 1.22 mg/dL (P = 0.92), although this is a global analysis in all patients, converting because of renal dysfunction or for other reasons. We can observe the same low difference when we analyze converted patients with plasma creatinine levels > 1.3 mg/dL (1.87 mg/dL and 1.73 mg/dL, P = 0.78). Only patients converted because of renal dysfunction with plasma creatinine levels > 1.5 mg/dL show a statistically significant improvement of renal function, with initial levels of 2.14 and final ones of 1.77 mg/dL (P = 0.02) (Figure 1).
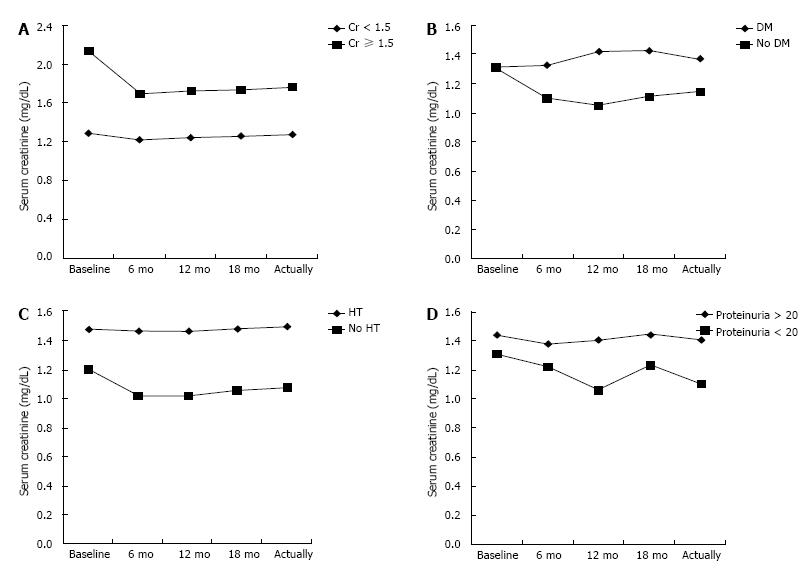
We next investigated whether the mTORi effect is less in recipients with diabetes mellitus and/or high blood pressure (HT) (Figure 2). Subgroup analysis of only those mTORi patients with DM shows no improvement of serum creatinine levels (1.31 mg/dL to 1.37 mg/dL) compared with non DM patients (1.31 mg/dL to 1.15 mg/dL) (P = 0.01) and it is the same when comparing HT patients (1.48 mg/dL to 1.5 mg/dL) with non HT patients (1.21 mg/dL to 1.08 mg/dL) and patients with proteinuria (1.44 mg/dL to 1.41 mg/dL) and no proteinuria (1.31 mg/dL to 1.11 mg/dL).
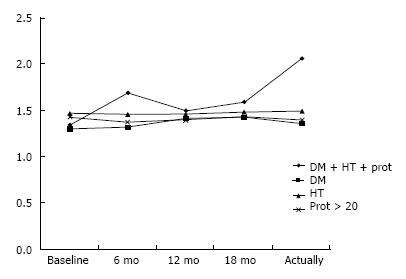
Finally, we considered patients with DM, HT and proteinuria (Figure 3). Although converting to mTORi, these patients have worsening renal function (1.35 mg/dL to 2.07 mg/dL) compared with patients when only one of these factors is present (P = 0.04).
Our study shows retrospectively that mTORi conversion resulted in an improvement in renal function in patients with plasma creatinine levels above 1.5 mg/dL. In patients with better renal function, conversion therapy involves no improvement. This improvement has been described in several published studies but none have shown that the worse the renal function, the greater the improvement after conversion[9,10,14-17].
MTORi was started a median of eight months after OLT for a variety of reasons. Plasma levels of creatinine at the start of the study were comparable in both mTORi and CNI cohorts. A personal history of risk factors for renal damage, such as diabetes mellitus and arterial hypertension, was comparable in both mTORi and CNI cohorts and considered in a multivariate model. Patients with hepatocellular carcinoma were adjusted in the mTORi cohort because treatment with chemotherapy may have affected serum creatinine. Despite this, it could be possible that some confounders are distributed unevenly in both groups. Further randomized trials may be necessary to avoid this problem.
Furthermore, we have segregated groups of patients with DM, hypertension and proteinuria and patients with all three diseases. We have seen how renal function does not improve after conversion to mTORi in these patients but it stops the progressive deterioration secondary to calcineurin inhibitors. However, in patients with DM, hypertension and proteinuria, renal function worsens despite conversion to mTORi.
Nephropathy is a major complication of type 1 and type 2 diabetes mellitus, along with CNI toxicity, end-stage renal dysfunction and hemodialysis[18]. Chronic nephropathy is also worsened by arterial hypertension. Diabetic nephropathy is first characterized by microalbuminuria and later by glomerular sclerosis. Podocytes play an important role in preventing proteinuria. Podocyte damage and reduction in the number of these cells contribute to the development of diabetic nephropathy[19]. mTOR plays a very important role in podocyte growth and size control. This molecule forms two different functional complexes, mTORC1 and mTORC2. Sirolimus and everolimus selectively inhibit mTORC1 but not mTORC2. In the first stages of diabetic damage in the kidney, an increased mTORC1 activity and podocyte hypertrophy can be observed. Moreover, there are some studies that report mTORi treatment to prevent diabetic nephropathy in animal models. Paradoxically, sirolimus and everolimus cause proteinuria and glomerular sclerosis in some patients[19,20]. In our study, we observed that these experimental findings are corroborated clinically in liver transplant patients with diabetic nephropathy.
There are no studies linking mTORi effectiveness in patients with hypertensive nephropathy. In our series, we showed how renal function, although not improved after conversion to mTORi, stabilizes after this change in immunosuppression regimen.
Proteinuria is a frequent side effect after switching from CNI to mTORi treatment in another solid transplant patient as a kidney graft recipient[21-26]. Wadei et al[21] shows that patients who developed massive proteinuria had a 3.3-fold increased risk of further renal insufficiency after mTORi conversion and proteinuria less than 1000 mg/d do not present with this association. This author indicates that a higher mTORi level after OLT diabetes and a lower eGFR at time of mTORi switching were observed with the appearance of very important urinary protein excretion after mTORi treatment. This study is in concordance with other articles that show a dose-dependent effect of mTORi on proteinuria and podocyte protein expression[12,27-31]. Higher proteinuria before mTORi treatment has also been correlated with massive proteinuria after switching.
Our results do not support these studies as we have shown that in patients with proteinuria, mTORi conversion leads to a stabilization of this proteinuria as well as serum levels of creatinine.
We recognize some limitations in this study. We do not routinely measure eGFR levels with Modification of Diet in Renal Disease or Cockcroft-Gault equations because this measurement is not very precise and not validated in OLT recipients.
In conclusion, we observed that, after OLT, switching from a CNI-based immunosuppression regimen to mTORi-based treatment improves renal function, when compared with recipients who did not switch, when creatinine levels are ≥ 1.5 mg/dL. In patients with diabetes or hypertensive nephropathy, conversion to mTORi does not improve renal function but stabilizes plasma levels of creatinine. Proteinuria is not a contraindication to conversion to mTORi, it also stabilizes renal function. Only patients with diabetes, hypertension and proteinuria should avoid conversion to mTORi because it worsens. Complete understanding of the effects of mTORi in liver transplant recipients derived from randomized, controlled trials will help better use of this immunosuppression regimen after OLT.
This study shows how mammalian target of rapamycin inhibitors (mTORi) based immunosuppression therapy in liver transplant recipients with diabetic and/or hypertensive renal dysfunction, even in patients with proteinuria, preserves renal function and plasma levels of creatinine.
MTORi based immunosuppression therapy in liver transplant patients and renal chronic disease.
Observational study in diabetic and hypertensive liver transplant patients and those with proteinuria.
This study helps to choose immunosuppression treatment in patients with renal dysfunction after liver transplant.
mTORi (mTOR inhibitors like sirolimus and everolimus, immunosuppression drugs for transplanted patients).
The manuscript observed the effect of mTORi-based immunosuppression therapy on diabetes mellitus, arterial hypertension and proteinuria for analysis of the potency of mTORi to renal function. This may be useful for clinical therapy.
| 1. | Post DJ, Douglas DD, Mulligan DC. Immunosuppression in liver transplantation. Liver Transpl. 2005;11:1307-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 381] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 3. | Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003;9:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Sehgal SN. Rapamune (Sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995;17:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 219] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3063] [Cited by in RCA: 3199] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 6. | Diekmann F, Waiser J, Fritsche L, Dragun D, Neumayer HH, Budde K. Conversion to rapamycin in renal allograft recipients with biopsy-proven calcineurin inhibitor-induced nephrotoxicity. Transplant Proc. 2001;33:3234-3235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Johnson RW, Kreis H, Oberbauer R, Brattström C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001;72:777-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 347] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, Lang P, Touraine JL, Claesson K, Campistol JM, Durand D. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999;67:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 668] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Kniepeiss D, Iberer F, Grasser B, Schaffellner S, Tscheliessnigg KH. Sirolimus in patients after liver transplantation. Transplant Proc. 2003;35:815-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Neff GW, Montalbano M, Slapak-Green G, Meyer D, Berney T, Safdar K, Schiff ER, Tzakis AG. Sirolimus therapy in orthotopic liver transplant recipients with calcineurin inhibitor related chronic renal insufficiency. Transplant Proc. 2003;35:3029-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Sanchez EQ, Martin AP, Ikegami T, Uemura T, Narasimhan G, Goldstein RM, Levy MF, Chinnakotla S, Dawson S, Randall HB. Sirolimus conversion after liver transplantation: improvement in measured glomerular filtration rate after 2 years. Transplant Proc. 2005;37:4416-4423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Campbell MS, Rai J, Kozin E, Bloom RD, Markmann JF, Olthoff KM, Shaked A, Rajender Reddy K. Effects of sirolimus vs. calcineurin inhibitors on renal dysfunction after orthotopic liver transplantation. Clin Transplant. 2007;21:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Neff GW, Montalbano M, Tzakis AG. Ten years of sirolimus therapy in orthotopic liver transplant recipients. Transplant Proc. 2003;35:209S-216S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Alamo JM, Bernal C, Marín LM, Suárez G, Serrano J, Barrera L, Sousa JM, Padillo FJ, Gómez-Bravo MA. Antitumor efficacy of mammalian target of rapamycin inhibitor therapy in liver transplant recipients with oncological disease: a case-control study. Transplant Proc. 2012;44:2089-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Alamo JM, Barrera L, Casado MD, Bernal C, Marin LM, Suarez G, Sanchez-Moreno L, Jimenez R, Suarez-Grau JM, Sousa JM. Efficacy, tolerance, and safety of mammalian target of rapamycin inhibitors as rescue immunosuppressants in liver transplantation. Transplant Proc. 2009;41:2181-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Mártinez JM, Pulido LB, Bellido CB, Usero DD, Aguilar LT, Moreno JL, Artacho GS, Díez-Canedo JS, Gómez LM, Bravo MA. Rescue immunosuppression with mammalian target of rapamycin inhibitor drugs in liver transplantation. Transplant Proc. 2010;42:641-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kushwaha SS, Khalpey Z, Frantz RP, Rodeheffer RJ, Clavell AL, Daly RC, McGregor CG, Edwards BS. Sirolimus in cardiac transplantation: use as a primary immunosuppressant in calcineurin inhibitor-induced nephrotoxicity. J Heart Lung Transplant. 2005;24:2129-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Rossing P, de Zeeuw D. Need for better diabetes treatment for improved renal outcome. Kidney Int Suppl. 2011;S28-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121:2181-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 20. | Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 475] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 21. | Wadei HM, Zaky ZS, Keaveny AP, Rosser B, Jones M, Mai ML, Bulatao I, Gonwa TA. Proteinuria following sirolimus conversion is associated with deterioration of kidney function in liver transplant recipients. Transplantation. 2012;93:1006-1012. [PubMed] |
| 22. | Diekmann F, Gutiérrez-Dalmau A, López S, Cofán F, Esforzado N, Ricart MJ, Rossich E, Saval N, Torregrosa JV, Oppenheimer F. Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: comparison of two CNI-free protocols. Nephrol Dial Transplant. 2007;22:2316-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Stephany BR, Augustine JJ, Krishnamurthi V, Goldfarb DA, Flechner SM, Braun WE, Hricik DE, Dennis VW, Poggio ED. Differences in proteinuria and graft function in de novo sirolimus-based vs. calcineurin inhibitor-based immunosuppression in live donor kidney transplantation. Transplantation. 2006;82:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Aliabadi AZ, Pohanka E, Seebacher G, Dunkler D, Kammerstätter D, Wolner E, Grimm M, Zuckermann AO. Development of proteinuria after switch to sirolimus-based immunosuppression in long-term cardiac transplant patients. Am J Transplant. 2008;8:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Morard I, Dumortier J, Spahr L, Hadengue A, Majno P, Morel P, Mentha G, Giostra E. Conversion to sirolimus-based immunosuppression in maintenance liver transplantation patients. Liver Transpl. 2007;13:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Thomusch O, Tittelbach-Helmrich D, Seifert G, Pisarski P. Late-onset proteinuria after antithymocyte globulin induction and de novo sirolimus monotherapy in kidney transplant recipients. Transplantation. 2011;92:e62-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Stallone G, Infante B, Pontrelli P, Gigante M, Montemurno E, Loverre A, Rossini M, Schena FP, Grandaliano G, Gesualdo L. Sirolimus and proteinuria in renal transplant patients: evidence for a dose-dependent effect on slit diaphragm-associated proteins. Transplantation. 2011;91:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Izzedine H, Brocheriou I, Frances C. Post-transplantation proteinuria and sirolimus. N Engl J Med. 2005;353:2088-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Sartelet H, Toupance O, Lorenzato M, Fadel F, Noel LH, Lagonotte E, Birembaut P, Chanard J, Rieu P. Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant. 2005;5:2441-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Bumbea V, Kamar N, Ribes D, Esposito L, Modesto A, Guitard J, Nasou G, Durand D, Rostaing L. Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant. 2005;20:2517-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Letavernier E, Bruneval P, Mandet C, Duong Van Huyen JP, Péraldi MN, Helal I, Noël LH, Legendre C. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol. 2007;2:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
P- Reviewer: He JY, Luo GH S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/