Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.105597
Revised: March 23, 2025
Accepted: April 15, 2025
Published online: December 18, 2025
Processing time: 294 Days and 17.8 Hours
Transplantectomy has long been considered the preferred treatment for spon
We describe the case of a 37-year-old sensitized XY patient who experienced early spontaneous graft rupture after receiving his second deceased donor kidney transplant. Following temporary hemodynamic stabilization and abdominal con
Amid organ shortages and sensitized patients, graft nephrectomy is reserved for severe injuries; repair using sealants and mesh is effective.
Core Tip: Spontaneous kidney graft rupture represents a life-threatening surgical complication, typically presenting with abdominal pain, impaired renal function, and shock. Contrast-enhanced computed tomography scan is the gold standard imaging modality for diagnosis, while aggressive resuscitation and prompt surgical exploration are key factors for optimized recipient and transplant outcomes. For many years, first-line graft nephrectomy has been considered the standard of care. However, there is now evidence supporting a more conservative approach. Indeed, recent reports show that suture-free repair offers several advantages over direct suturing and extended graft manipulation. We describe a case successfully repaired with sealants application and resorbable mesh wrapping.
- Citation: Campioli E, Sikharulidze A, Cenzi LM, Spagnoletti G, Ferraresso M, Favi E. Suture-free polyglactin 910 mesh repair of kidney graft rupture: A case report and review of literature. World J Transplant 2025; 15(4): 105597
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/105597.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.105597
Spontaneous graft rupture (SGR), though rare, is a critical condition that can result in kidney transplant (KT) loss or death[1]. More often, this dreadful complication arises in the peri-operative course, but delayed presentations have also been recorded[2,3]. Main contributing factors include antibody- or cell-mediated rejection, severe acute tubular necrosis, and renal vein thrombosis[4]. While transplant nephrectomy has been considered the default response for many years, there is now evidence that conservative approaches are feasible in selected clinical scenarios[5]. We herein describe a case of SGR in a sensitized deceased donor KT recipient. The rupture was successfully managed with external compression, app
A 37-year-old XY subject with end-stage renal disease secondary to bilateral cystic dysplasia was admitted to our center for a deceased donor KT in April 2023. The very early post-transplant course was uneventful, with excellent urinary output (> 2000 mL/day) and rapid amelioration of renal function (serum creatinine concentration, 2.3 mg/dL). Three days after the procedure, the patient reported a sudden-onset, sharp, left-sided abdominal pain, mainly located above the surgical incision and unresponsive to analgesics. There were no fluids flowing out from the drain placed in the surgical site and no signs of hemodynamic instability were detected.
The patient was hospitalized as a KT candidate. The kidney donor was a 50-year-old XY individual who had died from intra-cranial hemorrhage. The donor and recipient were blood group compatible and had six human leukocyte antigen mismatches. Pre-operative class-I and class-II panel reactive antibody tests were both 50%. Complement dependent cytotoxicity and flow cytometry assays were negative. Macroscopically, the kidney was normal and medium sized, with two arteries, one vein, and one ureter. It was extra-peritoneally transplanted into the left iliac fossa. The procedure was carried out smoothly, with neglectable blood loss, optimal graft reperfusion, and immediate urinary output. Cold and warm ischemia time were seven hours and 45 minutes, respectively. As induction, the patient received intravenous thymoglobulin 5/mg/kg total-dose and methylprednisolone 500 mg/day for three days. Maintenance immunosuppression consisted of oral lifecycle pharma-tacrolimus (0.15 mg/kg/day), mycophenolic acid 2000 mg/day, and prednisone 20 mg/day.
The comorbidities of the recipient included chronic kidney disease (bilateral cystic dysplasia) on renal replacement therapy (hemodialysis), systemic hypertension, secondary hyperparathyroidism, and previous KT failed due to Pseudomonas aeruginosa deep surgical site infection, further complicated by right external iliac arteritis, right external iliac vein thrombosis, and massive bleeding requiring multiple transfusions, graft nephrectomy, local debridement, and endovascular stenting.
Besides the comorbidities, the personal and family history was unremarkable.
Physical examination upon admission did not identify any abnormal findings.
Laboratory tests performed to assess the post-operative abdominal pain showed a substantial decrease in hemoglobin levels (9.6 g/dL).
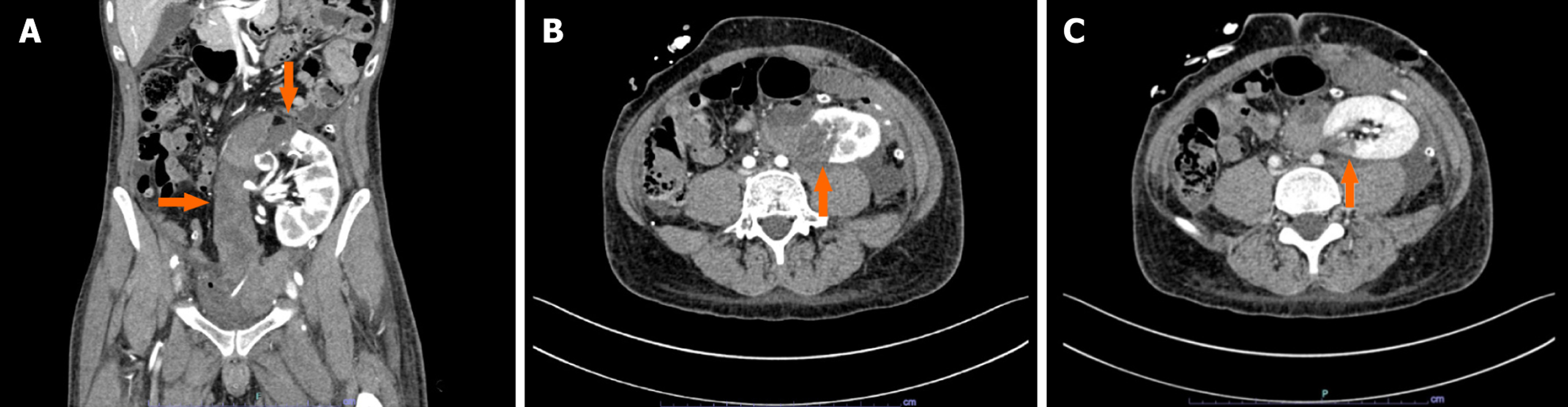
After a brief bedside assessment with a Doppler ultrasound (US) scan (showing a well-perfused graft surrounded by a large retro-peritoneal fluid collection), the recipient underwent immediate contrast-enhanced computed tomography (CT) evaluation. The imaging study demonstrated a large (9.6 cm × 4.3 cm × 17 cm), retro-peritoneal hematoma (Figure 1A) with features of active bleeding from the upper pole of the transplanted kidney (Figure 1B). A focal laceration of the renal cortex not involving the calyceal system and a 3.8-cm-wide area of parenchymal hypoperfusion were noticed in proximity to the blushing spot (Figure 1C). Therefore, a plan was made for surgical exploration.
Intra-operatively, we found a voluminous, partially organized, retro-peritoneal hematoma surrounding the graft, displacing the bladder, and tearing the fascia of the homolateral psoas muscle. Overall, the kidney appeared viable and well perfused, with a 3-cm-wide and 2-cm-deep laceration in the upper pole. There were no signs of leakage at the vascular or ureteral anastomotic sites. The renal vein was patent as much as the two renal arteries. Besides, we noticed a thrombotic occlusion of a superior branch of the main renal artery, likely determining the ischemic area detected by the CT scan.
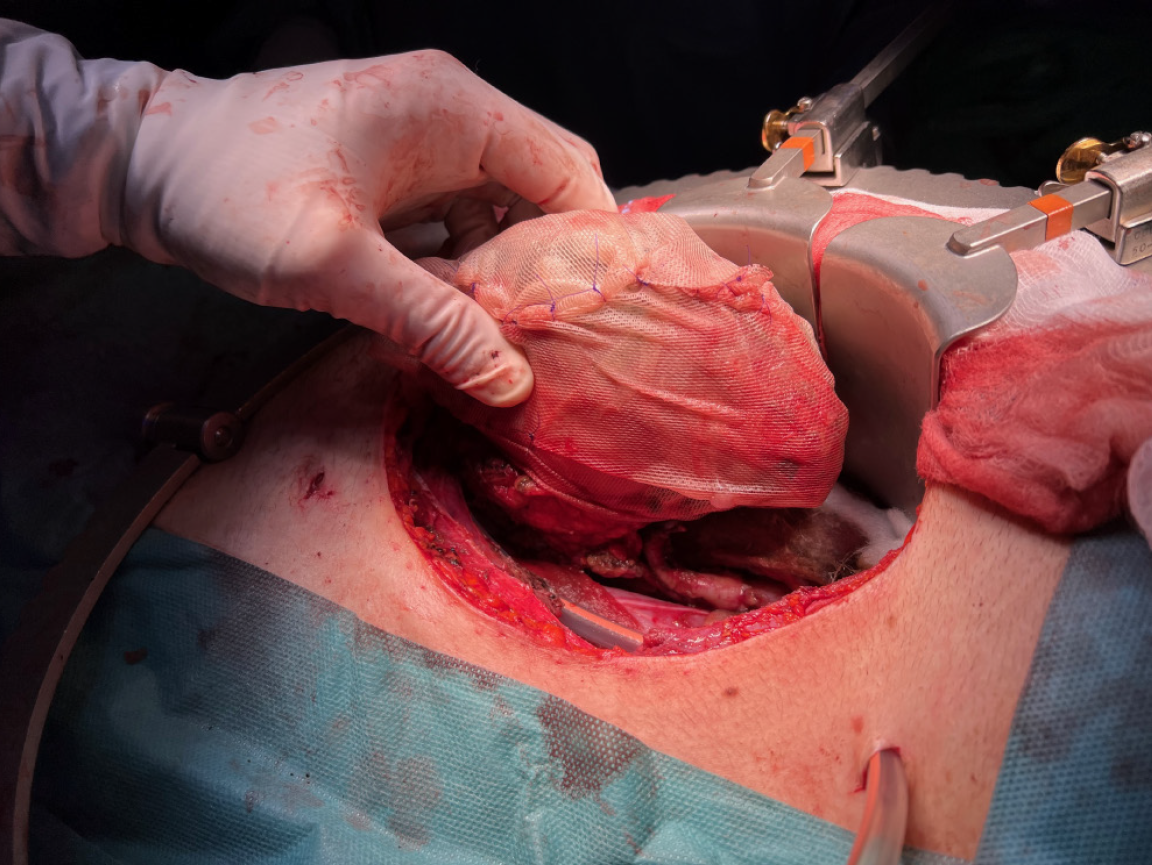
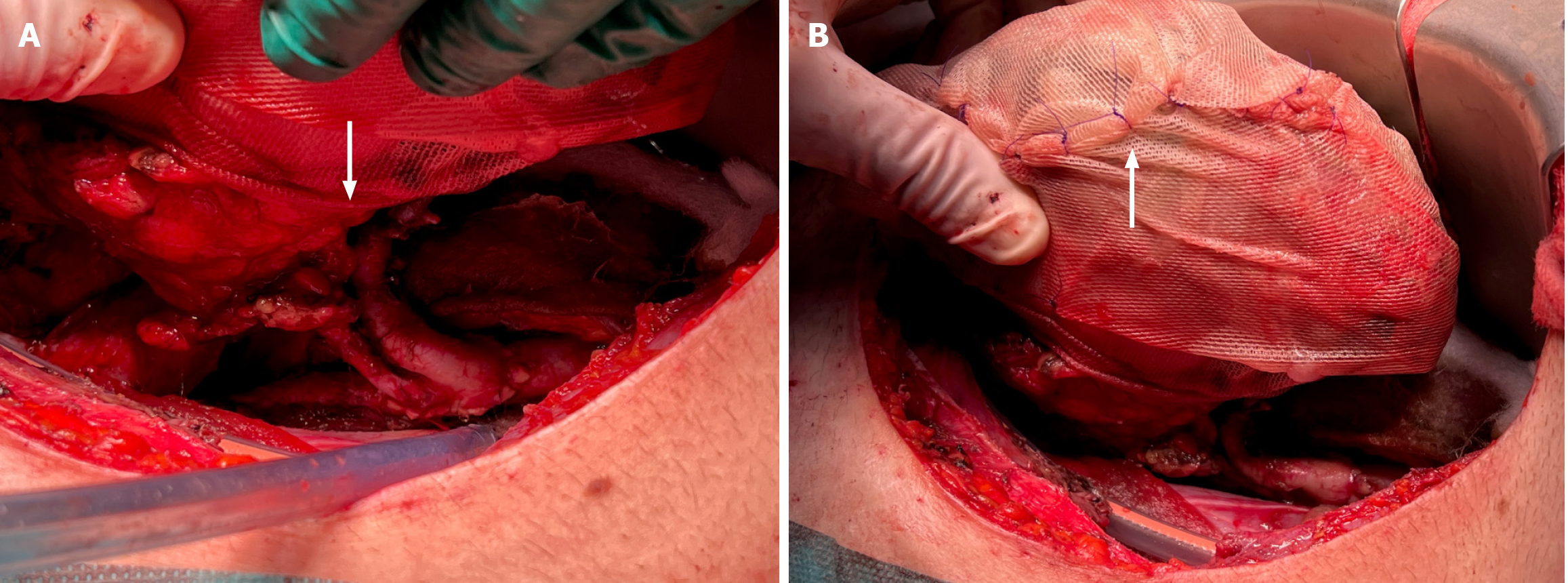
Relevant bleeding and oozing sources located in the surgical site were easily controlled using bi-polar electrocautery and topical hemostats. The parenchymal injury of the graft was managed as follows. The edges and the bottom of the laceration were covered and filled up with a fibrin sealant (Tisseel®, Baxter International, Deerfield, Illinois). After that, we applied a gentle compression on the edges of the lesion. As shown in Figure 2, the surface of the kidney was sprinkled with a fibrin sealant and wrapped in a tailored polyglactin 910 knitted mesh (Vicryl™ Knitted Mesh, Ethicon Inc., Raritan, New Jersey). The mesh was cut in the center and trimmed to let the renal vessels perfectly fit in the gap (Figure 3A). The extremities of the mesh were gradually tightened and tied together with multiple polydioxanone sutures (Figure 3B), avoiding vascular compression while achieving the desired hemostatic effect. The entire procedure took 245 minutes, with an estimated blood loss of 250 mL.
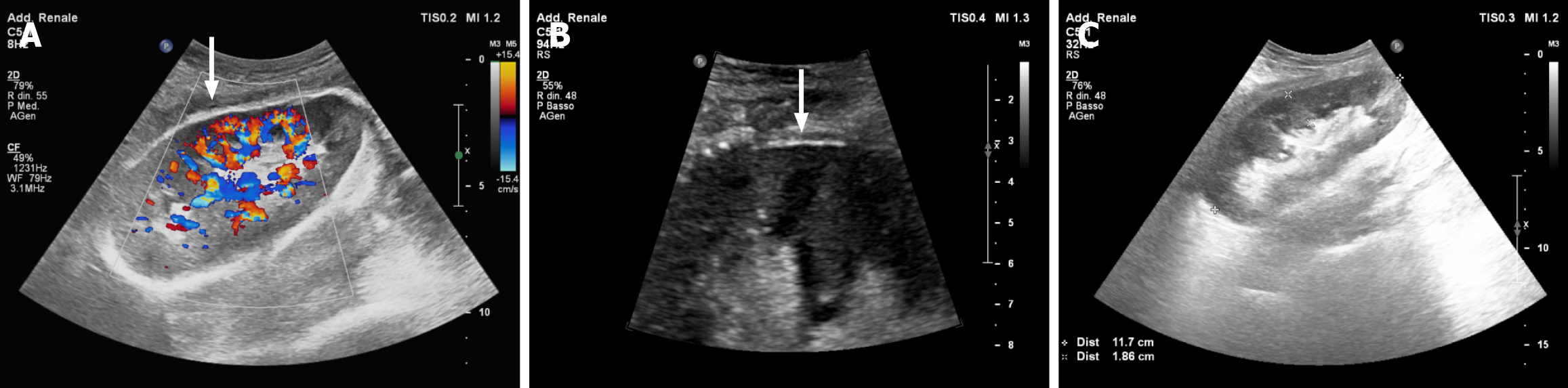
Following the operation, the patient was transferred to the ward with stable vital parameters, reassuring hemoglobin levels, normal urinary output, and excellent renal function. No further complications were recorded, and he was safely discharged from the hospital 14 days after the transplant. At 20 months of follow up, the recipient is doing very well, with a serum creatinine concentration of 1.17 mg/dL. As demonstrated by serial US scans of the surgical site, the polyglactin 910 mesh was gradually reabsorbed, disappearing within six months of implantation. Relevantly, we observed that the prosthetic device did not preclude the possibility of thoroughly evaluating the vascularization of the graft with doppler US techniques (Figure 4).
SGR, marked by an overt parenchymal laceration without pre-existing macroscopic evidence of tissue injury at the time of organ retrieval, benching, or transplantation, represents an uncommon yet potentially fatal surgical complication[1]. Reported incidence varies from 0.4% to 9.6%, depending on the series[6,7]. Rejection is currently recognized as the leading cause of atraumatic graft rupture. Less frequent etiologies include renal vein thrombosis, severe acute tubular necrosis, ischemic or hemorrhagic infarction of the renal cortex, ureteral obstruction with hydronephrosis, pyelonephritis, renal abscess, and neoplasms[2,8]. In our case, the rupture, located in the upper pole of the kidney, was likely caused (or perhaps favored) by the thrombotic event involving the superior branch of the main renal artery. To date, no specific donor- or recipient-related risk factors have been identified[3,9]. However, it has been shown that delayed graft function (defined as the need for dialysis within seven days of transplant) is associated with a substantial increase in the risk of rupture (unadjusted risk ratio, 3.0, 95% confidence interval 2.4-3.8; P < 0.001)[3].
As herein reported, SGR mostly occurs within the first two post-transplant weeks, but later cases have been recorded[4,10]. Common clinical features are sudden-onset abdominal pain with possible irradiation to the lumbar region, sweating, reduced urinary output, tachycardia, hypotension, and hemorrhagic shock[5]. US scan certainly represents the preferred first-line imaging modality for the evaluation of the transplanted kidney. Nevertheless, in the emergency setting, contrast-enhanced CT scan remains the gold standard technique[11]. Typical radiological findings include kidney enlargement, cortical oedema, parenchymal disruption, active pooling of contrast material within or around the graft, and hematoma-like fluid collections located in the retro-peritoneal space.
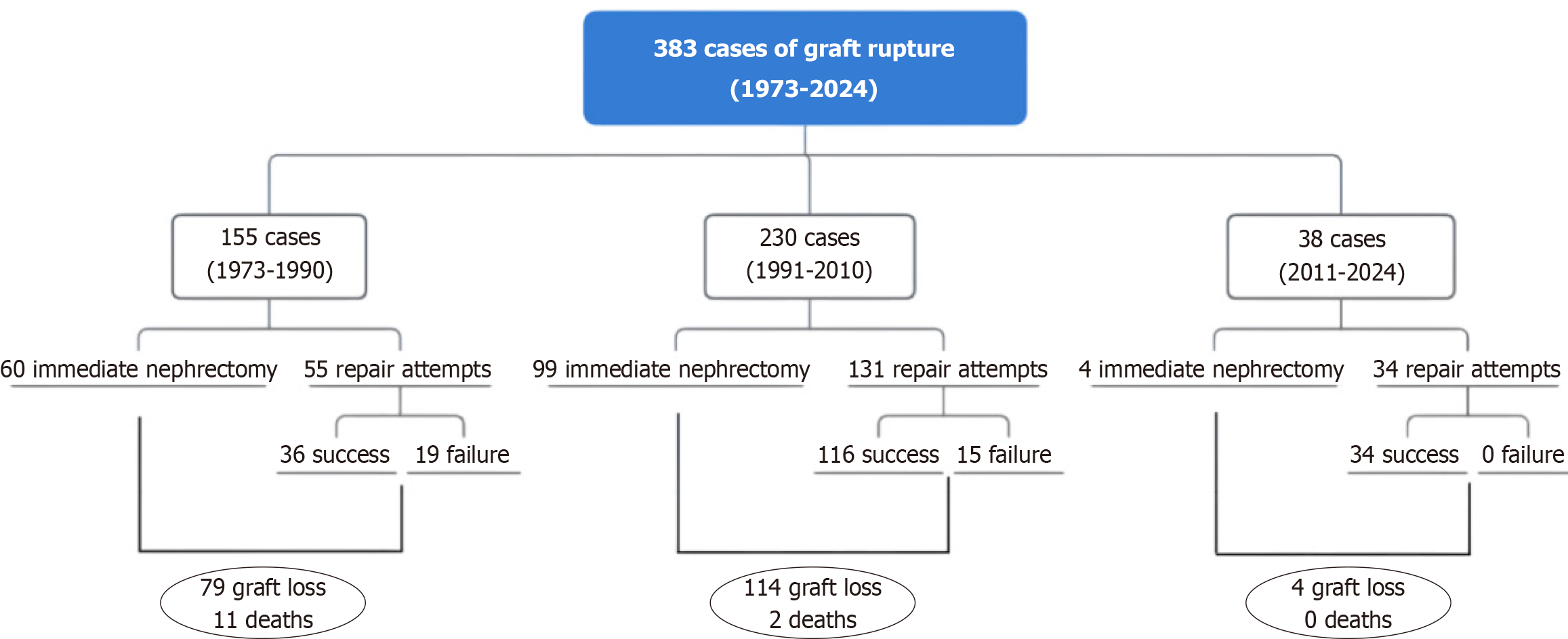
Prompt diagnosis and aggressive fluid resuscitation represent key factors for optimized recipient management. While immediate transplant nephrectomy was once considered the preferred strategy for SGR, recent evidence supports a more conservative approach, showing excellent salvage rates and long-term graft survivals. According to our literature review (Table 1), from 1973 to 2024, 383 cases of SGR have been reported, with 163 immediate transplant nephrectomies and 220 surgical repairs. Among the latter, 34 attempts eventually failed, leading to a concerning overall record of 197 graft losses and 13 deaths. However, sorting the cases of SGR for publication era (1973-1990, 1991-2010, and 2011-2024), it can be noticed that, over the last 50 years, there has been a paradigm shift in the treatment of SGR, as much as a substantial improvement in repair-related outcomes (Figure 5). Indeed, with all the due limitations of a merely descriptive analysis performed on summaries based on individual cases, comparing overall graft losses (immediate graft nephrectomies plus graft nephrectomies following failed repairs) between the three publication eras, we can observe a striking difference in the relative number of successful graft salvages: 36/115 (1973-1990) vs 116/230 (1991-2010) vs 34/38 (2011-2024). Therefore, in current clinical practice, it appears as first-line graft nephrectomy can be justified only by refractory hemodynamic instability, disseminated intravascular coagulation, irreversible rejection, or complex graft injuries with very limited chances of recovery. The graft salvage attempt should be also guided by the specific characteristics of the recipient, including age, comorbidities, and future transplant options. Relevantly, the latest reports show that recurrent rupture, the most dreadful complication of attempted graft repair, occurs in less than 10% of the patients and does not compromise the prognosis.
| Ref. | SGR/KT (n) | P.O. day (range) | Follow up (months) | Graftectomy | Repair | Repair technique | Graft salvage | Death |
| Lord et al[1], 1973 | 1/280 | 14 | < 1 | 1 | 0 | - | - | 1 |
| Ghose et al[20], 1973 | 6/71 | - | - | 3 | 3 | Porcine skin gelatin + sutures | 3 | 0 |
| Fjeldborg et al[21], 1974 | 7/200 | - | - | 4 | 3 | - | 1 | 2 |
| Libertino et al[22], 1974 | 1/- | 1 | 32 | 1 | 0 | - | - | 0 |
| Anderson et al[23], 1976 | 1/- | 5 | 9 | 0 | 1 | Autogenous musculofascial tissue + sutures | 1 | 0 |
| Van Cangh et al[24], 1977 | 9/325 | - | - | 6 | 3 | Autogenous fat bolster + sutures | 0 | 0 |
| Brekke et al[12], 1978 | 16/448 | - | - | 10 | 6 | Direct sutures | 1 | 0 |
| Goldman et al[25], 1978 | 1/- | 8 | 1 | 1 | 0 | - | - | 0 |
| Susan et al[26], 1978 | 4/474 | 5-16 | 15 | 0 | 4 | Oxidized cellulose + sutures | 2 | 0 |
| Prompt et al[27], 1979 | 8/327 | 2-11 | < 1 | 6 | 2 | - | 0 | 0 |
| Dryburgh et al[6], 1979 | 9/93 | 1-18 | 22 | 7 | 2 | - | 1 | 2 |
| van der Vliet et al[28], 1980 | 1/211 | - | - | 1 | 0 | - | - | 0 |
| Goldman et al[29], 1982 | 7/350 | 3-7 | 58 | 3 | 4 | - | 4 | 0 |
| Thukral et al[13], 1982 | 3/100 | 6-8 | 60 | 0 | 3 | Direct sutures or direct sutures + oxcel mesh | 3 | 0 |
| Ajao et al[2], 1984 | 1/- | 1475 | 1 | 1 | 0 | - | - | 1 |
| Yadav et al[30], 1985 | 11/152 | 7-14 | 30 | 3 | 8 | Porcine skin gelatin + sutures | 8 | 3 |
| Serrallach et al[31], 1985 | 5/66 | 4-10 | 15 | 0 | 5 | Compression + lyophilized human dura | 5 | 0 |
| Schwartz et al[32], 1986 | 5/80 | 5-17 | - | 4 | 1 | - | 0 | 1 |
| Chopin et al[33], 1989 | 4/85 | 5-32 | 12 | 0 | 4 | Synthetic glue + polyglactin 910 mesh | 3 | 0 |
| Richardson et al[34], 1990 | 15/791 | 3-14 | 72 | 9 | 6 | Topical hemostatic agent + sutures | 4 | 1 |
| Odocha et al[35], 1991 | 1/- | 5 | - | 1 | 0 | - | - | 0 |
| Robertson et al[36], 1992 | 6/384 | 4-38 | 43 | 0 | 6 | Oxidized cellulose + sutures | 6 | 0 |
| Rosario et al[37], 1993 | 3/571 | 2-4 | - | 0 | 3 | Polyglycolic acid mesh | 3 | 0 |
| Yadav et al[38], 1994 | 15/237 | - | 120 | 4 | 11 | Gelatin matrix + sutures | 11 | - |
| Said et al[39], 1994 | 3/75 | 4-11 | 10 | 2 | 1 | Oxidized cellulose + sutures | 1 | 0 |
| Heimbach et al[18], 1995 | 8/238 | 8-17 | 94 | 1 | 7 | Fibrin sealant + collagen foam + polyglactin 910 mesh | 7 | 0 |
| Guleria et al[17], 1995 | 1/- | 5 | - | 0 | 1 | - | 1 | 0 |
| Azar et al[3], 1996 | 12/331 | 4-21 | - | 12 | 0 | - | - | 1 |
| Zadrozny et al [40], 1997 | 8/112 | - | - | 2 | 6 | Fibrin sealant + polyester mesh or cyanoacrylate + polyester mesh or gelatin matrix + sutures | 3 | 0 |
| Pontones Moreno et al[41], 1998 | 21/868 | - | - | 4 | 16 | Lyophilized human dura or autogenous fascia lata or lyophilized human dura + autogenous fascia lata or polyglycolic acid mesh | 16 | 0 |
| Szenohradszky et al[42], 1999 | 53/628 | - | - | 37 | 16 | - | 16 | - |
| Chan et al[43], 1999 | 1/- | 5 | 1 | 0 | 1 | Collagen sheets coated with hemostats | 1 | 0 |
| Philipneri et al[44], 2000 | 1/- | 7 | < 1 | 0 | 1 | - | 1 | 0 |
| Ramos et al[45], 2000 | 11/934 | 2-13 | - | 10 | 1 | Fibrin sealant + collagen foam | 1 | 0 |
| Millwala et al[46], 2000 | 4/145 | - | 6 | 2 | 2 | Porcine skin gelatine + polyglycolic acid mesh or collagen foam + fibrin sealant + lyophilized human dura | 2 | 1 |
| Ahmed et al[8], 2001 | 1/- | 1099 | 3 | 0 | 0 | - | - | 0 |
| Hochleitner et al[4], 2001 | 14/1811 | 3-23 | 111 | 5 | 9 | Direct sutures or fibrin sealant + infrared and/or laser coagulation or absorbable mesh | 9 | 0 |
| Zakir et al[10], 2003 | 1/- | 644 | < 1 | 1 | 0 | - | - | 0 |
| Guleria et al[47], 2003 | 3/172 | 5-7 | - | 0 | 3 | Autogenous muscle patch (transversus abdominis/internal oblique muscle) | 3 | 0 |
| He et al[48], 2003 | 38/1000 | - | 60 | 2 | 31 | Sutures + external oblique aponeurosis pledgets | 29 | 0 |
| Sanchez de la Nieta et al[9], 2004 | 10/657 | 1-10 | - | 5 | 5 | Direct sutures or Polyglycolic acid mesh | 3 | 0 |
| Busi et al[49], 2004 | 4/778 | 4-13 | < 1 | 3 | 1 | - | 1 | 0 |
| Shahrokh et al[7], 2005 | 6/1682 | 4-13 | 60 | 3 | 3 | Autogenous fat bolster + sutures or oxidized cellulose + sutures | 2 | 0 |
| Basaran et al[50], 2009 | 5/1379 | 6-32 | - | 5 | 0 | - | - | - |
| Askandarani et al[51], 2011 | 1/- | 1950 | - | 1 | 0 | - | - | - |
| McCausland et al[52], 2011 | 1/- | 11 | 7 | 0 | 1 | Human thrombin and animal gelatin + fibrin sealant | 1 | 0 |
| Han et al[15], 2012 | 26/1851 | - | 78 | 0 | 26 | Autogenous fat bolster + resorbable bundle or autogenous muscle patch + resorbable bundle or sutures + autologous cubic muscle pledgets or sutures + external oblique aponeurosis pledgets | 26 | 0 |
| Lai et al[53], 2014 | 1/- | 9 | 24 | 0 | 1 | Oxidized cellulose + sutures + hem-o-lok | 1 | 0 |
| Sin et al[54], 2014 | 1/- | 20 | - | 1 | 0 | - | - | 0 |
| Almarastani et al[55], 2014 | 1/- | 4 | 1 | 0 | 1 | Human fibrinogen and thrombin + bovine albumin-glutaraldehyde | 1 | 0 |
| Favi et al[5], 2015 | 1/- | 4 | 96 | 0 | 1 | Human thrombin and animal gelatin + polyglactin 910 mesh | 1 | 0 |
| Ray et al[56], 2017 | 1/- | 6 | < 1 | 0 | 1 | Human fibrinogen and thrombin + sutures + hem-o-lok | 1 | 0 |
| Ko et al[57], 2019 | 1/- | 6 | 20 | 0 | 1 | Human thrombin and animal gelatin + oxidized cellulose | 1 | 0 |
| Fazeli et al[58], 2021 | 1/- | 5 | < 1 | 0 | 1 | Sutures + fat bolster | 1 | 0 |
| Montali et al[59], 2022 | 1/- | 14 | 2 | 1 | 0 | - | - | 0 |
| Godara et al[60], 2022 | 1/- | 7 | - | - | 1 | Sutures + oxidized cellulose | 1 | 0 |
| Konopa et al[61], 2024 | 1/- | 4 | 30 | 1 | 0 | - | - | 0 |
In this specific case, we opted for graft repair for the following reasons: (1) The quality of the kidney was above average; (2) Considering the past surgical history of the patient and the previous immunization, the chances of receiving another transplant were relatively low; (3) The recipient was young, fit (no diabetes mellitus, no cardiac conditions, no autoimmune disorders), hemodynamically stable, and with no signs of coagulopathy at the time of the second operation; (4) As shown by the contrast-enhanced CT scan performed before surgery, the laceration was in the upper pole of the graft, with a neglectable risk of post-operative ureteral ischemia or necrosis; (5) The imaging study demonstrated that the rupture was superficial (limited to the renal cortex), without involvement of the calyceal system (minimal risk of post-operative urinary leakage); (6) Intra-operatively, the kidney appeared viable and well perfused, with no signs of vascular thrombosis or ureteral leakage; (7) As suggested by the CT scan (area of parenchymal hypoperfusion close to the bleeding spot), and in line with the intra-operative findings (thrombotic occlusion of a small superior branch of the main renal artery), the rupture did not recognize an immunological cause (severe rejection episode or thrombotic microangiopathy) possibly jeopardizing long-term graft survival or function; (8) All bleeding and oozing sources in the surgical site were easily controlled using bi-polar electrocautery and topical hemostats; (9) The hemorrhage caused by the laceration stopped short after the application of the fibrin sealant and external compression; and (10) During the wrapping maneuver with the polyglactin 910 mesh, there were no signs of recurrent bleeding from the ruptured area of the kidney.
Graft repair techniques have also evolved over time (Table 1). In the past, direct suturing of the ruptured edges of the transplanted kidney was common practice[12,13]. Nowadays, most surgeons prefer avoiding unnecessary manipulation of the graft, opting for the primary use of topical hemostatic agents or sealants[5], such as oxidized regenerated cellulose, absorbable gelatin matrix, microfibrillar collagen, microporous polysaccharide spheres, topical thrombin, fibrin sealant, human thrombin plus animal gelatin, bovine albumin-glutaraldehyde tissue adhesive, and dual polyethylene glycol[14]. As a matter of fact, it has been demonstrated that suture-free repairs are easier, faster, and less traumatic than traditional suture-based techniques, with reduced peri-operative blood loss[15]. Since the graft of our patient was frankly edematous and extremely fragile, in line with current trends, we decided to apply a fibrin sealant at the base of the rupture and over the damaged margins of the graft. To generate and maintain an external compression, we also wrapped the kidney with a polyglactin 910 knitted mesh, anchored to the surface of the organ with a fibrin sealant, and progressively tightened with interrupted resorbable sutures (Supplementary material). Indeed, renal corsetage offers several additional advantages as it supports hemostasis and reduces the risk of further laceration[16]. Reported options include human tissues such as external oblique aponeurosis, fascia lata, and lyophilized dura or synthetic devices like polypropylene, polyglycolic acid, and polyglactin meshes[15,17]. We believe that a polyglactin 910 knitted mesh represents the optimal choice because it is readily available, cheap, flexible, resistant, non-antigenic, non-pyrogenic, and completely resorbable within three to six months of use. Furthermore, compared to non-absorbable synthetic materials, polyglactin 910 entails a reduced risk of infection and it is associated with less scarring[5,18]. These characteristics are particularly relevant in the setting of renal graft repair as transplant patients are more susceptible to infectious complications than the general population[19], are routinely followed up with US scans or graft biopsies and may require further surgical procedures in the long term.
SGR represents a serious complication of KT. Most cases occur in the peri-operative course and usually present with abdominal pain and hemorrhagic shock. Contrast enhanced CT scan is the gold standard diagnostic modality. Aggressive resuscitation and prompt surgical exploration are key factors for optimized recipient and graft outcomes. For many years, graft nephrectomy has been considered the default treatment. However, current literature supports first-line salvage attempts. As confirmed by our experience, suture-free surgical techniques offer an easy and reproducible repair option, with excellent results and no impact on post-transplant long-term management.
We thank Cesina Tamburri for logistical support.
| 1. | Lord RS, Effeney DJ, Hayes JM, Tracy GD. Renal allograft rupture: cause, clinical features and management. Ann Surg. 1973;177:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Ajao OG, Callender CO, Stevens J, Sampson C. Spontaneous renal allograft rupture 4 years after transplantation. Urol Int. 1984;39:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Azar GJ, Zarifian AA, Frentz GD, Tesi RJ, Etheredge EE. Renal allograft rupture: a clinical review. Clin Transplant. 1996;10:635-638. [PubMed] |
| 4. | Hochleitner BW, Kafka R, Spechtenhauser B, Bösmüller C, Steurer W, Königsrainer A, Margreiter R. Renal allograft rupture is associated with rejection or acute tubular necrosis, but not with renal vein thrombosis. Nephrol Dial Transplant. 2001;16:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Favi E, Iesari S, Cina A, Citterio F. Spontaneous renal allograft rupture complicated by urinary leakage: case report and review of the literature. BMC Urol. 2015;15:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Dryburgh P, Porter KA, Krom RA, Uchida K, West JC, Weil R 3rd, Starzl TE. Should the ruptured renal allograft be removed? Arch Surg. 1979;114:850-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Shahrokh H, Rasouli H, Zargar MA, Karimi K, Zargar K. Spontaneous kidney allograft rupture. Transplant Proc. 2005;37:3079-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ahmed S, Batiuk TD. Broken kidney: traumatic fracture of a renal allograft. Am J Kidney Dis. 2001;37:E33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Sanchez de la Nieta MD, Sánchez-Fructuoso AI, Alcázar R, Pérez-Contin MJ, Prats D, Grimalt J, Blanco J. Higher graft salvage rate in renal allograft rupture associated with acute tubular necrosis. Transplant Proc. 2004;36:3016-3018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Zakir AH, Woodside KJ, Feliberti EC, Rajaraman S, Gugliuzza KK, Daller JA. Late renal allograft rupture in a patient with small vessel vasculitis following discontinuation of immunosuppression. Transpl Int. 2003;16:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Goiffon RJ, Depetris J, Dageforde LA, Kambadakone A. Radiologic evaluation of the kidney transplant donor and recipient. Abdom Radiol (NY). 2025;50:272-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Brekke I, Flatmark A, Laane B, Mellbye O. Renal allograft rupture. Scand J Urol Nephrol. 1978;12:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Thukral R, Mir AR, Jacobson MP. Renal allograft rupture: a report of three cases and review of the literature. Am J Nephrol. 1982;2:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Allotey JK, King AH, Kumins NH, Wong VL, Harth KC, Cho JS, Kashyap VS. Systematic review of hemostatic agents used in vascular surgery. J Vasc Surg. 2021;73:2189-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Han XW, He B, Zhang YH, Amin B, Yan W, Tian Y. A novel technique for suture-free repair of renal allograft rupture. Ann Transplant. 2012;17:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Wainstein MA, Resnick MI. Use of polyglycolic acid mesh to support parenchymal closure following partial nephrectomy. J Urol. 1997;158:526-527. [PubMed] |
| 17. | Guleria S, Sinha S, Dorairajan LN, Khazanchi RK, Saxena S, Aggarwal SK, Tiwari SC, Dash SC. Spontaneous renal allograft rupture: still a threat. Nephron. 1995;70:385-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Heimbach D, Miersch WD, Buszello H, Schoeneich G, Klehr HU. Is the transplant-preserving management of renal allograft rupture justified? Br J Urol. 1995;75:729-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Sommerer C, Schröter I, Gruneberg K, Schindler D, Behnisch R, Morath C, Renders L, Heemann U, Schnitzler P, Melk A, Della Penna A, Nadalin S, Heeg K, Meuer S, Zeier M, Giese T; Transplant Cohort of the German Center for Infection Research (DZIF Transplant Cohort) Consortium. Incidences of Infectious Events in a Renal Transplant Cohort of the German Center of Infectious Diseases (DZIF). Open Forum Infect Dis. 2022;9:ofac243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 20. | Ghose MK, Kest LM, Cohen SM, Roza O, Berman LB, Lidsky I, Teitelbaum S, Galvin JB. Spontaneous rupture of renal allotransplants. J Urol. 1973;109:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Fjeldborg O, Kim CH. Spontaneous rupture of renal transplant. Scand J Urol Nephrol. 1974;8:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Libertino JA, Merino MJ, Zinman L, Takacs FJ. Spontaneous rupture of renal transplants. Urology. 1974;3:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 23. | Anderson B, Sampson C, Callender CO. Spontaneous renal allograft rupture without rejection: a case report. J Urol. 1976;115:745-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Van Cangh PJ, Ehrlich RM, Smith RB. Renal rupture after transplantation. Urology. 1977;9:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Goldman MH, Leapman SB, Handy RD, Best DW. Renal allograft rupture with iliofemoral thrombophlebitis. Arch Surg. 1978;113:204-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Susan LP, Braun WE, Banowsky LH, Straffon RA, Valenzuela R. Ruptured human renal allograft. Pathogenesis and management. Urology. 1978;11:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Prompt CA, Johnson WH, Ehrlich RM, Lee DB, Smith RB, Schultze RG. Nontraumatic rupture of renal allografts. Urology. 1979;13:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | van der Vliet JA, Kootstra G, Tegzess AM, Meijer S, Krom RA, Slooff MJ, Hooykaas JA, Mensink HJ. Management of rupture in allografted kidneys. Neth J Surg. 1980;32:45-48. [PubMed] |
| 29. | Goldman M, de Pauw L, Kinnaert P, Vereerstraeten P, van Geertruyden J, Toussiant C. Renal Allograft Rupture—Possible Causes and Results of Surgical Conservative Management. J Urology. 1982;127:397-398. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Yadav RV, Sinha R, Chugh KS, Sakuja VK, Datta BN. Renal allograft rupture and its management. Urol Int. 1985;40:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Serrallach N, Gutierrez R, Serrate R, Aguilò F, Muñoz J, Franco E, Griño J, Gil-Vernet S, Alsina J, Caralps A. Renal allograft rupture: surgical treatment by renal corsetage with lyophilized human dura. J Urol. 1985;133:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Schwartz A, Podzimek A, Valenta J, Klecka J, Opatrný K. Spontaneous renal allograft rupture. Clinical and pathological patterns. Int Urol Nephrol. 1986;18:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Chopin DK, Abbou CC, Lottmann HB, Popov Z, Lang PR, Buisson CL, Belghiti D, Colombel M, Auvert JM. Conservative treatment of renal allograft rupture with polyglactin 910 mesh and gelatin resorcin formaldehyde glue. J Urol. 1989;142:363-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Richardson AJ, Higgins RM, Jaskowski AJ, Murie JA, Dunnill MS, Ting A, Morris PJ. Spontaneous rupture of renal allografts: the importance of renal vein thrombosis in the cyclosporin era. Br J Surg. 1990;77:558-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Odocha O, Callender CO, Pinn-Wiggins VW. Spontaneous rupture of the renal allograft. J Natl Med Assoc. 1991;83:171-174. [PubMed] |
| 36. | Robertson AJ, Francis DM, Millar RJ, Clunie GJ, Walker RG. Spontaneous renal allograft rupture: a disappearing phenomenon in the cyclosporine era? Aust N Z J Surg. 1992;62:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Rosario PG, Greenstein SM, Schechner RS, Tellis VA. Spontaneous rupture of human renal allografts. Urology. 1993;41:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Yadav RV, Sinha R. Graft repair: the treatment of choice for renal allograft rupture. J Urol. 1994;151:1498-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Said R, Duarte R, Chaballout A, el Boghdadly S, Nezamuddin N, Mattoo T. Spontaneous rupture of renal allograft. Urology. 1994;43:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Zadrozny D, Pirski MI, Draczkowski T, Gacyk W. The treatment of renal allograft rupture. Transplant Proc. 1997;29:156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Pontones Moreno JL, Rodrigo Aliaga M, Monserrat Monfort JJ, Guillén Navarro M, Sánchez Plumed J, Jiménez Cruz JF. [Post-transplantation renal rupture]. Actas Urol Esp. 1998;22:840-5; discussion 846. [PubMed] |
| 42. | Szenohradszky P, Smehák G, Szederkényi E, Marofka F, Csajbók E, Morvay Z, Ormos J, Iványi B. Renal allograft rupture: a clinicopathologic study of 37 nephrectomy cases in a series of 628 consecutive renal transplants. Transplant Proc. 1999;31:2107-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Chan YH, Wong KM, Lee KC, Li CS. Spontaneous renal allograft rupture attributed to acute tubular necrosis. Am J Kidney Dis. 1999;34:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Philipneri M, Solomon H, Garvin PJ, Bastani B. Delayed graft function complicated by spontaneous renal allograft rupture without acute rejection. Am J Nephrol. 2000;20:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Ramos M, Martins L, Dias L, Henriques AC, Soares J, Queirós J, Sarmento AM. Renal allograft rupture: a clinicopathologic review. Transplant Proc. 2000;32:2597-2598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Millwala FN, Abraham G, Shroff S, Soundarajan P, Rao R, Kuruvilla S. Spontaneous renal allograft rupture in a cohort of renal transplant recipients: a tertiary care experience. Transplant Proc. 2000;32:1912-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Guleria S, Khazanchi RK, Dinda AK, Aggarwal S, Gupta S, Bhowmik D, Aggarwal SK, Tiwari SC, Dash SC, Mandal S. Spontaneous renal allograft rupture: is graft nephrectomy an option? Transplant Proc. 2003;35:339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | He B, Rao MM, Han X, Li X, Guan D, Gao J. Surgical repair of spontaneous renal allograft rupture: a new procedure. ANZ J Surg. 2003;73:381-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Busi N, Capocasale E, Mazzoni MP, Benozzi L, Valle RD, Cambi V, Sianesi M. Spontaneous renal allograft rupture without acute rejection. Acta Biomed. 2004;75:131-133. [PubMed] |
| 50. | Basaran C, Donmez FY, Tarhan NC, Coskun M, Haberal M. Multidetector computed tomography findings of spontaneous renal allograft ruptures. Clin Radiol. 2009;64:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Askandarani S, Aloudah N, Al Enazi H, Alsaad KO, Altamimi A. Late Renal Allograft Rupture Associated with Cessation of Immunosuppression following Graft Failure. Case Rep Transplant. 2011;2011:512893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | McCausland FR, Varma MC, Goes NB, Heher EC, Markmann JF, Cosimi AB, Elias N, Ko DS, Kawai T, Smith RN, Rubin MF. Renal allograft rupture: a strategy for graft preservation. Transplantation. 2011;91:e67-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Lai Q, Rizza V, Di Clemente L, Iesari S, Bellobono M, Bianchi Z, Clemente K, Famulari A, Pisani F. A new proposal of surgical suture in case of spontaneous renal allograft rupture. Transplant Proc. 2014;46:2207-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Sin YH, Kim YJ, Oh JS, Lee JH, Kim SM, Kim JK. Graft rupture after high-dose intravenous immunoglobulin therapy in a renal transplant patient. Nephrology (Carlton). 2014;19 Suppl 3:35-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Almarastani M, Aloudah N, Hamshow M, Hegab B, Alsaad KO. Salvaging of severely ruptured living-related renal allograft secondary to acute antibody mediated rejection. Int J Surg Case Rep. 2014;5:723-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Ray DS, Thukral S. Spontaneous Renal Allograft Rupture Caused by Acute Tubular Necrosis: A Case Report and Review of the Literature. Case Rep Transplant. 2017;2017:9158237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Ko K, Jeong HC, Jeon HJ, Ko K, Lee S. Temporary Renal Artery Clamping With Hemostatic Materials in Diffuse Type of Spontaneous Renal Allograft Rupture: A Case Report. Transplant Proc. 2019;51:2845-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Fazeli F, Yasseri AF, Kazem Aghamir SM. Successful salvage of renal allograft rupture: A case report. Urol Case Rep. 2021;35:101538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Montali F, Annicchiarico A, Grisales P, Panarese A, Pisani F. Hypothermic Machine Perfusion and Spontaneous Kidney Allograft Rupture: Causation or Correlation? A Case Report and Review of Pertinent Literature. Transplant Proc. 2022;54:2716-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Godara S, Saraf K. Spontaneous renal allograft rupture due to acute rejection in early post-transplant period – A case report. Indian J Transplant. 2022;16:138-141. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Konopa J, Komorowska-Jagielska K, Łaski D, Chamienia A, Budyńko Ł, Dębska-Ślizień A. Kidney Transplant Loss Due to May-Thurner Syndrome: Case Report and Review of the Literature. Transplant Proc. 2024;56:972-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/