Published online Dec 24, 2014. doi: 10.5500/wjt.v4.i4.299
Revised: July 8, 2014
Accepted: October 31, 2014
Published online: December 24, 2014
Processing time: 214 Days and 22.3 Hours
AIM: To investigate the effects of 1400W-a selective inducible nitric oxide synthase (iNOS) inhibitor in a model of donation after circulatory death (DCD) kidneys.
METHODS: Porcine kidneys were retrieved after 25 min warm ischemia. They were then stored on ice for 18 h before being reperfused ex vivo with oxygenated autologous blood on an isolated organ perfusion system. The selective iNOS inhibitor 1400W (10 mg/kg) was administered before reperfusion (n = 6) vs control group (n = 7). Creatinine (1000 μmol/L) was added to the system, renal and tubular cell function and the level of ischemia reperfusion injury were assessed over 3 h of reperfusion using plasma, urine and tissue samples.
RESULTS: Kidneys treated with 1400W had a higher level of creatinine clearance (CrCl) [area under the curve (AUC) CrCl: 2.37 ± 0.97 mL/min per 100 g vs 0.96 ± 0.32 mL/min per 100 g, P = 0.004] and urine output [Total: 320 ± 96 mL vs 156 ± 82 mL, P = 0.008]. There was no significant difference in levels of fractional excretion of sodium (AUC, Fr ex Na+: Control, 186.3% ± 81.7%.h vs 1400W, 153.4% ± 12.1%.h, P = 0.429). Levels of total protein creatinine ratio were significantly lower in the 1400W group after 1 h of reperfusion (1h Pr/Cr: 1400W 9068 ± 6910 mg/L/mmol/L vs Control 21586 ± 5464 mg/L/mmol/L, P = 0.026). Levels of 8-isoprostane were significantly lower in the 1400W group [8-iso/creatinine ratio: Control 239 ± 136 pg/L/mmol/L vs 1400W 139 ± 47 pg/L/mmol/L, P = 0.041].
CONCLUSION: This study demonstrated that 1400W reduced ischaemia reperfusion injury in this porcine kidney model of DCD donor. Kidneys had improved renal function and reduced oxidative stress.
Core tip: It is important to examine the effects of therapies that can reduce ischemia reperfusion injury particularly in donation after circulatory death donor kidneys. The biological role of inducible nitric oxide synthase (iNOS) is somewhat controversial. This study uses a large animal ex vivo model to assess the effects of 1400W, an iNOS inhibitor. The model provides a functional assessment of each kidney, providing a close simulation to clinical transplantation. The study found that 1400W improved early renal function and reduced oxidative stress.
-
Citation: Hosgood SA, Yates PJ, Nicholson ML. 1400W reduces ischemia reperfusion injury in an
ex-vivo porcine model of the donation after circulatory death kidney donor. World J Transplant 2014; 4(4): 299-305 - URL: https://www.wjgnet.com/2220-3230/full/v4/i4/299.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i4.299
The pathophysiology of ischemia reperfusion (I/R) injury is a complex action involving many intercellular and molecular processes. It is characterised by the up-regulation of inflammatory processes, activation of endothelial cells, generation and release of reactive oxygen species (ROS), migration of inflammatory leucocytes, cellular oedema, cell membrane damage, apoptosis and necrosis[1-3]. Severe I/R injury causes significant disruption to the microcirculation and is associated with high rates of delayed graft function, primary non function and acute rejection after kidney transplantation[4,5]. This is of particular significance in kidneys from marginal or donation after circulatory death (DCD) donors that sustain both a period of warm and cold ischemic injury prior to transplantation. It is therefore important to investigate therapies to alleviate injury to improve the outcome of DCD transplantation.
Nitric oxide (NO) is an important mediator of normal biological processes. It is a free radical produced by all mammalian cells from the synthesis of L-arginine and oxygen, by the enzyme NO synthase (NOS)[6]. It is capable of regulating local blood flow, scavenging free radicals and inhibiting platelet and leukocyte activation[6,7]. There are three different isoforms of NO; neuronal, endothelial (eNOS) and inducible (iNOS)[8].
The biological role of iNOS is somewhat controversial[9]. iNOS is known to be up-regulated by certain disease states such as inflammation, ischemia and during reperfusion after transplantation[10]. Although NO is generally regarded as cytoprotective, excess NO derived from iNOS during these states can contribute to the injury process[11,12]. NO can augment I/R injury by reacting with superoxide generated by excess ROS to form peroxynitrite, causing severe oxidative damage ands cellular injury[10]. It also has a role in the mediation of neutrophil activation, although the processes are not fully understood[9].
Evidence suggests that the effects and role of iNOS are influenced by the microenvironment and bioavailability of the other forms of NO[9] and iNOS inhibitors have been shown to reduce I/R injury[11-13]. However, these have principally been studied in small animal models after a sole period of warm ischemic injury and reperfusion. The aim of this study was to assess the effects of 1400W a selective iNOS inhibitor on I/R injury in a model of the DCD donor using porcine kidneys.
Under Home Office regulations (Scientific Act 1986, Schedule 1 procedure) female large white pigs (60-70 kg) were killed by electrocution followed by exsanguination. Approximately 2 L of blood was collected into a sterile receptacle containing 25000 units of heparin (Multiparin®; CP Pharmaceuticals, Wrexham, United Kingdom). The blood was then transferred into CPDA-1 blood bags (Baxter Healthcare, Thetford, United Kingdom) for storage at 4 °C.
The kidneys were retrieved after 25 min of in situ warm ischemia and flushed with 500 mL of hyperosmolar citrate (Soltran; Baxter Healthcare) at 4 °C infused at a hydrostatic pressure of 100 cm H2O. Kidneys were then placed in ice for a period of 18 h.
After the preservation period kidneys were prepared for ex vivo reperfusion. The renal artery, vein and ureter were cannulated and kidneys flushed with Ringer’s at 4 °C (Baxter Healthcare, United Kingdom) to remove the preservation solution before being placed immediately on the isolated organ preservation system. They were then reperfused with oxygenated autologous blood for 3 h at a temperature of 38 °C and set mean arterial pressure of 85 mmHg. The system has been previously described[14]. Creatinine (Sigma-Aldrich, Steinheim, Germany) was added to the perfusate to achieve an initial circulating concentration of 1000 µmol/L.
1400W (Sigma-Aldrich) -a highly selective iNOS inhibitor was prepared before use and stored at -20 °C until required.
Kidneys were divided into two groups; Control (n = 7) and 1400W at a dose of 10 mg/kg per kidney weight (n = 6). 1400W was added as a bolus to the arterial arm of the circuit 15 min before reperfusion of the kidney.
Renal blood flow (RBF) and mean arterial pressure (MAP) were recorded continuously and intrarenal resistance (IRR) calculated (MAP/RBF). Urine output was also measured during reperfusion.
Biochemical analysis of serum and urine samples was carried out at hourly intervals. The following parameters were calculated:
Creatinine clearance (urinary creatinine × urinary volume/plasma creatinine), fractional excretion of sodium [(urinary sodium × urine volume) / (glomerular filtration rate × plasma sodium) × 100)] and the urinary total protein (mg/L) to creatinine (mmol/L) ratio.
Blood gas analysis was used to record PaO2, PvO2 and acid-base homeostasis. Oxygen consumption [(PaO2-venous PvO2) × flow rate/weight] was calculated
Urine samples were taken at 1 and 3 h of reperfusion and stored at -80 °C until analyses. Levels of urine 8-isoprostane were determined by ELISA (Cayman Chemical Co, MI, United States). Urine samples were centrifuged at 10000 g for 2 min and the supernatant taken for analysis. Samples were diluted 10 fold prior to analysis. The sample and standards were added in duplicate to the ELISA plate together with an 8-isoprostane-acetylcholinesterase (AChE) conjugate and incubated for 18 h at 4 °C. During incubation 8-isoprostane present in the sample competed with the 8-isoprostane AChE conjugate for the 8-isoprostane rabbit antiserum binding sites on the pre-coated plate. The plate was then washed and developed by the addition of the substrate to AChE. The plate was read at 405 nm after colour development for 90 min.
Plasma samples were taken pre and 3 h after reperfusion and urine samples taken at 1 and 3 h of reperfusion and stored at -80 °C until analyses. Urine levels of NO were quantified using the total NO test kit (Assay Designs, MI, United States) according to the manufacturers’ instructions. This assay is based on the conversion of NO to nitrate and the subsequent conversion of nitrate to nitrite by the enzyme nitrate reductase. Nitrite is then detected colorimetrically at 540 nm as an azodye product of the Griess reaction. Briefly, plasma and urine sample were centrifuged at 10000 g and the supernatant withdrawn. Fifty µL of each sample were added in duplicate to a micro titre test plate. Twenty-five µL NADH and 25 µL nitrate reductase were added to each well and incubated at 37 °C for 30 min. One hundred µL Griess reagents (sulphanilamide and N-(l-Naphthyl) ethylenediamine in 2M HCl) were then added and incubated at room temperature for 10 min. Optical density was then read at 540 nm using a spectrophotometer and the concentration calculated using standards.
Wedge biopsies were taken after 25 min warm ischemia and after 3 h of reperfusion, fixed in 10% formal saline, dehydrated and embedded in paraffin wax. Sections of 4 µm were cut and stained with haematoxylin and eosin for evaluation using light microscopy. Sections were scored over five fields, assessing changes in four morphological variables; Tubular dilation, Tubular debris, vaculolation and interstitial oedema. Samples were scored from 0 to 3 according to the level of damage; 0 representing normal, 1 representing mild, 2 representing moderate and 3 representing severe morphological changes.
Immunohistochemical staining of MPO, a marker mainly for neutrophil granulocytes, was undertaken on post reperfusion paraffin sections using a DAKO ChemMate EnVision™ Detection Kit (DAKO, Glostrup, Denmark). The sections were digested by 40 µg/mL proteinase K for 15 min at 37 °C then blocked by peroxidase-blocking reagent. The sections were labelled by an anti-MPO antibody (1:600, DAKO) at 4 °C overnight. The antibody binding was revealed by 3’-amino-9-ethylcarbazole. MPO+ cells in the tubular, interstitial and glomeruli were semi-quantitatively scored by counting the number of positive cells in 20 fields at 400 × magnification.
Values are presented as mean ± SD. Levels of continuous variables such as RBF were plotted against time and the area under the curve (AUC) for individual perfusion experiments was calculated using Excel® software (Microsoft, Reading, United Kingdom) and Graphpad Prism (GraphPad Software, San Diego California, United States).
Mean AUC values were compared using Mann Whitney U-Test (GraphPad InStat version 3.00 for Windows 95, GraphPad Software, San Diego California, United States). Correlations between parameters were made with Spearman’s non parametric rank correlation. P < 0.050 was taken as statistically significant.
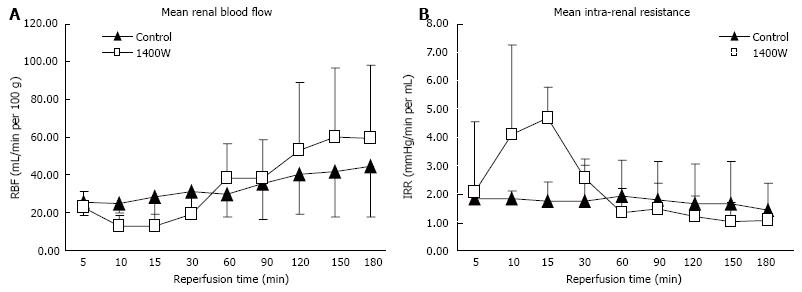
There was a significant fall in the level of RBF and an increase in intra-renal resistance in the 1400W group after 10 and 15 min of reperfusion compared to the control kidneys (RBF, P = 0.002 and 0.005, respectively; IRR, P = 0.005 and 0.014, respectively; Figure 1A and B). RBF then recovered and IRR fell with no significant difference between the groups throughout the rest of the reperfusion period (AUC, RBF: Control 270 ± 86 mL/min/100 g.h vs 1400W 274 ± 143 mL/min/100 g.h, P = 0.999; IRR: Control 13.4 ± 7.3 mmHg/min.h vs 1400W 17.8 ± 8.5 mmHg/min.h, P = 0.234). The level of oxygen consumption after reperfusion was higher in the 1400W group after 3 h of reperfusion but this did not reach statistical significance (3 h: Control 28.0 ± 13.9 mL/min/g vs 1400W 36.7 ± 22.8 mL/min/g, P = 0.731).
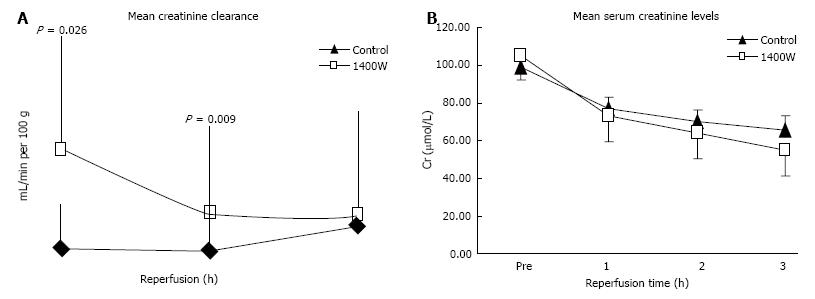
Levels of creatinine clearance were significantly higher after 1 and 2 h of reperfusion in the 1400W group compared to the control (P = 0.026 and 0.009 respectively; Figure 2A) and the AUC creatinine clearance was significantly higher (AUC, CrCl: 1400W 2.37 ± 0.97 mL/min/100 g.h vs Control 0.96 ± 0.32 mL/min/100 g.h, P = 0.004). Levels of serum creatinine fell more quickly in the 1400W group but the difference with controls was only marginally significant at the end of reperfusion (P = 0.073; Figure 2B).
There was no significant difference in levels of fractional excretion of sodium (AUC, Fr ex Na+: Control 186. 3% ± 81.7%.h vs 1400W 153.4% ± 12.1%.h, P = 0.429), although total urine output was significantly higher in the 1400W group (Total urine output: 1400W 320 ± 96 vs Control, 156 ± 83 mL, P = 0.008).
Levels of total protein creatinine ratio were significantly lower in the 1400W group after 1 h of reperfusion (1h Pr/Cr: 1400W 9068 ± 6910 mg/L/mmol/L vs Control 21586 ± 5464 mg/L/mmol/L, P = 0.026). There was no further difference in the levels between the groups after 2 and 3 h of reperfusion (P = 0.662 and 0.628, respectively).
Levels of pH fell significantly in both groups with no significant difference between groups at 3 h (P = 0.100; Table 1). There was also no significant difference in levels of bicarbonate or potassium after 3 h (P = 0.628 and 0.295, respectively; Table 1). Pre levels of potassium were significantly lower but within normal range in the control group compared to 1400W (P = 0.002; Table 1).
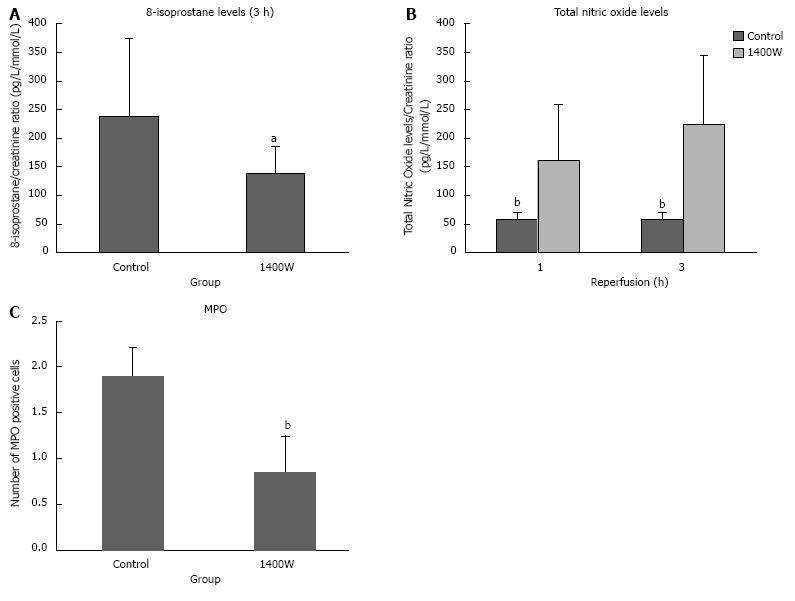
Urinary levels of 8-isoprostane were significantly lower in the 1400W group after 3 h of reperfusion compared to the control group (P = 0.041; Figure 3A).
There was no significant difference in the pre or 3 h reperfusion plasma concentrations of total NO (Pre: Control 73.6 ± 43.1 pg/mL, 1400W 79.9 ± 14.5 pg/mL; 3h: Control 48.7 ± 21.7 pg/mL, 1400W 63.9 ± 20.2 pg/mL). Urinary levels of total nitric oxide were significantly higher in the 1400W group after 1 and 3 h of reperfusion (P = 0.002 and 0.002, respectively; Figure 3B).
There was a significantly higher amount of MPO positive cells in the control group compared to the 1400W (P = 0.002; Figure 3C). Positive cells were largely localised in the interstitium.
Baseline biopsies showed an increased level of tubular dilatation in the 1400W group compared to the control (P = 0.001; Table 2) and a higher level of interstitial oedema in the control group compared to the 1400W (P = 0.032; Table 2). After 3 h of reperfusion there was a significant increase in tubular dilatation and vacuolation in the control group (P = 0.0003 and 0.033, respectively; Table 2) and tubular debris, vacuolation and interstitial oedema in the 1400W group (P = 0.003, 0.040 and 0.011, respectively; Table 2). The 1400W group had a significantly higher level of tubular debris after reperfusion compared to the control (P = 0.0001; Table 2).
This study demonstrated that the administration of 1400W, a selective iNOS inhibitor, reduced the level I/R injury in porcine kidneys that were subjected to warm and cold ischemic injury. Kidneys had a higher level of creatinine clearance, reduced oxidative stress and neutrophil infiltration during reperfusion compared to untreated kidneys.
NO is generally regarded as cytoprotective: scavenging free radicals, relaxing the endothelium, inhibiting platelet aggregation and reducing neutrophil adherence[6,15]. However, the biological effects of NO derived from iNOS can be either deleterious or beneficial, depending on the disease state[9]. iNOS is known to be upregulated during ischemia and reperfusion and is widely expressed throughout the vasculature, tubule cells and glomeruli in the kidney. It is also expressed on monocytes, macrophages and neutrophils[16].
Warm and cold ischemic injury sustained before transplantation exacerbates the level of I/R injury[4,14]. The anoxic conditions, depletion of adenosine triphosphate (ATP) and accumulation of toxic substances results in severe cellular disruption[5]. The level of warm and cold ischemic injury in this porcine kidney model of the DCD donor was sufficient to cause severe renal dysfunction, alteration of acid base homeostasis and histological change during reperfusion. Kidneys treated with 1400W showed some ameliorate of injury with higher levels of creatinine clearance, urine output and reduced levels of protein excretion and oxidative stress compared to untreated kidneys. However, iNOS inhibition did not improve tubular cell function, acid base balance or reduce the level of histological injury.
1400W is a selective inhibitor of iNOS. It is relatively long acting and has been used successfully in several rat I/R injury models to reduce injury[13,17]. Mark et al[17] found that 1400W administered 20 min before ischemia, improved renal function and reduced the level of tubular dysfunction. Another study compared the effects of 1400W and melatonin: an antioxidant, iNOS inhibitor and scavenger of peroxynitrite[13]. They found that both agents reduced the level of oxidative damage, albeit melatonin to a greater extent due to its scavenging properties. Other selective iNOS inhibitors such as, L-N6-(L-iminoethyl) lysine (L-NIL)[16] and the novel iNOS inhibitor GW274150 have also been used to improved glomerular and tubular function and reduce levels of NO in rat models of I/R injury[12] and FR260330 in Vervet monkeys[18].
A key role of NO is the modulation of blood flow and NO derived from eNOS is thought to be particularly important during early reperfusion[6-8]. In this present study there was a marked reduction in renal blood flow and increase in intra-renal resistance during the first 15 min of reperfusion with iNOS inhibition. This warrants further investigation but was possibly due to low levels of NO derived from eNOS during the early reperfusion phase as a result of the level of ischemic injury and inhibition of iNOS. This suggests an important role for iNOS in the control of homeostasis during this acute phase.
The activation of neutrophils during reperfusion is a principle mediator of I/R injury causing microcirculatory disruption and release of superoxide[19]. NO can inhibit the expression of P-selectin on endothelial cells, preventing rolling, and expression of intercellular and vascular cell adhesion molecules-1 (ICAM-1, VCAM-1) reducing neutrophil adhesion and infiltration[11,17]. NO derived from iNOS is thought to enhance endothelial-leukocyte activation and inhibitors have demonstrated a reduction in neutrophil activation[12]. Contrary to this, in a model of endotoxic shock, NO released by cNOS and iNOS reduced neutrophil migration due to decreased rolling and adhesion[19]. Levels of neutrophil infiltration were reduced by almost half after iNOS inhibition in this present study. Hickey et al[9] suggested that the role of iNOS varies according to the cell type and location in which it is expressed, and that leukocyte recruitment could alter according to the type of inflammatory response. Evidence from this study supports the findings of others that iNOS inhibition prevents neutrophil infiltration during I/R injury, although the exact mechanisms are still to be elucidated. Nonetheless, the activation of neutrophils has also an important role in regeneration and repair and it is likely that a balance is needed to ensure optimal graft function[20].
Plasma concentrations of total NO were not affected by iNOS inhibition in this study and perhaps real time analysis of NO or the measurement of eNOS and iNOS expression may have provided more information on the significance and bioavailability of NO in this model. Urinary levels of total NO were however, significantly increased during reperfusion after iNOS inhibition possibly indicating a higher level of proximal tubular cell injury. Nonetheless, high levels were not associated with tubular cell dysfunction. Urinary levels of 8-isoprostane, a marker of lipid peroxidation, generated by free radical catalyzed attack on arachidonic acid, were significantly lower after the administration of 1400W[21]. Lower levels of lipid peroxidation suggest less oxidative damage and formation of peroxynitrite during reperfusion possibly due to less neutrophil infiltration.
In conclusion, the administration of 1400W a selective inhibitor of iNOS improved renal function, reduced oxidative stress and neutrophil infiltration in this porcine kidney model of the DCD. This study supports the evidence of the deleterious effects of iNOS during I/R injury.
The shortage of organ donors has led to increasing use of marginal donors. Although a valuable source of kidneys for transplantation these kidneys have more injury and a high percentage do not function immediately after transplantation. This injury is in part, mediated by an inflammatory action immediately after transplantation: ischaemia reperfusion injury. Targeting this inflammatory process by using therapies may improve early graft function. Despite an abundance of research into such therapeutic agents, none are used clinically as part of standard practice.
Inducible nitric oxide synthase (iNOS) is produced naturally by the body and thought to play a role in the injury process after transplantation. 1400W is an iNOS inhibitor that has been shown to reduce injury and improve graft function. However, the research hotspot is that it has not been trialed in a clinically relevant model such as the porcine kidney with similar ischaemic insults that human kidneys are subject to.
iNOS inhibitors such as 1400W have previously been used to reduce injury and improve renal function. However, some studies have found no benefit in inhibiting iNOS. Furthermore, most of these studies have used small animal models which do not necessarily represent the effect in humans. In this present study the authors used a porcine model with similar periods of ischaemic injury to assess the effects of 1400W. Porcine kidneys have similar anatomy to human kidneys and their physiological response to ischaemic injury is also comparable. The authors found that 1400W significantly reduced the injury processes and improved renal function. This suggests that iNOS plays an important role in the injury process after transplantation.
This study suggests that iNOS inhibitors are a potential therapy for reducing renal ischaemia reperfusion injury after transplantation.
Ischaemia reperfusion injury is a natural inflammatory like reaction that a transplanted organ suffers. It involves a cascade of events that can cause irreversible cellular damage. This can reduce renal function and also limit graft survival. Nitric oxide synthase (NOS) is a gaseous molecule that is produced naturally in the body. There are three different forms of NOS. Generally it has a protective role however iNOS is associated with inflammatory disease states.
Ischaemia reperfusion injury is a critical problem in the transplant field. This study reported that 1400W reduced ischaemia reperfusion injury in a porcine model of the donation after circulatory death donor. This paper is well written and the results of renal function, oxidative stress and histology in 1400W reveal the protection from I/R injury.
| 1. | Ponticelli C. Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014;29:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 2. | de Vries DK, Lindeman JH, Tsikas D, de Heer E, Roos A, de Fijter JW, Baranski AG, van Pelt J, Schaapherder AF. Early renal ischemia-reperfusion injury in humans is dominated by IL-6 release from the allograft. Am J Transplant. 2009;9:1574-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738-747. [PubMed] |
| 4. | Gok MA, Shenton BK, Pelsers M, Whitwood A, Mantle D, Cornell C, Peaston R, Rix D, Jaques BC, Soomro NA. Ischemia-reperfusion injury in cadaveric nonheart beating, cadaveric heart beating and live donor renal transplants. J Urol. 2006;175:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 770] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Vos IH, Joles JA, Rabelink TJ. The role of nitric oxide in renal transplantation. Semin Nephrol. 2004;24:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Yates PJ, Hosgood SA, Nicholson ML. A biphasic response to nitric oxide donation in an ex vivo model of donation after cardiac death renal transplantation. J Surg Res. 2012;175:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Joubert J, Malan SF. Novel nitric oxide synthase inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:537-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Hickey MJ, Granger DN, Kubes P. Inducible nitric oxide synthase (iNOS) and regulation of leucocyte/endothelial cell interactions: studies in iNOS-deficient mice. Acta Physiol Scand. 2001;173:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Joles JA, Vos IH, Gröne HJ, Rabelink TJ. Inducible nitric oxide synthase in renal transplantation. Kidney Int. 2002;61:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Korkmaz A, Kolankaya D. Inhibiting inducible nitric oxide synthase with rutin reduces renal ischemia/reperfusion injury. Can J Surg. 2013;56:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Chatterjee PK, Patel NS, Sivarajah A, Kvale EO, Dugo L, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Britti D. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63:853-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Ersoz N, Guven A, Cayci T, Uysal B, Turk E, Oztas E, Akgul EO, Korkmaz A, Cetiner S. Comparison of the efficacy of melatonin and 1400W on renal ischemia/reperfusion injury: a role for inhibiting iNOS. Ren Fail. 2009;31:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Hosgood SA, Bagul A, Yang B, Nicholson ML. The relative effects of warm and cold ischemic injury in an experimental model of nonheartbeating donor kidneys. Transplantation. 2008;85:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Lo Faro ML, Fox B, Whatmore JL, Winyard PG, Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Legrand M, Almac E, Mik EG, Johannes T, Kandil A, Bezemer R, Payen D, Ince C. L-NIL prevents renal microvascular hypoxia and increase of renal oxygen consumption after ischemia-reperfusion in rats. Am J Physiol Renal Physiol. 2009;296:F1109-F1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Mark LA, Robinson AV, Schulak JA. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. J Surg Res. 2005;129:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Qi S, Xu D, Ma A, Zhang X, Chida N, Sudo Y, Tamura K, Daloze P, Chen H. Effect of a novel inducible nitric oxide synthase inhibitor, FR260330, in prevention of renal ischemia/reperfusion injury in vervet monkeys. Transplantation. 2006;81:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Dal Secco D, Paron JA, de Oliveira SH, Ferreira SH, Silva JS, Cunha Fde Q. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide. 2003;9:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, De Broe ME. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 283] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Waller HL, Harper SJ, Hosgood SA, Bagul A, Yang B, Kay MD, Kaushik M, Nicholson ML. Biomarkers of oxidative damage to predict ischaemia-reperfusion injury in an isolated organ perfusion model of the transplanted kidney. Free Radic Res. 2006;40:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
P- Reviewer: Tomohide H S- Editor: Yu J L- Editor: A E- Editor: Lu YJ