Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.110496
Revised: June 18, 2025
Accepted: September 12, 2025
Published online: December 18, 2025
Processing time: 164 Days and 12.9 Hours
Liver transplantation (LT) is the only curative treatment for end-stage liver disease. Although Mexico has made important strides in surgical capacity and institutional development, the country continues to report one of the lowest LT rates in Latin America. Multiple challenges remain, including inequitable access to care, limited organ donation, and structural inefficiencies in allocation systems. To review the current status of LT in Mexico, describe historical trends, highlight significant barriers to progress, and discuss potential opportunities for program expansion. We conducted a narrative review incorporating data from the National Transplant Center (Centro Nacional de Trasplantes in Spanish), relevant peer-reviewed literature, and global benchmarks. The analysis focused on trends in liver transplant volume, donor types, etiology shifts, institutional disparities, and the impact of the coronavirus disease 2019 (COVID-19) pandemic. LT activity in Mexico increased from 25 transplants in 1999 to 297 in 2023. However, over 68% of transplants are concentrated in Mexico City, and only eight centers perform more than ten LTs per year. Deceased donors account for most grafts, while living donor transplants remain rare and mostly limited to private institutions. The national waiting list functions primarily as a registry rather than a priority-based allocation system. The COVID-19 pandemic further disrupted transplant programs, particularly in the public sector. Innovative approaches such as donation after circulatory death, hepatitis C virus-positive donor utilization, and advanced perfusion technologies are currently unavailable or underutilized in Mexico. Mexico's LT system faces geographic, regulatory, and resource-related limitations. To improve outcomes and ensure equitable access, strategic reforms focused on donor expansion, centralized allocation, perfusion technologies, and standardization of care are urgently needed.
Core Tip: Liver transplantation in Mexico remains disproportionately concentrated in a few urban centers and significantly lags behind regional benchmarks. This mini-review highlights the historical development of the transplant system, ongoing challenges such as low donor rates and fragmented healthcare structures, and opportunities for innovation through donation after circulatory death protocols, hepatitis C virus-positive graft utilization, and machine perfusion strategies. Implementing national reforms could bridge the gap between transplant capacity and patient need.
- Citation: Avila-Rojo JA, Martínez-Sánchez FD, Rosales-Rentería LA, Aguirre-Villarreal D, Contreras AG, Cruz-Martinez R, Servin-Rojas M, Ramírez-del Val A, Zamora-Valdés D, Leal-Leyte P, Aguirre-Valadez J, Paez-Zayas VM, Sánchez-Cedillo AI, Lugo-Baruqui A, Covarrubias-Esquer JD, García-Juárez FI, Ruiz I, García-Juárez I. Overcoming barriers and expanding opportunities in liver transplantation in Mexico. World J Transplant 2025; 15(4): 110496
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/110496.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.110496
Cirrhosis, the end stage of chronic liver disease, represents a major public health concern, contributing to over one million deaths globally each year[1]. In Mexico, liver-related illnesses ranked as the fifth leading cause of death among the economically active population as of 2023[2]. Despite its profound impact on healthcare systems, cirrhosis remains under-recognized and underprioritized in health policies. Liver transplantation (LT) is currently the only effective curative therapy for patients with advanced liver disease.
National mortality data indicate a consistent upward trend in liver-related deaths, from 31528 cases in 2008 to 40052 in 2023, with a median of 36311 deaths per year (interquartile range: 32647–40184). Notably, 72.4% of the deceased were male, and 59.8% were between 25 and 64 years of age[2].
LT has significantly improved survival and quality of life in patients with end-stage liver disease. Globally, liver transplant activity increased by 6.5% from 2020 to 2021, reaching 34694 procedures performed[3]. The United States and the European Union lead in deceased donor liver donation (DDLD), with reported rates of 28.4 and 21.9 per million population (pmp), respectively. In Latin America, Uruguay reported the highest average DDLD rate during 2018–2022 (20.1 pmp), followed by Brazil (16.1 pmp), Argentina (15.1 pmp), and Chile (8.0 pmp)[3]. In contrast, Mexico, Peru, and Paraguay reported much lower average DDLD rates of 2.99 pmp, 1.36 pmp, and 1.2 pmp, respectively[3].
While Mexico has made progress in promoting organ donation and enhancing medical training related to LT, tran
LT was first attempted in Mexico in 1976, about a decade after Dr. Thomas Starzl performed the world's first successful liver transplant[4]. Motivated by early international successes, Dr. Héctor Orozco-Zepeda[4] conducted Mexico’s first LT on a 44-year-old woman with cirrhosis. Unfortunately, the patient did not survive the postoperative period. In response, Dr. Orozco-Zepeda and Dr. Héctor Diliz pursued advanced surgical training under Dr. Thomas Starzl in the United States. Upon their return to Mexico, they established an experimental dog transplant program at the Instituto Nacional de Ciencias Médicas y Nutrición "Salvador Zubirán" in Mexico City, successfully mastering the technique within a year[4]. In March 1985, they performed the country’s first successful orthotopic liver transplant. By the end of 1990, 11 LTs had been completed at the same institution[5].
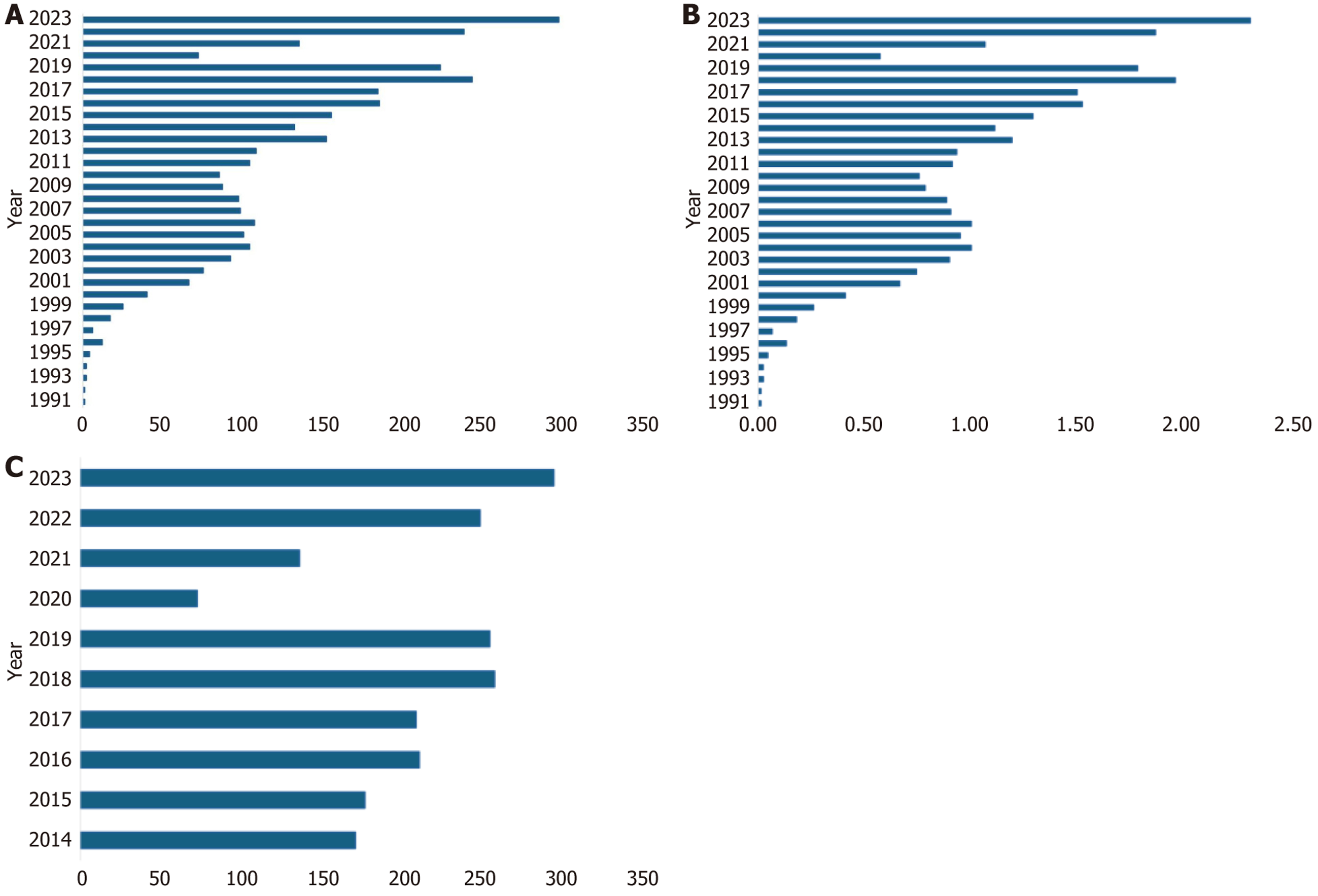
This early success positioned LT as a viable therapeutic option for end-stage liver disease in Mexico[3]. In 1999, the federal government established the National Transplant Center [Centro Nacional de Trasplantes in Spanish (CENATRA)] to oversee and regulate nationwide organ donation and transplantation activities[6]. Over the following decades, the annual number of LTs rose from 25 in 1999 to 297 in 2023, while the national transplant rate increased from 0.01 pmp to 2.3 pmp during the same period (Figure 1A and B). Despite this progress, LT activity in Mexico has plateaued since 2013, and current transplant rates remain among the lowest in Latin America[6].
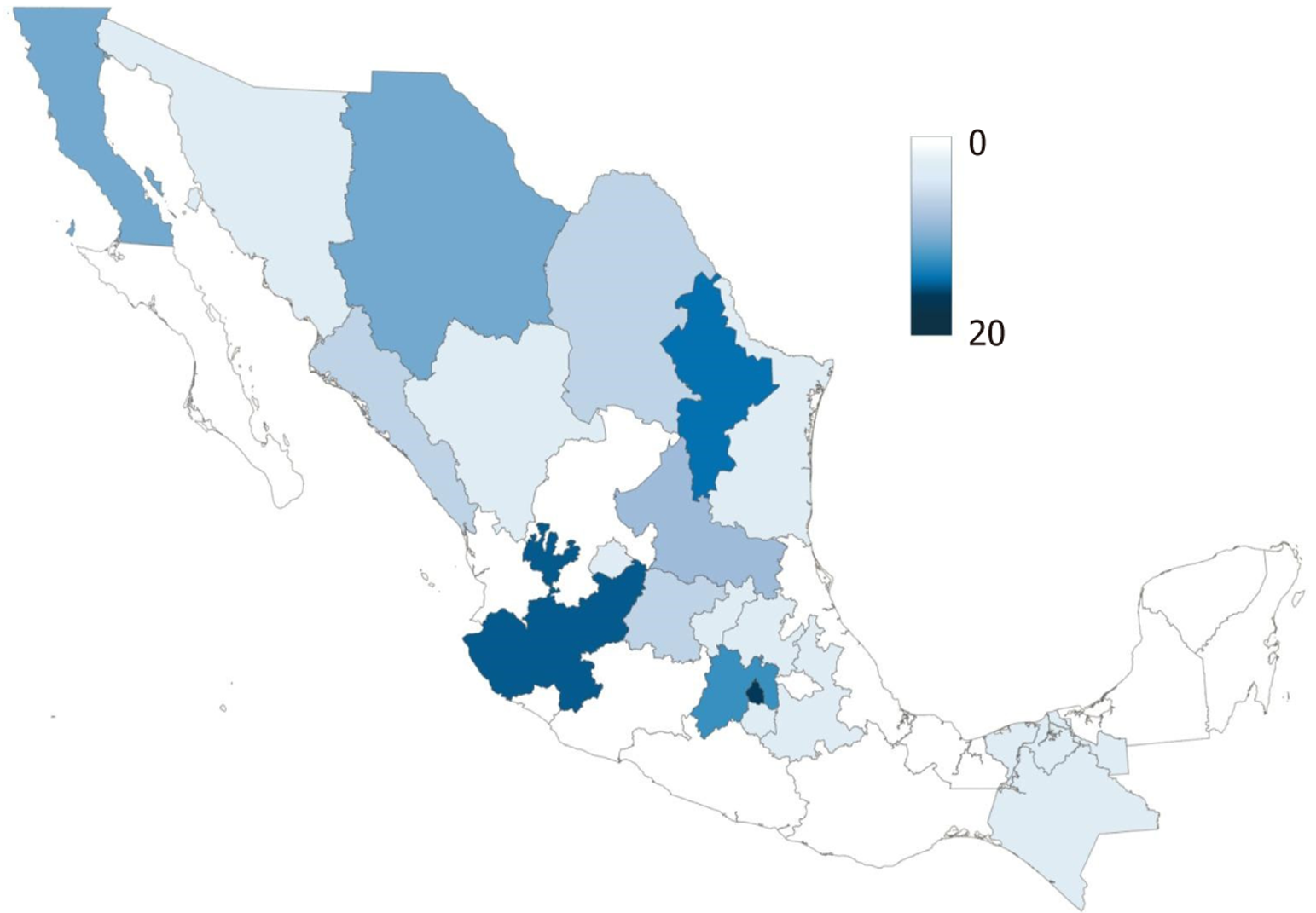
As of 2024, there are 84 CENATRA-accredited liver transplant centers across Mexico, with the majority located in Mexico City, Monterrey, and Guadalajara (Figure 2). Although this reflects ongoing efforts to expand transplant accessibility, the distribution is uneven. Most of these centers belong to the private sector, and only eight perform ten or more transplants per year, predominantly clustered in Mexico City. This geographic concentration limits access to tran
A critical limitation of the Mexican LT system is the lack of long-term post-transplant follow-up data. Only a few centers systematically report survival outcomes. Among these, 1-year, 3-year, and 5-year survival rates have been reported as 94%, 88%, and 88%, respectively[3,6].
The introduction of direct-acting antivirals (DAAs) and the global rise in obesity have significantly altered the etiological landscape of cirrhosis, particularly in high-income countries such as those in Europe and the United States[7,8]. Hepatitis C virus (HCV), previously the leading global cause of cirrhosis, is now considered a curable infection, resulting in a steady decline in HCV-related cirrhosis cases. In contrast, metabolic-associated steatotic liver disease (MASLD) has emerged as the predominant cause of cirrhosis in many Western populations[7-9].
This trend is also reflected in the Mexican experience. A historical study conducted at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, one of the most active liver transplant centers in the country, reported that between 1976 and 2012, HCV was the leading cause of cirrhosis, followed by autoimmune liver disease and MASLD[3,10]. However, a more recent multicenter study involving five major transplant centers in Mexico City found that MASLD has become the leading etiology of cirrhosis, closely mirroring the shifts observed in Western nations. Alcoholic liver disease (ALD) and HCV were identified as the second and third most common causes, respectively[10].
These observational findings might suggest that the burden of MASLD will continue to rise in Mexico and globally[8,10]. This epidemiologic transition highlights the urgent need for tailored public health strategies to address the increasing prevalence of metabolic disorders (such as diabetes) and persistently high levels of alcohol consumption[11,12].
Mexico's healthcare system is divided into public and private sectors. The public sector comprises multiple institutions, including the Mexican Social Security Institute [Instituto Mexicano del Seguro Social in Spanish (IMSS)], which serves workers in the formal economy and their families; the Institute for Social Security and Services for State Workers (Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado in Spanish), which covers government employees; and the Institute of Health for Well-being (Instituto de Salud para el Bienestar in Spanish), which aims to provide care for individuals without social security, such as informal workers. Additionally, smaller systems serve specific populations, such as Mexican Petroleum Company (Petróleos Mexicanos in Spanish) and the armed forces (Fuerzas Armadas). The private sector includes a network of private hospitals and clinics that provide care for individuals with private insurance or the means to pay out of pocket. Although this dual framework was designed to provide broad coverage, disparities in access and quality of care between public and private sectors remain a major challenge[6].
Healthcare access, particularly LT, is highly variable across these subsystems. According to data from the CENATRA, 3246 LTs were performed in Mexico from 1991 through 2023, with most grafts originating from deceased donors. In 2023 Mexico recorded its highest annual number of LTs, reaching 297 transplants nationwide. These procedures were primarily concentrated in Mexico City (68.3%, n = 203), followed by Nuevo León (14.8%, n = 44), Jalisco (14.0%, n = 42), Sonora (1.6%, n = 5), Querétaro (0.9%, n = 2), and the State of Mexico (0.4%, n = 1), reflecting the geographic concentration of healthcare infrastructure and economic resources[6].
Concerning institutional participation, 44% (n = 132) of LTs were performed in Secretaría de Salud (SSA) hospitals, 36% (n = 107) in IMSS facilities, and 20% (n = 58) in private hospitals. The Hospital General de Mexico Dr. Eduardo Liceaga led with 60 transplants, followed by the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (n = 52), the Hospital de Especialidades Dr. Antonio Fraga Mouret at Centro Médico Nacional La Raza (IMSS) (n = 27), Hospital de Especialidades No. 25 (IMSS) (n = 26), and Centro Medico Nacional de Occidente (IMSS) (n = 16)[6].
Despite progress, Mexico continues to report one of the lowest organ donation rates pmp in Latin America and globally[3]. From 2014 to 2023, liver donations remained limited (Figure 1C). In 2023, only 300 Liver donors were registered, despite the identification of over 400 hospitals with the potential to serve as organ donation centers[3,6]. Most liver grafts came from deceased donors, with CENATRA recording 275 deceased and only 22 Living donors. Of the deceased donor grafts, 47% (n = 130) were allocated to SSA, 36% (n = 99) to IMSS, and 17% (n = 46) to private institutions. Geographically, most transplants occurred in Mexico City (n = 197), followed by Nuevo León (n = 41), Jalisco (n = 30), Sonora (n = 5), and Querétaro (n = 2)[6].
Living donor LT (LDLT) remains uncommon in Mexico, with only 22 cases reported in 2023. Notably, 55% of LDLTs were performed in private hospitals, demonstrating the disparities in access to complex surgical procedures across the public and private healthcare systems.
Undoubtedly, the coronavirus disease 2019 (COVID-19) pandemic profoundly impacted LT outcomes and healthcare practices worldwide. A Brazilian study found that 38.9% of LT recipients infected with COVID-19 needed intensive care unit care, with a 64.3% mortality rate for those critically ill. In comparison, no fatalities were noted among patients with mild symptoms[13]. Furthermore, a systematic review showed that LT recipients faced similar risks of severe COVID-19 outcomes as non-transplant patients[14]. However, data from the United States revealed an increase in inpatient mortality and elevated rates of transplant rejection during the pandemic[15]. In addition, a European study indicated a 15% mor
Importantly, vaccination was shown to reduce the severity of COVID-19 in liver transplant recipients, as demonstrated by a study conducted in Mexico[17]. The pandemic also led to a strategic modification in LT programs, prioritizing patients with hepatocellular carcinoma, while the number of transplants for ALD increased[18,19].
There was a substantial decline in LT activity in Mexico, particularly within public healthcare institutions. Globally, the reduction in transplantation procedures was more pronounced in public institutions (89%) compared to private ones (62%)[20]. This pattern extended to kidney, cornea, liver, and heart transplants, all of which experienced greater declines in the public sector.
Specifically, in Mexico, the number of liver transplants dropped dramatically from 224 in 2019 to only 72 in 2020, re
Improving LT programs in Mexico requires more uniform access to healthcare to decrease disparities, and more specifically, commitment and accountability in the public sector. In addition, there is an urgent need to increase the donation rate so that donation after circulatory death (DCD) can be implemented[11,22-24], as well as investments in other technologies such as normothermic or hypothermic perfusion[25-28]. These innovations would not only increase transplant rates but could also help address geographic disparities[23].
DCD is a promising avenue for expanding the liver donor pool in Mexico. The modified Maastricht classification[29] provides a framework for categorizing DCD scenarios from controlled (Maastricht III) to uncontrolled settings (Maastricht I and II). By implementing this classification, Mexico can more effectively identify and utilize potential DCD donors, particularly in controlled hospital environments, where logistics can be better managed.
A study analyzing Organ Procurement and Transplantation Network data from 1993 to 2007 revealed inferior outcomes for DCD liver transplants compared with donation after brain death. DCD livers exhibited lower one-year graft survival (79% vs 85%), higher rates of primary non-function (7%–10% vs 2%–3%), and more biliary complications. These findings highlight the increased susceptibility of DCD livers to ischemic injury, which can compromise the long-term graft viability[30].
A consensus by the International Liver Transplantation Society, published in 2021, identified several donor risk factors that negatively impact DCD outcomes. Factors such as prolonged warm ischemia time, older donor age, and high body mass index were associated with increased graft failure. For instance, warm ischemia times exceeding 30 minutes were associated with a 2.4-fold higher risk of graft failure. This emphasizes the critical need for meticulous management of the DCD process to mitigate these risks[31]. While DCD liver transplants in the United States saw a significant increase (147%) from 2009 to 2019, these only accounted for 14% of all liver transplants. An important obstacle is the lack of effective preservation mechanisms, leading to nearly 30% of DCD grafts being discarded[3]. This rate is likely even higher in Mexico.
In Mexico, the utilization of HCV-positive liver donors remains limited, and little data suggests that a significant number of livers are discarded due to HCV positivity. Globally, however, the use of HCV-positive donor organs for tran
The main barriers to using HCV-positive donors are likely related to infrastructure and access to DAAs, as well as a lack of awareness of new therapeutic strategies[25]. Overcoming these challenges would involve investing in antiviral medications and establishing protocols designed to manage patients receiving HCV-positive grafts.
HCV-positive donors can be categorized as chronic or resolved infections [anti-HCV antibody (Ab)+ with or without nucleic acid amplification testing (NAT)+] or suspected acute HCV (Ab−/NAT+). A retrospective study by Myers et al[26], Analyzing United Network for Organ Sharing data from 2015–2019 demonstrated that HCV-positive livers in DCD transplants were non-inferior to HCV-negative livers in terms of patient and graft survival at 1 year and 3 years[28,29]. This suggests that HCV-positive status may not be a contraindication for DCD LT.
Initially, it was believed that patients with low model for end-stage liver disease (MELD) scores were ideal candidates for HCV-positive liver disease. However, a recent study (Bekki et al[27]), published in Transplantation, found that applying more stringent criteria for these patients resulted in significantly higher one-year graft survival rates (97.1% vs 91.8%) compared to recipients of HCV-negative livers[27]. This suggests that the general selection criteria for DCD HCV-negative donors can be utilized for HCV-positive livers.
Perfusion technologies, such as hypothermic oxygenated perfusion (HOPE), normothermic regional perfusion (NRP), and normothermic machine perfusion (NMP), are cornerstones in the preservation of livers obtained from donations after DCD[32,33]. These techniques mitigate ischemia–reperfusion injury, which is a common challenge in DCD liver tran
These strategies present significant advantages for DCD liver transplants by addressing ischemia–reperfusion injury and expanding the donor pool, especially in resource-limited settings such as Mexico[22,35]. However, implementing these strategies requires substantial technology, training, and infrastructure investment.
To our knowledge, no DCD liver transplants have been performed in Mexico. The primary barriers include limited awareness among healthcare providers, lack of advanced perfusion technologies, and an absence of regulatory fra
Decentralizing LT services in Mexico is essential to improve access and equity. Currently, most LT procedures are concentrated in major cities such as Mexico City, Monterrey, and Guadalajara, leading to pronounced geographic dis
Mexico’s current organ allocation system presents multiple structural challenges. The General Health Law allows donor-generating hospitals substantial autonomy in selecting recipients, resulting in a decentralized allocation process. Unlike centralized systems in other countries, where allocation follows a unified national waiting list and is based on transparent criteria (e.g., MELD score), Mexico lacks such standardization, which hampers fairness and accountability in organ distribution.
The absence of a centralized and prioritized national waiting list makes equitable organ allocation difficult. Fur
Additionally, the logistics and financial burden associated with organ procurement are considerable. Despite Mexico’s moderate geographic extension, the fragmented healthcare infrastructure and lack of coordinated transportation networks make organ retrieval and distribution costly and inefficient. These burdens often fall on individual centers, particularly in the public sector, with limited financial and technical support.
The deployment of advanced perfusion technologies, such as NMP and HOPE, is an enhancement and a vital pro
Improving regulatory oversight and standardization across LT centers in Mexico is essential to enhance programmatic quality and expand the scope of transplant indications. This includes the adoption of HCV-positive donors for HCV-negative recipients, supported by the widespread availability of DAAs, and the broader use of extended-criteria donors. The integration of liver perfusion technologies, such as hypothermic oxygenated and NMP, may further improve graft quality and post-transplant outcomes, benefiting patients, transplant programs, and healthcare providers.
These strategic advances are urgently needed to address the significant mismatch between the number of liver transplants performed and the actual demand, particularly given the growing number of certified transplant centers. Additionally, reducing pre-transplant mortality will require optimizing the process for patient inclusion on the transplant waiting list. The current registry system fails to reflect the national demand, underestimating the number of needy pa
The authors gratefully acknowledge the Centro Nacional de Trasplantes’ invaluable contributions to ensuring public access to national transplant data, which significantly informed and supported the development of this mini-review. We also thank the Secretaría de Salud for its ongoing commitment to improving healthcare delivery in Mexico, which provided the foundation for several of the analyses presented in this article.
| 1. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1184] [Cited by in RCA: 1120] [Article Influence: 186.7] [Reference Citation Analysis (5)] |
| 2. | Instituto Nacional de Estadística y Geografía (INEGI). Características de las defunciones registradas en México durante enero a agosto de 2020 [Internet]. 2021. Available from: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2021/EstSociodemo/DefuncionesRegistradas2020_Pnles.pdf. |
| 3. | Aguirre-Villarreal D, Servin-Rojas M, Sánchez-Cedillo A, Chávez-Villa M, Hernandez-Alejandro R, Arab JP, Ruiz I, Avendaño-Castro KP, Matamoros MA, Adames-Almengor E, Diaz-Ferrer J, Rodriguez-Aguilar EF, Paez-Zayas VM, Contreras AG, Alvares-da-Silva MR, Mendizabal M, Oliveira CP, Navasa M, García-Juárez I. Liver transplantation in Latin America: reality and challenges. Lancet Reg Health Am. 2023;28:100633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Orozco-Zepeda H. [A little history about liver transplantation]. Rev Invest Clin. 2005;57:124-128. [PubMed] |
| 5. | Carlos CN, Antonio OMM, Paulino LVR, Ángel MDM, Javier AF, Eitan P, Mónica DC, Héctor OZ. Programa de Trasplante Hepático en el Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Rev Gastroenterol Mex. 2003;68:83-86. |
| 6. | Centro Nacional de Trasplantes. CENATRA [Internet]. 2025. Available from: https://www.gob.mx/cenatra. |
| 7. | Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, Fritz J, Feurstein B, Popp W, Karam V, Muiesan P, O'Grady J, Jamieson N, Wigmore SJ, Pirenne J, Malek-Hosseini SA, Hidalgo E, Tokat Y, Paul A, Pratschke J, Bartels M, Trunecka P, Settmacher U, Pinzani M, Duvoux C, Newsome PN, Schneeberger S; European Liver and Intestine Transplant Association (ELITA). Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019;71:313-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 8. | Méndez-Sánchez N, Ramírez-Mejía MM, Zheng MH, Cortez-Hernández C, Tovar-Bojorquez EM, Contreras-Omaña R, Monsiváis-Morales JD, Córdova-Gallardo J, Castillo-Barradas M, Guzmán-Rodríguez N, González-Huezo MS, Sandez-Araiza A, Cerda-Reyes E, Cornejo-Hernández S, Barranco-Fragoso B, Cano-Contreras AD, Remes-Troche JM, Higuera-de-la-Tijera F, Pérez-Hernández JL, Chávez-Tapia N, Valentin-Cortez FJ, Montalvo-Gordon I, Lozano-Salazar RR, Sabanes-Hernández M, Borbolla-Schega I, Rodríguez-Hernández H. Changing Landscape of Liver Cirrhosis Etiologies. Arch Med Res. 2025;56:103240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, Ahmed A, Racila A, Henry L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19:580-589.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (1)] |
| 10. | Gonzalez-Chagolla A, Olivas-Martinez A, Ruiz-Manriquez J, Servín-Rojas M, Kauffman-Ortega E, Chávez-García LC, Juárez-León O, Cordova-Gallardo J, Díaz-García JD, Gonzalez-Huezo MS, Milanés-Lizarraga G, Paez-Zayas VM, Castillo-Barradas M, Cobos-Quevedo OJ, García-Juárez FI, Romero-Lozanía JA, Toapanta-Yanchapaxi L, Sánchez-Avila JF, Avila-Rojo JA, Bonilla-Salas A, Dirthurbide-Hernández M, Ruiz I, Valenzuela-Vidales AK, García-Juárez I. Cirrhosis etiology trends in developing countries: Transition from infectious to metabolic conditions. Report from a multicentric cohort in central Mexico. Lancet Reg Health Am. 2022;7:100151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 747] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 12. | Younossi ZM, Golabi P, Price JK, Owrangi S, Gundu-Rao N, Satchi R, Paik JM. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients With Type 2 Diabetes. Clin Gastroenterol Hepatol. 2024;22:1999-2010.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 193] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 13. | Neves MSSE, Paiva JHHGL, Ferreira NSA, Queiroz FPA, Limeira CBB, Veras CM, Carvalho TMT, Freitas TVS, Esmeraldo RM, Brasil IRC. Impact of COVID-19 on liver transplant recipients during the first pandemic wave, in a tertiary hospital, in Northeastern Brazil. Rev Inst Med Trop Sao Paulo. 2022;64:e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, Kumar P, Sharma M, Rao PN, Reddy DN. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Inayat F, Patel P, Ali H, Afzal A, Tahir H, Chaudhry A, Ishtiaq R, Rehman AU, Darji K, Afzal MS, Nawaz G, Giammarino A, Satapathy SK. Impact of COVID-19 on liver transplant recipients: A nationwide cohort study evaluating hospitalization, transplant rejection, and inpatient mortality. World J Transplant. 2024;14:90866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Polak WG, Fondevila C, Karam V, Adam R, Baumann U, Germani G, Nadalin S, Taimr P, Toso C, Troisi RI, Zieniewicz K, Belli LS, Duvoux C. Impact of COVID-19 on liver transplantation in Europe: alert from an early survey of European Liver and Intestine Transplantation Association and European Liver Transplant Registry. Transpl Int. 2020;33:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Azamar-Llamas D, Arenas-Martinez JS, Olivas-Martinez A, Jimenez JV, Kauffman-Ortega E, García-Carrera CJ, Papacristofilou-Riebeling B, Rivera-López FE, García-Juárez I. Impact of COVID-19 vaccination on liver transplant recipients. Experience in a reference center in Mexico. PLoS One. 2024;19:e0301198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Kuo YF, Kwo P, Wong RJ, Singal AK. Impact of COVID-19 on Liver Transplant Activity in the USA: Variation by Etiology and Cirrhosis Complications. J Clin Transl Hepatol. 2023;11:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Servin-Rojas M, Olivas-Martinez A, Ramirez Del Val F, Torres-Gomez A, Navarro-Vargas L, García-Juárez I. Transplant trends in Mexico during the COVID-19 pandemic: Disparities within healthcare sectors. Am J Transplant. 2021;21:4052-4060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Ozturk NB, Muhammad H, Gurakar M, Aslan A, Gurakar A, Dao D. Liver transplantation in developing countries. Hepatol Forum. 2022;3:103-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (2)] |
| 21. | De Carlis R, Paolo Muiesan, Taner B. Donation after circulatory death: Novel strategies to improve the liver transplant outcome. J Hepatol. 2023;78:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Eden J, Sousa Da Silva R, Cortes-Cerisuelo M, Croome K, De Carlis R, Hessheimer AJ, Muller X, de Goeij F, Banz V, Magini G, Compagnon P, Elmer A, Lauterio A, Panconesi R, Widmer J, Dondossola D, Muiesan P, Monbaliu D, de Rosner van Rosmalen M, Detry O, Fondevila C, Jochmans I, Pirenne J, Immer F, Oniscu GC, de Jonge J, Lesurtel M, De Carlis LG, Taner CB, Heaton N, Schlegel A, Dutkowski P. Utilization of livers donated after circulatory death for transplantation - An international comparison. J Hepatol. 2023;78:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 23. | Sasaki K, Nair A, Firl DJ, McVey JC, Moro A, Diago Uso T, Fujiki M, Aucejo FN, Quintini C, Kwon CD, Eghtesad B, Miller CM, Hashimoto K. Conditional probability of graft survival in liver transplantation using donation after circulatory death grafts - a retrospective study. Transpl Int. 2021;34:1433-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Croome KP, Mathur AK, Aqel B, Yang L, Taner T, Heimbach JK, Rosen CB, Paz-Fumagalli R, Taner CB. Classification of Distinct Patterns of Ischemic Cholangiopathy Following DCD Liver Transplantation: Distinct Clinical Courses and Long-term Outcomes From a Multicenter Cohort. Transplantation. 2022;106:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 25. | Delman AM, Ammann AM, Shah SA. The current status of virus-positive liver transplantation. Curr Opin Organ Transplant. 2021;26:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Myers B, Bekki Y, Kozato A, Crismale JF, Schiano TD, Florman S. DCD Hepatitis C Virus-positive Donor Livers Can Achieve Favorable Outcomes With Liver Transplantation and Are Underutilized. Transplantation. 2023;107:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Bekki Y, Crismale JF, Myers B, Schiano TD, Florman S. Varying Utilization Rates but Superior Outcomes in Liver Transplantation From Hepatitis C-positive Donors in the United States: An Analysis of the OPTN/UNOS Database. Transplantation. 2022;106:1787-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 28. | Thuong M, Ruiz A, Evrard P, Kuiper M, Boffa C, Akhtar MZ, Neuberger J, Ploeg R. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 29. | Ruch B, Kumm K, Arias S, Katariya NN, Mathur AK. Donation After Circulatory Death Liver Transplantation: Early Challenges, Clinical Improvement, and Future Directions. Surg Clin North Am. 2024;104:27-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Schlegel A, Foley DP, Savier E, Flores Carvalho M, De Carlis L, Heaton N, Taner CB. Recommendations for Donor and Recipient Selection and Risk Prediction: Working Group Report From the ILTS Consensus Conference in DCD Liver Transplantation. Transplantation. 2021;105:1892-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Haque O, Yuan Q, Uygun K, Markmann JF. Evolving utilization of donation after circulatory death livers in liver transplantation: The day of DCD has come. Clin Transplant. 2021;35:e14211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Manara A, Shemie SD, Large S, Healey A, Baker A, Badiwala M, Berman M, Butler AJ, Chaudhury P, Dark J, Forsythe J, Freed DH, Gardiner D, Harvey D, Hornby L, MacLean J, Messer S, Oniscu GC, Simpson C, Teitelbaum J, Torrance S, Wilson LC, Watson CJE. Maintaining the permanence principle for death during in situ normothermic regional perfusion for donation after circulatory death organ recovery: A United Kingdom and Canadian proposal. Am J Transplant. 2020;20:2017-2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 33. | Schlegel A, Kron P, Dutkowski P. Hypothermic machine perfusion in liver transplantation. Curr Opin Organ Transplant. 2016;21:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Schlegel A, Porte R, Dutkowski P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J Hepatol. 2022;76:1330-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 35. | Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, Barton D, Curbishley S, Wilkhu M, Neil DAH, Hübscher SG, Muiesan P, Isaac JR, Roberts KJ, Abradelo M, Schlegel A, Ferguson J, Cilliers H, Bion J, Adams DH, Morris C, Friend PJ, Yap C, Afford SC, Mirza DF. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 368] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 36. | Gaurav R, Butler AJ, Kosmoliaptsis V, Mumford L, Fear C, Swift L, Fedotovs A, Upponi S, Khwaja S, Richards J, Allison M, Watson CJE. Liver Transplantation Outcomes From Controlled Circulatory Death Donors: SCS vs in situ NRP vs ex situ NMP. Ann Surg. 2022;275:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/