Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.109968
Revised: June 14, 2025
Accepted: July 16, 2025
Published online: December 18, 2025
Processing time: 174 Days and 1.9 Hours
Kidney transplantation is increasingly more common due to the ongoing shortage of deceased donors. However, anatomical challenges, such as a short renal artery, can complicate surgical procedures and increase complication risk, including thro
We present a case of kidney transplantation complicated by a short donor renal artery. To address the discrepancy between arterial length and diameter mismatch, the recipient’s inferior epigastric artery was used as a cuff interposition for arterial reconstruction. Following standard laparoscopic donor nephrectomy, vascular reconstruction was performed on the back table. The use of the inferior epigastric artery as a cuff allowed for successful elongation and size matching of the donor renal artery, enabling a tension-free anastomosis to the recipient’s exter
Interposition of the epigastric artery offers an innovative technique for managing short donor renal arteries, reducing the risk of early thrombosis and long-term complications as size mismatch and intimal hyperplasia.
Core Tip: Due to the ongoing shortage of deceased donors, kidney transplantation has increased as a strategy to expand the donor pool. However, this rise has brought increased encounters. We report a successful transplantation involving a kidney with a short renal artery from a living donor, using inferior epigastric artery as a cuff interposition for arterial reconstruction. To our knowledge, this is the first documented case of using epigastric artery in this manner. This novel technique appears to be a promising solution to prevent early thrombosis caused by vessel size discrepancy, and mid- to long-term anastomotic stenosis due to myo-intimal hyperplasia.
- Citation: Lekehal B, Ait Youssef N, Lekehal M, Bakkali T, Jdar A, Bounssir A. Inferior epigastric artery cuff interposition for short renal artery in living-donor kidney transplantation: A case report and review of literature. World J Transplant 2025; 15(4): 109968
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/109968.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.109968
Kidney transplantation remains the treatment of choice for end-stage renal disease, offering significant improvements in both life expectancy and quality of life for recipients. First described in 1951, this surgical technique has undergone only modest evolution over the past six decades[1].
kidney transplantation has emerged as a major therapeutic option, with graft survival rates comparable to or even exceeding those of deceased-donor transplants[2].
In this report, we describe a novel reconstruction technique for a short renal artery in kidney transplantation, utilizing a cuff made from the recipient’s inferior epigastric artery.
A 30-year-old woman with end-stage renal disease of unknown etiology was admitted for a scheduled living-donor kidney transplantation.
The patient had been undergoing hemodialysis for 3 years for end-stage renal disease of unknown etiology. She was evaluated for kidney transplantation, and a living-related donor, her sibling, was found to be a compatible match.
The patient had no history of prior abdominal surgeries or other significant medical conditions.
The patient was a non-smoker and did not consume alcohol. Family history was significant for diabetes mellitus in both parents, with no known hereditary renal diseases.
The patient was alert and oriented, with stable vital signs. There was no peripheral edema nor clinical evidence of fluid overload. Overall, the physical examination was unremarkable.
Preoperative laboratory evaluation revealed the following: Serum creatinine: 8.1 mg/dL (normal range: 0.6-1.3 mg/dL), blood urea nitrogen (commonly known as blood urea nitrogen): 72 mg/dL (normal range: 7-20 mg/dL), hemoglobin: 10.5 g/dL (normal range: 12-15.5 g/dL), and blood glucose: 145 mg/dL (normal range: 70-99 mg/dL).
All other routine pre-transplant laboratory values were within acceptable ranges.
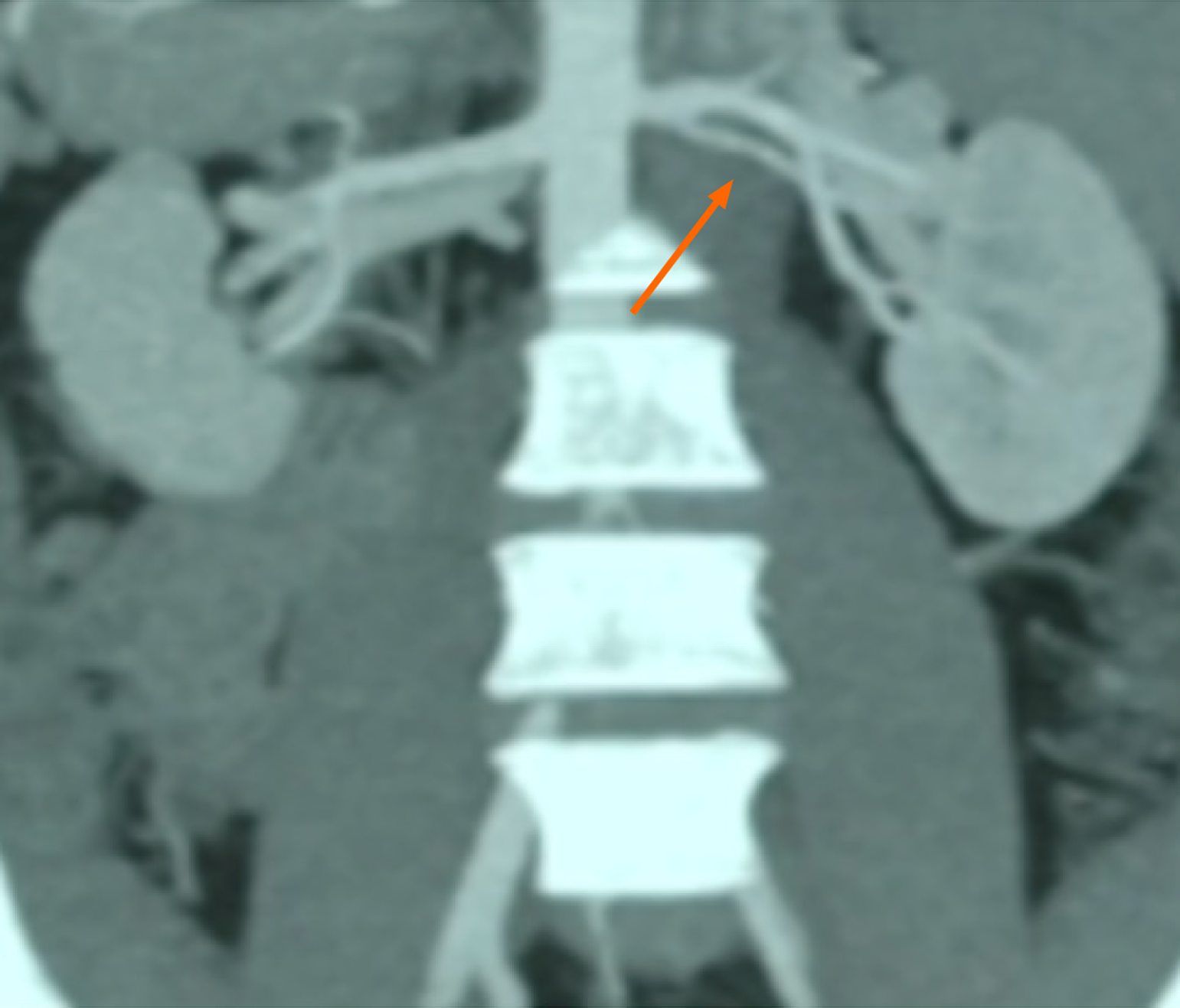
Preoperative abdominal computed tomography (CT) angiography confirmed suitable iliac vessel anatomy in the recipient for transplantation. However, the donor kidney demonstrated a short right renal artery (Figure 1).
The transplant team, including nephrologists, urologists, and vascular surgeons, reviewed the case. A consensus was made to proceed with transplant. The short length of the donor renal artery was identified on preoperative imaging and discussed during the multidisciplinary team meeting. The team agreed that the vascular anatomy was suitable for transplantation, and potential reconstruction strategies were considered to address any intra-operative challenges.
End-stage renal disease of unknown etiology requiring renal transplantation.
A standard hard-assisted laparoscopic living donor nephrectomy was performed to retrieve the left kidney. Preoperative CT imaging revealed that the donor kidney had a renal artery with an early bifurcation (Figure 1). During the procedure, an endovascular stapler was applied distal to the bifurcation, resulting in a reduction of the arterial length by approximately 10-15 mm (Figure 2).
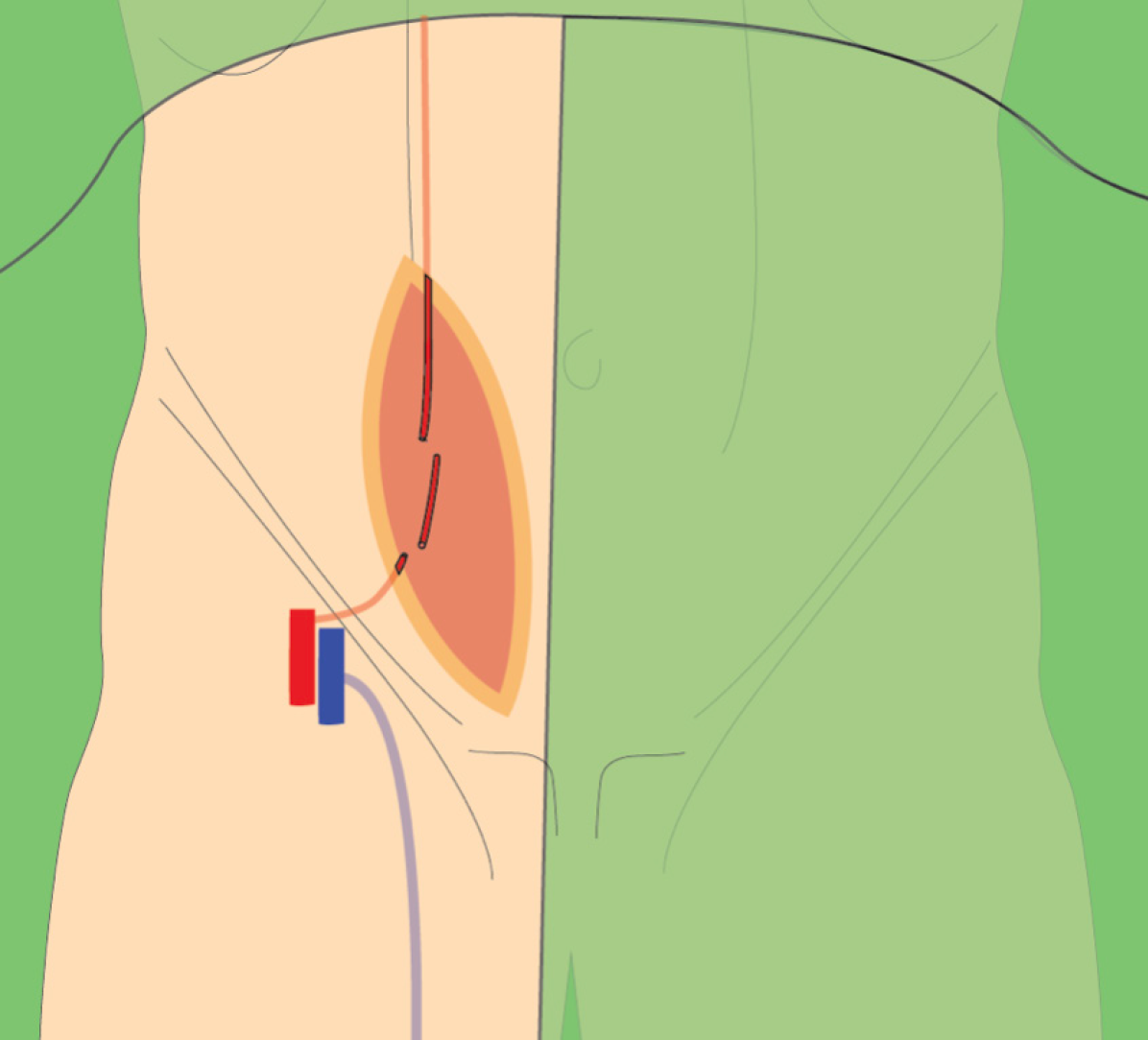
The renal transplant was carried out using a right para-rectal approach. A vertical incision was made along the medial border of the rectus abdominis muscle, providing adequate exposure of the external iliac vessels and urinary bladder. This access facilitated the vascular and ureteral anastomoses.
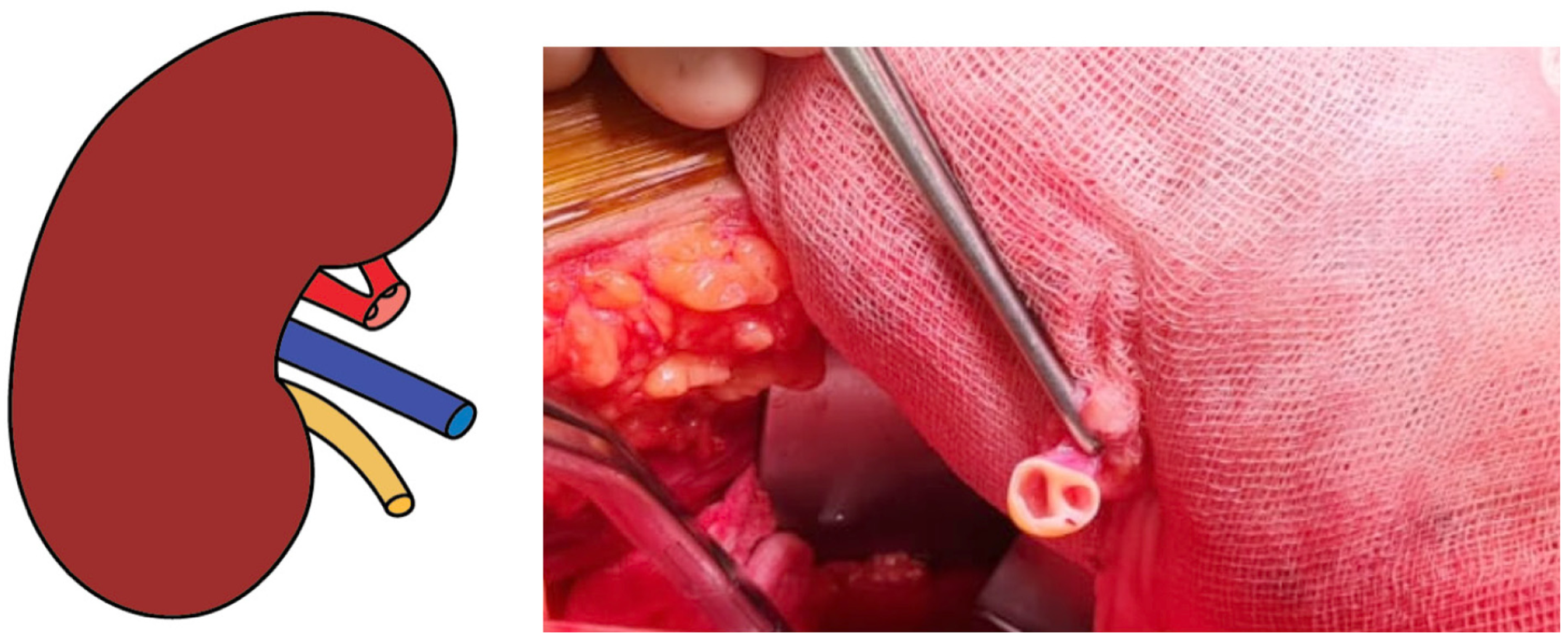
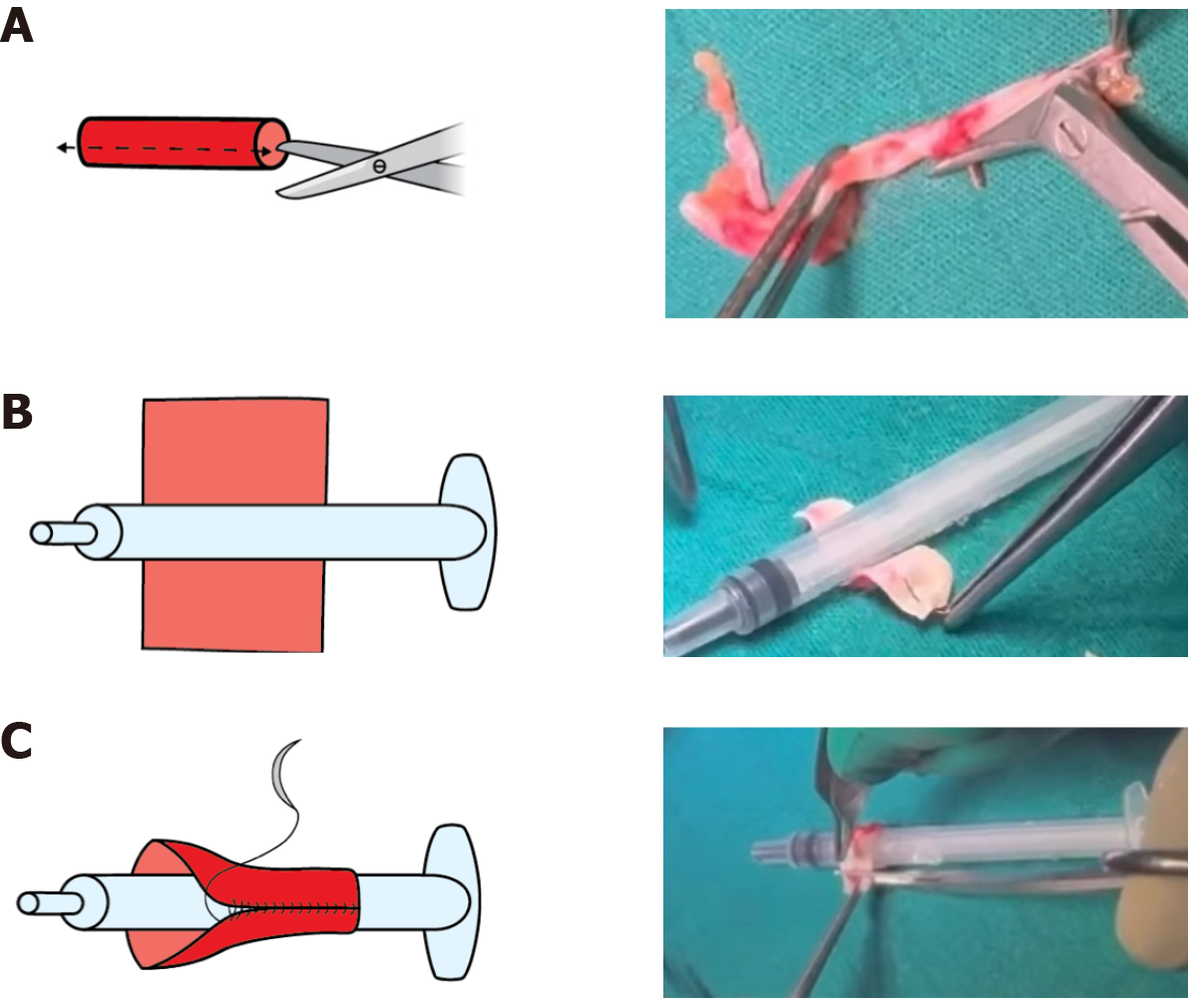
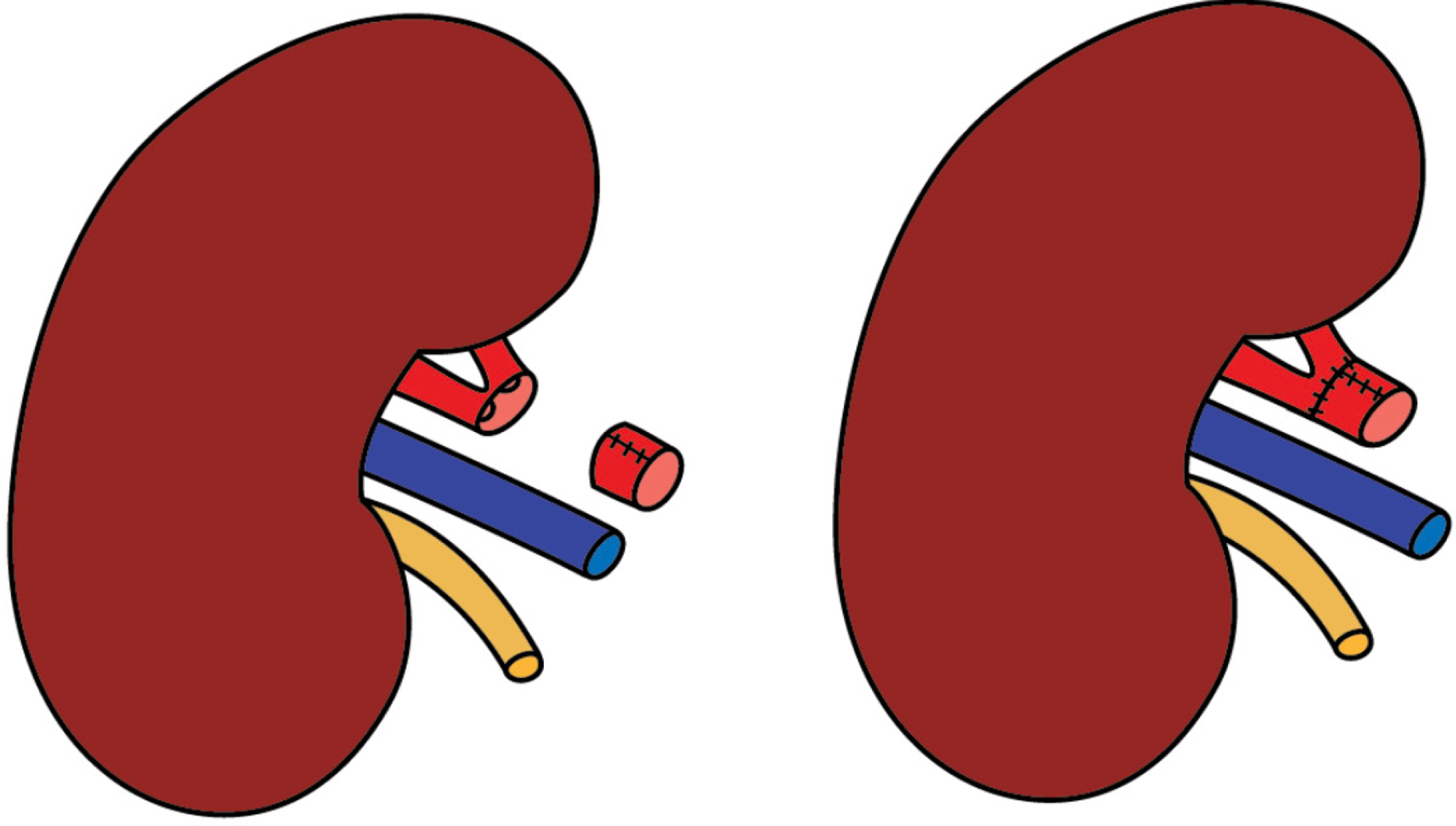
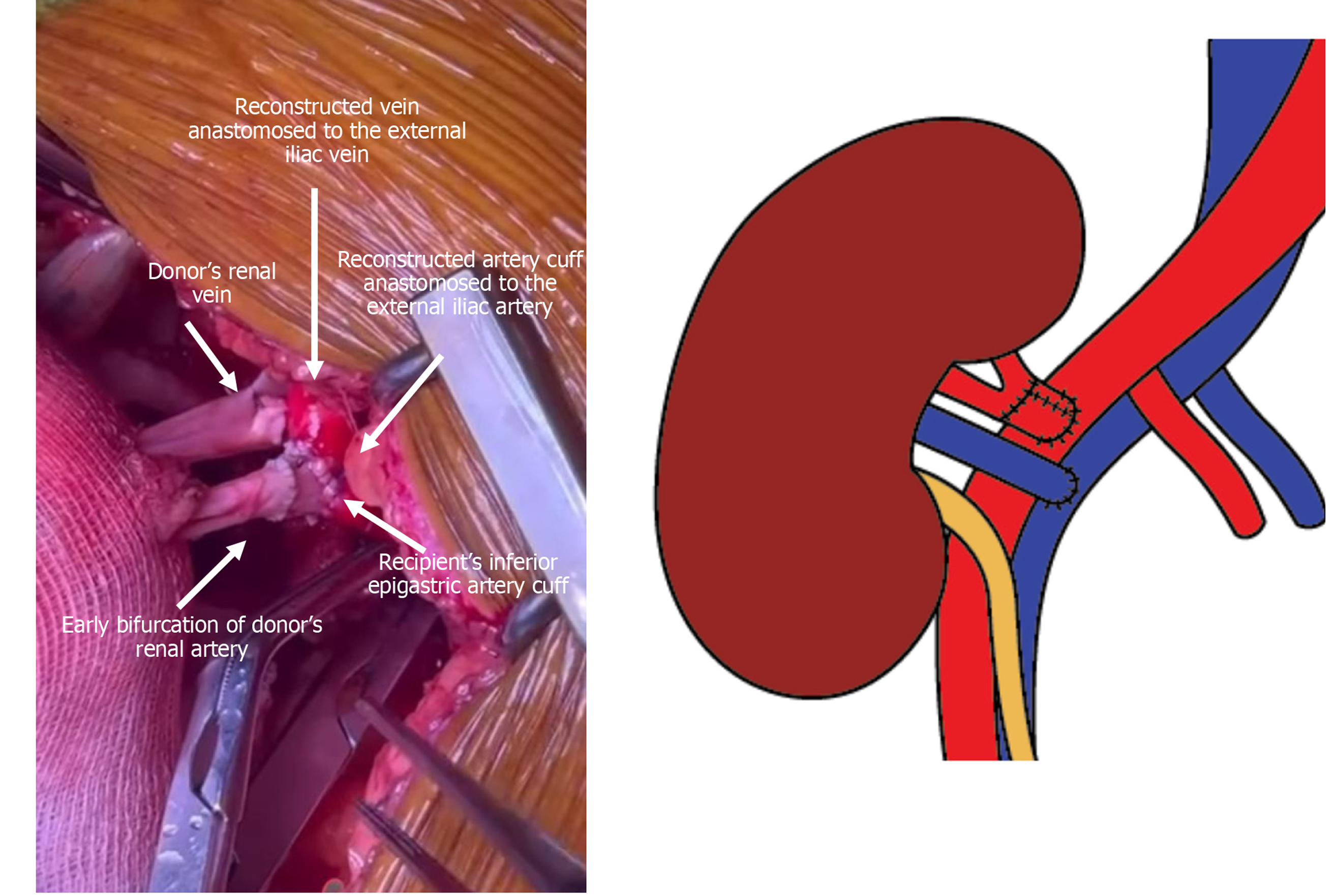
Back-table evaluation confirmed that the renal artery was short, measuring approximately 2 cm, and exhibited a gun-barrel-like bifurcation with a very short common trunk, which complicated vascular reconstruction (Figure 2). After careful assessment, the surgical team opted to use the recipient’s inferior epigastric artery as an interposition graft. This approach enabled an end-to-side anastomosis between the donor renal artery and the recipient’s external iliac artery. The inferior epigastric artery was harvested through the primary surgical field, eliminating the need for an additional incision (Figure 3). The harvested artery was opened longitudinally and fashioned into a cuff by folding it over a syringe to maintain its shape (Figure 4). It was then anastomosed using 7-0 Prolene suture (Figure 5). Subsequently, the extended renal artery and vein of the allograft were anastomosed to the recipient’s right external iliac artery and vein, respectively, using 6-0 Prolene suture (Figure 6). After vascular reconstruction, the clamps were released, confirming excellent perfusion of the kidney, regardless of its position. The ureter was anastomosed to the mucosal layer of the urinary bladder with the aid of a double J catheter. A Redon drain was placed to monitor for potential bleeding.
The procedure was completed without technical complications. The kidney was reperfused successfully, demonstrating immediate diuresis and good perfusion. Intraoperative Doppler ultrasound confirmed satisfactory blood flow in all vessels. The postoperative course was uneventful, and the patient has maintained stable renal function, with serum creatinine levels remaining within normal range at the 6-month follow-up.
Renal allografts with anatomical variations or vascular injury pose significant technical challenges to the transplant surgeon[3]. Vascular anomalies, pre-existing vessel disease, or iatrogenic injury during organ procurement may require ex vivo vascular reconstruction or repair to render the allograft suitable for transplantation[4,5].
Unlike deceased donor kidneys, living donor kidneys often lack extra vessel length, which can complicate surgery, especially if further shortened by anatomical variation, technique, or intra-operative injury[6]. In contrast, in deceased-donor kidney transplants, the renal artery is typically harvested with an aortic patch, providing additional length to facilitate anastomosis or to compensate for arterial injury[7].
In living-donor kidney transplantation, or in cases where a suitable aortic patch is unavailable, multiple graft arteries can be reshaped into a patch plasty using polytetrafluoroethylene (PTFE) or autologous tissue to facilitate vascular anastomosis in the recipient[8,9].
In this case, vascular stapling shortened the main renal artery at an early bifurcation. Therefore, we used the inferior epigastric artery as a cuff between the donor renal artery and recipient external iliac artery. The cuff technique was used not just to address artery shortening but primarily to reduce early thrombosis risk from size mismatch and to prevent long-term stenosis from myointimal hyperplasia.
Transplant renal artery stenosis is a common vascular complication within the first 6 months post-transplant and is a leading cause of graft loss and early mortality in recipients[10]. This condition may arise at various sites, including: (1) Pre-anastomotic stenosis of the recipient iliac artery; (2) Stenosis at the suture line; and (3) Stenosis of the donor renal artery[11]. Identified causes include donor artery atheroma, technical factors related to suture techniques, trauma during organ procurement or transplantation, and immune-mediated vascular injury[11,12]. Although technical factors such as arterial traction, intimal injury, or suture-related complications can contribute to post-transplant arterial stenosis, we did not observe these in our case. Careful dissection and reconstruction likely minimized such risks[13-15].
The donor aortic cuff reduced the risk of anastomotic renal artery stenosis by providing a larger surface area and better diameter match, helping to minimize technical errors and luminal narrowing. It also distances the renal artery ostium from potential fibrotic or calcific changes at the suture line, aiding long-term patency[16-18].
The use of the inferior epigastric artery as a cuff for arterial anastomosis is rare and not well documented in renal transplantation. However, the cuff technique has been studied in animal models, particularly mice, to reduce thrombosis in vascular anastomoses[19]. While the cuff technique may reduce thrombosis risk, its use in human kidney tran
According to the literature, multiple donor renal arteries may be unified into a composite arterial patch using various conduits to facilitate vascular anastomosis in the recipient. These include PTFE[6], the recipient’s hypogastric artery, the deceased donor’s external iliac artery[22], the recipient’s inferior epigastric vein[24] and the saphenous vein[25]. Among these, the saphenous vein graft is commonly used for renal artery reconstruction[26] but has a documented risk of aneurysmal dilation, especially in aorto-renal bypass, with incidence rates of < 1% to 5%-8%[27]. The causes of vein graft aneurysm remain unclear but may involve pre-stenotic dilatation from anastomotic narrowing. Rarely, infectious pseudoaneurysms after renal transplantation can also contribute[28]. Additionally, the high-flow, low-resistance renal vascular environment may lead vein grafts to dilate and develop aneurysms over time[29].
Although the saphenous vein is a commonly used vascular conduit due to its favorable caliber and ease of harvest, we chose not to employ it in this case. Harvesting the vein requires an additional incision, relatively increases operative time, and may favor venous insufficiency in case of a deep venous thrombosis occur later in life. In contrast, the inferior epigastric artery was readily accessible within the same surgical field and offered a suitable caliber match, which enabled a pure arterial reconstruction, making it the preferred option in this context.
The cuff technique was initially developed for infra-genicular bypass surgery to manage diameter discrepancies between prosthetic grafts and small-caliber distal arteries[30]. By creating a more compliant transition zone, it helps reduce turbulent flow and shear stress at the anastomotic site—key contributors to myo-intimal hyperplasia, which is a major cause of thrombosis and mid- to long-term anastomotic stenosis. This technique ultimately facilitates a more physiologic hemodynamic profile, improving long-term graft patency[31].
Using the inferior epigastric artery as a cuff in renal transplantation offers several benefits, namely a high-flow, low-resistance conduit, a long straight segment for anastomosis, reduced vessel harvesting, low vasospasm risk, and minimal aneurysm risk—making it a promising option in select cases.
To the best of our knowledge, this is the first reported case in the literature of a living donor kidney with a short renal artery reconstructed using an interposition cuff fashioned from the recipient’s inferior epigastric artery. The recipient experienced an uneventful recovery, with normal renal function and no postoperative complications. In conclusion, this case highlights a novel and effective approach to managing complex challenges in kidney transplantation.
| 1. | Kuss R, Teinturier J, Milliez P. [Some attempts at kidney transplantation in man]. Mem Acad Chir (Paris). 1951;77:755-764. [PubMed] |
| 2. | Lee S, Kim J, Shin M, Kim E, Moon J, Jung G, Choi G, Kwon C, Joh J, Lee S, Kim S. Comparison of outcomes of living and deceased donor kidney grafts surviving longer than 5 years in Korea. Transplant Proc. 2010;42:775-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Ghazanfar A, Tavakoli A, Zaki MR, Pararajasingam R, Campbell T, Parrott NR, Augustine T, Riad HN. The outcomes of living donor renal transplants with multiple renal arteries: a large cohort study with a mean follow-up period of 10 years. Transplant Proc. 2010;42:1654-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | De Rosa P, Santangelo M, Ferrara A, Pelosio L, Vallefuoco DM, Caggiano L, Imperatore P. Suboptimal kidney: the experience of a single transplant unit. Transplant Proc. 2004;36:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Davari HR, Malek-Hossini SA, Salahi H, Bahador A, Rais-Jalali GA, Behzadi S, Roozbeh J, Javid R, Karbassi A. Sequential anastomosis of accessory renal artery to external iliac artery in the management of renal transplantation with multiple arteries. Transplant Proc. 2003;35:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Chang WB, Shin YH, Park HS, Kim DH, Lee T. The use of polytetrafluoroethylene graft for damaged renal artery in ABO-incompatible living donor kidney transplantation: a case report. Korean J Transplant. 2022;36:67-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Vicéns-Morton AJ, Callaghan C, Olsburgh J. Reconstruction of a Damaged Lower Polar Artery for Kidney Transplantation Using Tubularised Donor Aorta. Case Rep Transplant. 2017;2017:3532473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Barry JM. Renal transplant-recipient surgery. BJU Int. 2007;99:701-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Smith JA, Howards SS, Preminger GM. Hinman’s atlas of urologic surgery. 3rd ed. Amsterdam: Elsevier Health Sciences, 2012: 883-900. |
| 10. | Chen W, Kayler LK, Zand MS, Muttana R, Chernyak V, DeBoccardo GO. Transplant renal artery stenosis: clinical manifestations, diagnosis and therapy. Clin Kidney J. 2015;8:71-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Lacombe M. Arterial stenosis complicating renal allotransplantation in man: a study of 38 cases. Ann Surg. 1975;181:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Fervenza FC, Lafayette RA, Alfrey EJ, Petersen J. Renal artery stenosis in kidney transplants. Am J Kidney Dis. 1998;31:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Smellie WA, Vinik M, Hume DM. Angiographic investigation of hypertension complicating human renal transplantation. Surg Gynecol Obstet. 1969;128:963-968. [PubMed] |
| 14. | Morris PJ, Yadav RV, Kincaid-Smith P, Anderton J, Hare WS, Johnson N, Johnson W, Marshall VC. Renal artey stenosis in renal transplantation. Med J Aust. 1971;1:1255-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Greenstein SM, Verstandig A, McLean GK, Dafoe DC, Burke DR, Meranze SG, Naji A, Grossman RA, Perloff LJ, Barker CF. Percutaneous transluminal angioplasty. The procedure of choice in the hypertensive renal allograft recipient with renal artery stenosis. Transplantation. 1987;43:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Fung LC, McLorie GA, Khoury AE, Churchill BM. Donor aortic cuff reduces the rate of anastomotic arterial stenosis in pediatric renal transplantation. J Urol. 1995;154:909-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Gray DW. Graft renal artery stenosis in the transplanted kidney. Transplant Rev. 1994;8:15-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Agüera Fernández LG, Zudaire JJ, Isa WA, Sánchez de la Muela PL, Rosell D, de Castro F, Robles JE, Errasti P, Berian JM. [Vascular complications in 237 recipients of renal transplant from cadaver]. Actas Urol Esp. 1992;16:292-295. [PubMed] |
| 19. | Yu Y, Li J, Bi Z, Wu C, Yang S, Jiang Q, Deng R, Fu Q, Liu L, Wang C. Cuff Anastomosis of Both Renal Artery and Vein to Minimize Thrombosis: A Novel Method of Kidney Transplantation in Mice. J Invest Surg. 2022;35:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Dubernard JM, Pin J, Gignoux N, Perrin J. [Use of the epigastric artery in renal transplantation (author's transl)]. J Urol Nephrol (Paris). 1976;82:469-472. [PubMed] |
| 21. | Young JS, Rohr MS. Use of the inferior epigastric artery to revascularize a lower pole renal artery in renal transplant. Am Surg. 1995;61:185-186. [PubMed] |
| 22. | Tabbara MM, Guerra G, Riella J, Abreu P, Alvarez A, Vianna R, Chen L, Morsi M, Gaynor JJ, Gonzalez J, Ciancio G. Creating a Single Inflow Orifice From Living Donor Kidney Allografts With Multiple Renal Arteries. Transpl Int. 2022;35:10212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Vincent JG, van Son JA, Skotnicki SH. Inferior epigastric artery as a conduit in myocardial revascularization: the alternative free arterial graft. Ann Thorac Surg. 1990;49:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Fronek J, Hearfield H, Fassiadis N, Magrill D, Morsy M, Kessaris N. Inferior epigastric vein as an interposition graft for renal transplant artery reconstruction. Transplantation. 2010;90:1132-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | A Farooqui M, A O Hali W, Muaikeel M, Naeem Akhtar M, Qurashi S, Mubarak A. Surgical Treatment of Transplant Renal Artery Aneurysm: Case Report and Review of Literature. J Clin Exp Transplant. 2016;1:108. [DOI] [Full Text] |
| 26. | Posner MP, King AL, Brown KB, Lee HM. Long-term experience with surgical repair for transplant renal artery stenosis. In: Abouna GM, Kumar MSA, White AG, editors. Organ Transplantation 1990. Developments in Surgery, Dordrecht: Springer, 1991: 11. [DOI] [Full Text] |
| 27. | Stanley JC, Ernst CB, Fry WJ. Fate of 100 aortorenal vein grafts: characteristics of late graft expansion, aneurysmal dilatation, and stenosis. Surgery. 1973;74:931-944. [PubMed] |
| 28. | Chung MM, Chan YC, Law Y, Cheng SW. Infectious anastomotic pseudoaneurysm complicating renal allograft: case report and review of literature. Int J Nephrol Renovasc Dis. 2017;10:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Oelschlaeger M, Sokolakis I, Kalogirou C, Frey L, Riedmiller H, Kübler H, Kellersmann R, Vergho D. Repair of an Autologous Saphenous Vein Graft Aneurysm Ten Years after Renal Artery Reconstruction during Live Donor Renal Transplantation. Urol Int. 2018;101:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Miller JH, Foreman RK, Ferguson L, Faris I. Interposition vein cuff for anastomosis of prosthesis to small artery. Aust N Z J Surg. 1984;54:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 151] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Lemson MS, Tordoir JH, Daemen MJ, Kitslaar PJ. Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg. 2000;19:336-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/