Published online Sep 24, 2014. doi: 10.5500/wjt.v4.i3.188
Revised: June 5, 2014
Accepted: July 25, 2014
Published online: September 24, 2014
Processing time: 213 Days and 17.6 Hours
AIM: To analyze the impact of steroid maintenance on the outcomes in kidney transplant recipients stratified by induction agent received.
METHODS: Patients who underwent first-time deceased donor kidney transplantation between 2000 and 2008 after receiving induction therapy with rabbit-antithymocyte globulin (r-ATG), alemtuzumab or an interleukin-2 receptor blocker (IL-2B) and discharged on a calcineurin inhibitor (CNI)/mycophenolate mofetil (MMF)-regimen along with or without steroids were identified from the Organ Procurement and Transplant Network/United Network of Organ Sharing database. For each induction type, adjusted overall and death-censored graft as well as patient survivals were compared between patients discharged on steroid vs no steroid. Among r-ATG induced patients, analysis was repeated after splitting the group into low and high immune risk groups.
RESULTS: Among the 37217 patients included in the analysis, 17863 received r-ATG (steroid = 13001, no-steroid = 4862), 3028 alemtuzumab (steroid = 852, no-steroid = 2176) and 16326 IL-2B (steroid = 15008, no-steroid = 1318). Adjusted overall graft survival was inferior (HR = 1.16, 95%CI: 1.06-1.27, P = 0.002) with similar death-censored graft survival (HR = 0.99, 95%CI: 0.86-1.14, P = 0.86) for steroid vs no-steroid groups in r-ATG induced patients. Both adjusted overall and death-censored graft survivals for steroid vs no-steroid groups were similar in alemtuzumab (HR = 0.92, 95%CI: 0.73-1.15, P = 0.47 and HR = 0.87, 95%CI: 0.62-1.22, P = 0.43 respectively) and IL-2B (HR = 1.05, 95%CI: 0.91-1.21, P = 0.48 and HR = 0.94, 95%CI: 0.75-1.18, P = 0.60 respectively) induced groups. Adjusted patient survivals were inferior for steroid vs no-steroid groups in r-ATG induced (HR = 1.31, 95%CI: 1.15-1.49, P < 0.001) but similar in alemtuzumab (HR = 1.02, 95%CI: 0.75-1.38, P = 0.92) and IL-2B (HR = 1.17, 95%CI: 0.97-1.40, P = 0.10) induced patients. Among the r-ATG induced group there were 4346 patients in the low immune risk and 13517 patients in the high immune risk group. Adjusted overall graft survivals were inferior for steroid vs no steroid groups in both low immune (HR = 1.34, 95%CI: 1.09-1.64, P = 0.001) and high immune (HR = 1.18, 95%CI: 1.07-1.30, P = 0.005) risk groups. Adjusted death-censored graft survivals for steroid vs no steroid groups were similar in both low (HR = 1.06, 95%CI: 0.78-1.45, P = 0.70) and high (HR = 1.04, 95%CI: 0.98-1.20, P = 0.60) immune risk groups. Adjusted patient survivals were inferior for steroid vs no steroid groups in both low immune (HR = 1.54, 95%CI: 1.18-2.02, P < 0.001) and high immune (HR = 1.32, 95%CI: 1.16-1.51, P = 0.002) risk groups. Overall, there were significantly higher deaths from infections and cardiovascular causes in patients maintained on steroids.
CONCLUSION: Our study showed an association between steroid addition to a CNI/MMF-maintenance regimen and increased death with functioning graft in patients receiving r-ATG induction for first-time deceased donor kidney transplantation.
Core tip: This study critically looked at outcomes in a large recent cohort of first time deceased donor kidney transplant recipients from the Organ Procurement and Transplant Network/United Network of Organ Sharing database to assess the impact of triple maintenance immunosuppression after receiving various induction therapies. In multivariate analysis, we found an increased risk for death with functioning graft when steroid maintenance was added to calcineurin inhibitor/mycophenolate mofetil based regimen in patients who received powerful induction with rabbit-antithymocyte globulin (r-ATG), an effect that persisted even when patients were split into high and low immune risk groups. Based on these finding we feel that, one has to be cautious while maintaining intense immunosuppression by adding steroid to a calcineurin inhibitor/mycophenolate mofetil regimen in kidney transplant recipients who were selected for r-ATG induction.
- Citation: Sureshkumar KK, Hussain SM, Thai NL, Ko TY, Nashar K, Marcus RJ. Impact of steroid maintenance on the outcomes in first-time deceased donor kidney transplant recipients: Analysis by induction type. World J Transplant 2014; 4(3): 188-195
- URL: https://www.wjgnet.com/2220-3230/full/v4/i3/188.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i3.188
Corticosteroid has been an integral part of maintenance immunosuppression from the dawn of clinical kidney transplantation. Chronic steroid use can contribute to worsening of hypertension as well as dyslipidemia, and development of new onset diabetes mellitus, all risk factors for cardiovascular (CV) disease. Steroid therapy can also increase susceptibility to infections and accelerates bone loss. In one survey, when kidney transplant recipients were asked which drug they would like to discontinue, majority chose prednisone[1]. Attempts to develop steroid- free immunosuppression for kidney transplant recipients started nearly three decades ago but the enthusiasm waned when results of studies including the Canadian multicenter randomized clinical trial showed increased risk for acute rejection (AR) and graft loss associated with steroid withdrawal[2-4]. The availability of more potent maintenance immunosuppressive agents such as tacrolimus and mycophenolate mofetil (MMF) as well as induction agents like rabbit-antithymocyte globulin (r-ATG) resulted in a resurgence of the interest in early steroid withdrawal during the last decade. For instance, a recent registry analysis showed that the percentage of kidney transplant recipients discharged following the initial transplant admission on a steroid-free maintenance immunosuppression increased from 3.7% in the year 2000 to 32.5% as of 2006[5].
Perioperative induction therapy was utilized in greater than 70% of kidney transplant recipients in the United States during the last decade[6]. Calcineurin inhibitor (CNI)/MMF with or without steroid was the most commonly employed maintenance immunosuppression over the same time period. One could speculate that the degree of immunosuppression rendered by triple immunosuppression following powerful induction might be excessive in some patients with the possibility of adverse outcomes. The risk-benefit evaluation of steroid maintenance in kidney transplant recipients who receive powerful induction with agents such as r-ATG and potent maintenance immunosuppression with CNI/MMF would be beneficial. We aimed to compare the outcomes for steroid vs no steroid addition in patients who underwent a first deceased donor kidney (DDK) transplantation after receiving different induction agents and maintained on CNI/MMF based regimen.
The study was performed in accordance with the ethical standards laid down by the Declaration of Helsinki as well as Declaration of Istanbul and was approved by the Institutional Review Board. Patients ≥ 18 years of age who underwent a first-time DDK transplantation between January 1, 2000 and December 31, 2008 after receiving induction therapy with r-ATG, alemtuzumab or an interleukin-2 receptor blocker (IL-2B) agent (basiliximab or daclizumab) and discharged on a CNI/MMF based maintenance immunosuppression regimen with or without steroids were identified from the Organ Procurement and Transplant Network/United Network of Organ Sharing database. A DDK transplant was considered as a first transplant if there was no history of previous kidney transplant for the patient in the database. For each induction type, patients were divided into two groups: those who underwent early steroid withdrawal before the hospital discharge categorized as early steroid withdrawal (ESW) group and those who were discharged on steroid maintenance. The latter group was designated as chronic steroid maintenance (CSM) group. This is an intention to treat analysis using the maintenance immunosuppression regimen at the time of discharge from the initial transplant hospitalization as the basis for defining the groups. Changes in maintenance immunosuppression that occurred after initial discharge were not used to classify study subjects. Patients were excluded from the analysis if they underwent live donor kidney or multi-organ transplants or received no induction, more than one induction or a different induction agent.
Demographic data for patients who received different induction agents were collected. Graft was considered failed when one of the following occurred: need for maintenance dialysis, re-transplantation or patient death. Over all and death-censored graft as well as patient survivals were compared between ESW and CSM groups for each induction type after adjusting for pre-specified variables. An adjusted model was used in the analysis to account for substantial variations in the demographic features for ESW vs CSM in each induction type. Co-variates that can adversely impact graft outcome included in the model were donor related factors: age, gender, expanded criteria donor (ECD) kidney, donation after cardiac death (DCD) kidney, death from cerebrovascular accident(CVA); recipient related factors: age, African American race, diabetes mellitus, dialysis duration, peak panel reactive antibody (PRA) titer, number of human leukocyte antigen (HLA) mismatches; and transplant related factors: cold ischemia time (CIT), delayed graft function (DGF, defined as the need for dialysis within the first week after transplantation), 12 mo AR, and transplant year. The type of CNI agent used was not included in the model since most patients were discharged on tacrolimus. A further analysis was performed to compare the overall and death-censored graft as well as patient survivals between ESW and CSM groups for the r-ATG induced patients by splitting them into high and low immune risk groups. Patients were considered high immune risk if they met any of the following: African American recipient, peak PRA > 20%, CIT > 24 h, ECD kidney recipient, DCD kidney recipient, or developed DGF.
Group comparisons were done utilizing 2-tailed t-test for continuous variables and χ2 test for categorical variables. Mean ± SD, median with range or percentage was used to express values. For the purpose of analysis, the risk factors were considered absent when data for them were missing. About 20%-25% of the data were missing for the variable “treated acute rejection” but for the remainder of the variables used in the analysis, only fewer than 2% of the data were missing. Adjusted (multivariate, after correcting for the confounding variables listed above) over all and death-censored graft as well as patient survivals were calculated and were compared between CSM vs ESW groups within each induction type using a Cox regression model. This was done for the whole group, as well as high and low immune risk groups (defined above) among the r-ATG induced patients. HR and 95%CI were calculated. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software version 14.
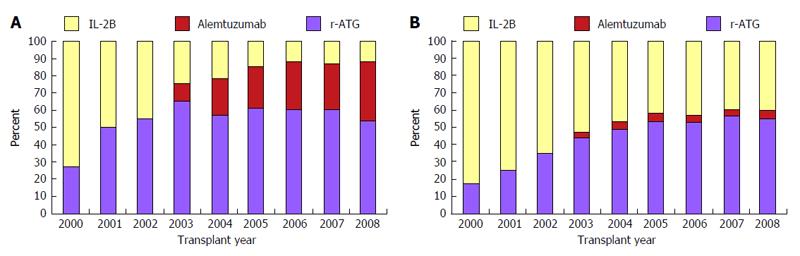
Median follow-up in months with range by induction type were as follows: r-ATG: 35.3 (21.8-55.1), alemtuzumab: 25.0 (13.3-46.0) and IL-2B agent: 46.4 (23.8-72.4). Pattern of induction therapy over the study period are shown in Figure 1. Alemtuzumab was used as an induction agent for the first time in 2003. Since then its use has gradually increased in ESW group but somewhat unchanged in CSM group. IL-2B induction was decreasingly utilized from 2000 to 2008 which was more marked in the ESW group. In the year 2008, roughly 50% patients received induction with r-ATG, 40% with alemtuzumab and 10% with an IL-2B agent in the ESW group. During the same year, about 55% of patients received r-ATG, 5% received alemtuzumab and 40% an IL-2B agent induction in the CSM group.
There were 37217 patients who received a first DDK transplant during the study period of January 2000 to December 2008 after receiving induction with r-ATG, alemtuzumab or an IL-2B agent followed by maintenance with CNI/MMF based regimen. Out of this, 8356 (22.5%) patients underwent early steroid withdrawal and 28861 (77.5%) patients were discharged on maintenance steroid. Distribution of patients by induction type and stratified by steroid use are shown in Table 1. Among all patients, 17863 (48%) received induction with r-ATG (steroid = 13001, no steroid = 4862), 3028 (8%) received alemtuzumab (steroid = 852, no steroid = 2176) and 16326 (44%) an IL-2B agent (steroid = 15008, no steroid = 1318). Among the r-ATG induced patients, there was a higher prevalence of females, African Americans, diabetics, tacrolimus use, pre-transplant dialysis requirement and duration, elevated PRA titer, donor death from CVA and development of DGF in CSM group. Recipient age was higher in ESW group. Among patients who received alemtuzumab, there was higher prevalence ECD/DCD kidney use, female gender, high peak PRA, HLA mismatch and DGF in the CSM group whereas recipient diabetes and tacrolimus use was higher in the ESW group. Among IL-2B induced patients, there was a higher prevalence of African Americans, dialysis requirement and duration, donor death from CVA and DGF development in CSM group while ESW group had higher recipient age, DCD kidney and tacrolimus use.
| r-ATG | Alemtuzumab | IL-2 receptor blocker | ||||
| Steroid | No steroid | Steroid | No steroid | Steroid | No steroid | |
| (n = 13001) | (n = 4862) | (n = 852) | (n = 2176) | (n = 15008) | (n = 1318) | |
| Donor age (yr) | 39 ± 17 | 39 ± 17 | 41 ± 18 | 40 ± 17 | 37 ± 17 | 37 ± 17 |
| Donor gender (M/F) (%) | 59/41 | 60/40 | 61/39 | 58/42 | 60/40 | 60/40 |
| Donor death from CVA (%) | 43 | 40b | 41 | 43 | 40 | 36b |
| ECD kidney (%) | 19 | 19 | 26 | 22b | 15 | 16 |
| DCD kidney (%) | 9.8 | 8.9 | 14.1 | 10.9a | 5.3 | 6.7a |
| Recipient age (yr) | 52 ± 13 | 53 ± 13d | 52 ± 13 | 53 ± 12 | 52 ± 13 | 54 ± 15d |
| Recipient gender (M/F) (%) | 57/53 | 63/37d | 56/44 | 60/40a | 63/37 | 64/36 |
| African American race (%) | 37 | 26d | 33 | 33 | 26 | 20d |
| Diabetes (%) | 34 | 20d | 31 | 38b | 34 | 34 |
| Pre-txp dialysis (%) | 91 | 88b | 88 | 88 | 91 | 87d |
| Dialysis duration (mo) | 48 ± 35 | 43 ± 36d | 47 ± 37 | 50 ± 34 | 43 ± 33 | 41 ± 32 |
| Peak PRA (%) | 18 ± 31 | 10 ± 22d | 19 ± 31 | 14 ± 27d | 9 ± 22 | 8 ± 20 |
| HLA mismatches | 3.9 ± 1.8 | 3.8 ± 1.8a | 3.9 ± 1.8 | 3.7 ± 1.7b | 3.6 ± 1.9 | 3.5 ± 2.0 |
| Cold ischemia (h) | 18.3 ± 8.2 | 18.5 ± 8.8 | 20.8 ± 8.7 | 20.5 ± 9.2 | 17.8 ± 7.9 | 17.4 ± 8.5 |
| Delayed graft function (%) | 28.9 | 19.5d | 29.1 | 21.2d | 20.2 | 16.5 |
| Discharged on tacrolimus (%) | 81 | 79b | 70 | 98d | 67 | 78d |
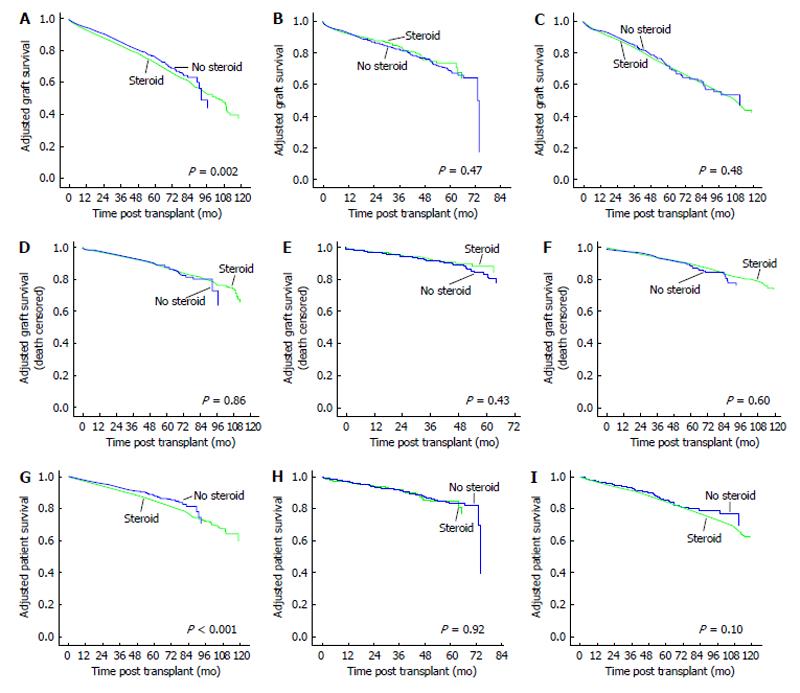
Adjusted overall and death censored graft survivals for CSM vs ESW groups for the three induction agents are shown in Figure 2. The adjusted overall graft survival was significantly inferior for CSM vs ESW groups in patients who underwent induction with r-ATG (HR = 1.16, 95%CI: 1.06-1.27, P = 0.002). However, adjusted death-censored graft survivals were similar between the groups. In alemtuzumab induced patients, there were no significant differences in both adjusted overall (HR = 0.92, 95%CI: 0.73-1.15, P = 0.47) and death-censored (HR = 0.87, 95%CI: 0.62-1.22, P = 0.43) graft survivals for CSM vs ESW groups. Similarly, adjusted overall (HR = 1.05, 95%CI: 0.91-1.21, P = 0.48) and death-censored (HR = 0.94, 95%CI: 0.75-1.18, P = 0.60) graft survivals were similar for CSM vs ESW groups in IL-2B induced patients.
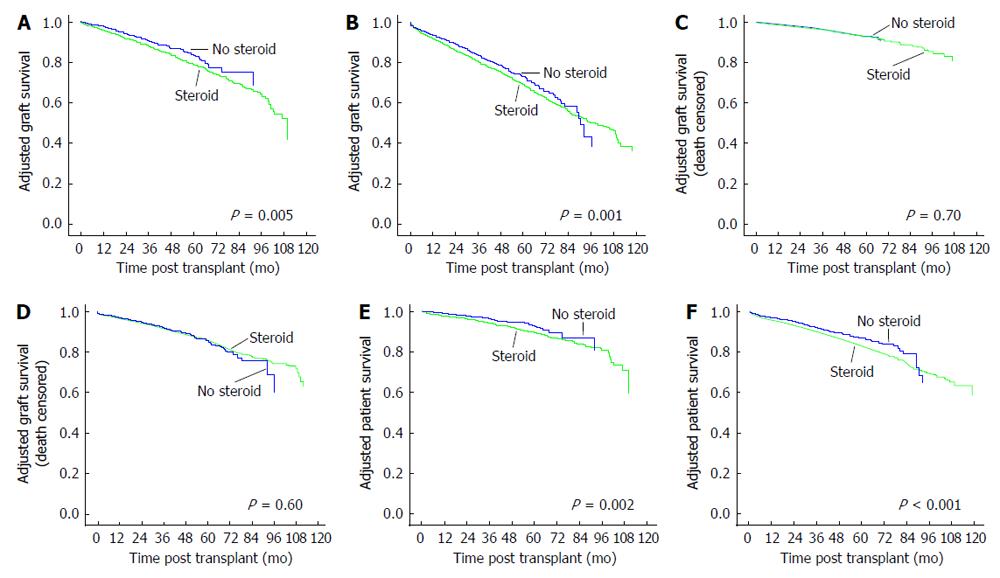
A further analysis was performed by splitting the r-ATG induced patients into low and high immune risk groups as explained in the methods section. There were 4346 patients in the low immune risk and 13517 patients in the high immune risk group. Adjusted overall and death-censored graft survivals for low and high immune risk groups are shown in Figure 3. Adjusted overall graft survivals were inferior for CSM vs ESW groups in both low immune (HR = 1.34, 95%CI: 1.09-1.64, P = 0.001) and high immune (HR = 1.18, 95%CI: 1.07-1.30, P = 0.005) risk patients. Adjusted death-censored graft survivals for CSM vs ESW groups were similar in both low (HR = 1.06, 95%CI: 0.78-1.45, P = 0.70) and high (HR = 1.04, 95%CI: 0.98-1.20, P = 0.60) immune risk groups.
Adjusted patient survivals for CSM vs ESW for the three different induction agents are shown in Figure 2. Patient survivals were inferior for CSM vs ESW groups in r-ATG induced (HR = 1.31, 95%CI: 1.15-1.49, P < 0.001) but similar in alemtuzumab (HR = 1.02, 95%CI: 0.75-1.38, P = 0.92) and IL-2B (HR = 1.17, 95%CI: 0.97-1.40, P = 0.10) induced patients. In r-ATG induced patients, adjusted patient survivals were inferior for CSM vs ESW groups in both low immune (HR = 1.54, 95%CI: 1.18-2.02, P < 0.001) and high immune (HR = 1.32, 95%CI: 1.16-1.51, P = 0.002) risk groups (Figure 3). We further analyzed the causes of recipient death for CSM vs ESW groups in r-ATG induced patients (Table 2). There were significantly higher deaths from infections and CV causes in CSM groups.
| Cause of death | CSM group | ESW group | P-value |
| n = 13001 (%) | n = 4862 (%) | ||
| Cardiovascular | 410 (3.2) | 97 (2.0) | < 0.0001 |
| Infections | 313 (2.4) | 42 (0.9) | < 0.0001 |
| Malignancy | 148 (1.1) | 55 (1.1) | 0.97 |
| Other causes | 916 (7) | 206 (4.2) | < 0.0001 |
Our study showed an association between increased overall graft failure as well as patient death risks and steroid addition to a CNI/MMF maintenance regimen in patients undergoing a first DDK transplantation following induction with r-ATG. Similar death-censored graft survival for steroid vs no-steroid maintenance in these patients suggests increased death with functioning graft in the steroid maintenance group. There were increased patient deaths from CV and infectious causes for steroid vs no-steroid groups.
Current data suggest that corticosteroids could be discontinued safely during the first week after transplantation in patients who are at low immunological risk and receive induction therapy[7]. Studies of early steroid withdrawal have shown outcomes comparable to steroid maintenance regimens[8-14]. Seven year results from a large prospective study performed within the frame work of the Collaborative Transplant Study involving predominantly Caucasian kidney transplant recipients showed a benefit for steroid withdrawal in terms of graft, patient and death-censored graft survival[15]. A five year prospective trial demonstrated similar graft survival and function as well as patient survival in kidney transplant recipients randomized to ESW vs CSM following induction with either r-ATG or IL-2B and tacrolimus/MMF maintenance[16]. There was increased incidence of mild biopsy proven AR in ESW group but favorable effects on CV risk factors including serum triglyceride levels, new onset diabetes after transplantation (NODAT) requiring insulin and weight gain. A meta-analysis involving 34 studies and 5637 patients showed a small increase in AR rates in steroid avoidance/withdrawal group, without any measurable adverse effect on graft or patient survival, while enjoying significant benefits in terms of CV risk factors[17]. A large single center retrospective analysis involving 1241 primary living and DDK transplant recipients showed similar patient, graft, death-censored graft, and AR free survival rates at 10 years for rapid steroid discontinuation group compared to historic controls on maintenance steroids[18]. All patients received induction with r-ATG and maintenance therapy consisting of a CNI agent along with either MMF or sirolimus. In the subgroup of patients who received DDK transplants, patient, graft and death-censored graft survivals were significantly higher in the ESW group. There were significant reductions in the incidences of NODAT, cataracts and avascular necrosis in rapid steroid discontinuation group. This study compared prednisone related side effects to historic control group maintained on relatively higher doses of prednisone (0.1-0.15 mg/kg). Our study showed superior patient and overall graft but not death-censored graft survival in the ESW groups indicating that the beneficial effects appear to be related to lower rate of death with functioning graft in ESW group.
The polyclonal antibody r-ATG is a powerful depleting induction agent commonly used in kidney transplant recipients. r-ATG contains antibodies to several human T-cell surface antigens including major histocompatibility antigens and causes apoptosis and cell lysis. r-ATG causes severe lymphocyte depletion with prolonged immunosuppressive effect with the drug detectable in blood at 90 d after initiation of therapy[19]. Our study showed inferior adjusted overall graft and patient survival in kidney transplant recipients continued on steroid maintenance after r-ATG induction when compared to patients in whom steroid was discontinued on hospital discharge. One could speculate that inferior outcomes in the CSM group could possibly be related to the enhanced immunosuppression related to the steroid containing triple immunosuppression following powerful induction with r-ATG. Similar death-censored graft survival between CSM and ESW groups along with the finding of significantly higher deaths from infectious and CV causes in CSM group is in support of this speculation. A highly significant association between maintenance steroid dose and death with functioning graft caused by CV disease or infection beyond the first year following DDK transplantation was reported recently in a study involving close to 42000 patients[20]. Such an association was observed with neither tacrolimus nor mycophenolic acid in that study. Adverse metabolic profile in patients on steroid therapy as shown in previous studies mentioned before could be contributing to CV complications and enhanced immunosuppression to infectious complications. These findings persisted when r-ATG induced patients in our study were split into high and low immune risk groups indicating that any potential benefits of steroid maintenance in terms of reducing immunological allograft injury even in high immune risk subgroup appear to be overshadowed by CV and infectious complications. These adverse outcomes associated with CSM after r-ATG induction was not seen in patients who were maintained on steroid following induction with IL-2B agent or alemtuzumab. IL-2B is a non-depleting monoclonal antibody with lesser degrees of immunosuppression. Alemtuzumab is a depleting monoclonal antibody typically administered as a single dose intra-operatively and can cause prolonged lymphopenia. It is not clear why inferior outcomes seen with CSM vs ESW following r-ATG induction was not observed with alemtuzumab. Alemtuzumab induced patients generally tolerate only lower doses of antiproliferative agent and many of them could have been taken off MMF completely due to low white blood cell counts[21]. This may decrease the degree of immunosuppression despite being on steroid maintenance. It should be noted that there were only 852 patients in the alemtuzumab group who were discharged on steroid maintenance thus limiting power of the comparison. A previous registry analysis showed superior outcomes with r-ATG induction when compared to induction with either alemtuzumab or IL-2B in DDK transplant recipients discharged on a steroid-free CNI/MMF regimen[21].
Large number of patients in a national cohort including several transplant centers and inclusion of high immune risk patients adds to the validity of the current analysis. One could argue that r-ATG induction and steroid maintenance is generally used in high risk patients with expectedly poor outcomes. Classification of r-ATG induced patients into high and low immune risk groups and the finding of inferior outcomes associated with CSM in both groups tend to mollify this criticism. Our findings are consistent with a previous registry analysis by Luan et al[5] which showed improved graft and patient survivals at years 1 and 4 post transplantation for patients in whom steroid was discontinued on discharge. That study evaluated both living and DDK transplant recipients and included first and subsequent transplants. We believe our findings are important since r-ATG is a commonly used induction agent and majority of the kidney transplant recipients are discharged on maintenance steroids. For instance, in the current analysis, 48% of patients received r-ATG out of which 73% were discharged on a steroid maintenance regimen. About 35% of all the study population received r-ATG induction followed by steroid maintenance.
Our study is not without limitations. Retrospective design can confirm association but cannot prove causation. Residual confounding likely exists despite utilizing an adjusted model and lack of granularity on some important details in the registry could likely have an effect on the outcomes tested. Center-specific differences in practice patterns lead to selection bias regarding different induction agents and steroid maintenance. Some early steroid withdrawal protocols discontinue steroids at seven days post-transplant and some others at day five. Discharging these patients from the hospital before the steroid withdrawal is complete would wrongly categorize them as being on steroids. Details on the doses of induction and maintenance immunosuppressive agents were not available which may have impact on the outcomes. Information on daily or cumulative doses of steroids used would be very helpful while analyzing the negative effects of steroids over long periods of time but unfortunately as mentioned dosing information was not available in the database. Data regarding changes in maintenance immunosuppressive regimen since initial hospital discharge were not available. This could be important since as many as 30% of patients initially discharged on no steroids may have been restarted on CSM mostly after rejection episodes. But this should in fact have diminished the strength of observed associations. Some patients initially discharged on steroid containing regimen may have undergone later steroid withdrawal since some transplant centers withdraw steroids late after transplantation. Despite these misclassifications, there is likely minimal impact on the results because of the non-differential influence of misclassification which tend to deflate the results toward the null[22]. The possibility of a type 1 error cannot be completely excluded.
In summary, our study showed an association of adverse outcomes with steroid addition to a CNI/MMF maintenance regimen in patients who received r-ATG induction for a first DDK transplantation. One has to be cautious while maintaining intense immunosuppression by adding steroid to a CNI/MMF regimen in kidney transplant recipients who were selected for r-ATG induction. Since r-ATG is a commonly used induction agent and majority of patients are maintained on steroid, this important observation needs to be further evaluated in future studies.
Part of this work was presented orally at the American Transplant Congress 2012, Boston, MA.
Analysis of the outcomes in kidney transplant recipients who were maintained on steroid and stratified by the induction type.
Enhanced immunosuppression with steroid maintenance in kidney transplant recipients who receive powerful induction therapy with rabbit-antithymocyte globulin may be associated with adverse outcomes.
In clinical transplantation.
The manuscript clearly describes the current situation about this problem representing therefore a subject of interest to the reader.
| 1. | Prasad GV, Nash MM, McFarlane PA, Zaltzman JS. Renal transplant recipient attitudes toward steroid use and steroid withdrawal. Clin Transplant. 2003;17:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Sinclair NR. Low-dose steroid therapy in cyclosporine-treated renal transplant recipients with well-functioning grafts. The Canadian Multicentre Transplant Study Group. CMAJ. 1992;147:645-657. [PubMed] |
| 3. | Hricik DE, O’Toole MA, Schulak JA, Herson J. Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: a meta-analysis. J Am Soc Nephrol. 1993;4:1300-1305. [PubMed] |
| 4. | Kasiske BL, Chakkera HA, Louis TA, Ma JZ. A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol. 2000;11:1910-1917. [PubMed] |
| 5. | Luan FL, Steffick DE, Gadegbeku C, Norman SP, Wolfe R, Ojo AO. Graft and patient survival in kidney transplant recipients selected for de novo steroid-free maintenance immunosuppression. Am J Transplant. 2009;9:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90:1511-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3:S1-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 1150] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 8. | Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Roza A. Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. Am J Transplant. 2003;3:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Matas AJ, Kandaswamy R, Gillingham KJ, McHugh L, Ibrahim H, Kasiske B, Humar A. Prednisone-free maintenance immunosuppression-a 5-year experience. Am J Transplant. 2005;5:2473-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Rostaing L, Cantarovich D, Mourad G, Budde K, Rigotti P, Mariat C, Margreiter R, Capdevilla L, Lang P, Vialtel P. Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation. 2005;79:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Kumar MS, Heifets M, Moritz MJ, Saeed MI, Khan SM, Fyfe B, Sustento-Riodeca N, Daniel JN, Kumar A. Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation. 2006;81:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Pascual J, Zamora J, Galeano C, Royuela A, Quereda C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2009;CD005632. [PubMed] |
| 14. | Schiff J, Cole EH. Renal transplantation with early steroid withdrawal. Pediatr Nephrol. 2009;24:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Opelz G, Döhler B, Laux G. Long-term prospective study of steroid withdrawal in kidney and heart transplant recipients. Am J Transplant. 2005;5:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564-577. [PubMed] |
| 17. | Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Rizzari MD, Suszynski TM, Gillingham KJ, Dunn TB, Ibrahim HN, Payne WD, Chinnakotla S, Finger EB, Sutherland DE, Kandaswamy R. Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation. Clin J Am Soc Nephrol. 2012;7:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Deeks ED, Keating GM. Rabbit antithymocyte globulin (thymoglobulin): a review of its use in the prevention and treatment of acute renal allograft rejection. Drugs. 2009;69:1483-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Opelz G, Döhler B. Association between steroid dosage and death with a functioning graft after kidney transplantation. Am J Transplant. 2013;13:2096-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Sureshkumar KK, Thai NL, Hussain SM, Ko TY, Marcus RJ. Influence of induction modality on the outcome of deceased donor kidney transplant recipients discharged on steroid-free maintenance immunosuppression. Transplantation. 2012;93:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488-495. [PubMed] |
P- Reviewer: Cantarovich F, Martins LSS S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ