Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.104675
Revised: March 24, 2025
Accepted: June 13, 2025
Published online: December 18, 2025
Processing time: 326 Days and 21.1 Hours
Aorto-hepatic conduits (AHCs) are an effective revascularization method for liver allografts when the native hepatic artery is unusable. Various studies have con
To investigate the published evidence on the outcomes according to different inflow site for AHCs.
A systematic search was conducted for studies reporting on AHCs in liver transplantation over the last 10 years (January 2014 onwards). Two independent reviewers selected articles, assessed quality, and evaluated bias in the included systematic reviews. The methodological quality of the included studies was assessed using the Newcastle-Ottawa Scale. The protocol was registered with PROSPERO (CRD42024545810). Review was conducted using the Preferred Re
Fourteen studies identified a total of 32486 deceased donor liver transplants, of which 1136 (3.5%) required AHCs. The most frequent indications for AHC use included poor arterial flow, intimal dissections, and hepatic artery thrombosis. Among all AHCs, 207 (18.2%) were supra-coeliac (SC) AHCs, 738 (65.0%) infra-renal (IR) AHCs, 25 (2.2%) iliac artery conduits, and 166 (14.6%) had unspecified origins. Pooled analysis revealed comparable demographic characteristics. The median follow-up duration ranged from 18 to 52 months. There were no sig
Considering these findings, there is no significant difference in early and late outcomes between SC and IR AHCs, although there is a discernible tendency towards higher late occlusion rates in the IR group.
Core Tip: This meta-analysis of fourteen studies identified aorta-hepatic conduits utilized in 3.5% of deceased donor liver transplantation, with the majority being infra-renal conduits, however, 5-year patency was better with supra-coeliac aortic conduits.
- Citation: Uragoda Appuhamilage B, Gupta S, Parente A, Srinivasan P, Menon K, Hakeem AR. Systematic review and meta-analysis of the role of aorto-hepatic conduits in liver transplant: Known knowns and known unknowns. World J Transplant 2025; 15(4): 104675
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/104675.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.104675
Effective vascularization of the liver allograft is crucial for the success of a liver transplant (LT). Hepatic artery-related complications, such as poor flow, dissections, thrombosis, and stenosis, are disastrous complications that can lead to graft failure and the need for retransplantation[1-5]. In some instances, it may not be possible to anastomose the native recipient hepatic artery to the donor hepatic artery. Alternatively, the splenic artery, gastroduodenal artery, and coeliac artery have been described as inflow sources[6-10]. However, these vessels often share a common origin from the aorta, which might also be affected by the same pathology. In such scenarios, aorto-hepatic conduits (AHC) can serve as a salvage technique.
AHC inflow sites are typically the supra-coeliac (SC) aorta, infra-renal (IR) aorta, or, in rare cases, the iliac arteries. Most studies report no difference in short- and long-term occlusion rates based on inflow sites[11,12]. However, each site has distinct advantages and disadvantages. SC inflow closely mimics natural anatomical inflow and requires a shorter conduit. However, accessing the SC aorta can be challenging in patients with portal hypertension and large varices. Additionally, aortic clamping may theoretically cause renal insufficiency and spinal neurological effects[11]. In contrast, the IR aorta provides easier access, although it can impact the renal arteries. This region of the aorta may also be prone to severe atherosclerosis, posing significant risks during or after clamping, including dissection, uncontrolled bleeding, and risk of graft or limb ischaemia[13]. Previous abdominal surgeries may complicate access to the IR aorta. The reversal of blood flow in the IR conduit is highly influenced by systemic hemodynamic changes, and over time, this may result in graft ischemia and thrombosis[12,13].
Different materials have been utilized in constructing AHCs depending on the setting and availability. The donor iliac artery is the main conduit material in the deceased donor setting, typically retrieved at the end of the procedure[14]. However, this artery can be compromised by atherosclerosis and aneurysmal dilatation, making it unsuitable as a conduit. In such cases, donor superior mesenteric artery, carotid arteries, or axillary arteries have been used as alternatives. In the living donor setting, the donor external jugular or greater saphenous vein (GSV) has been commonly utilized as a conduit[15-19].
While some aspects of their efficacy and safety are well-documented, uncertainties remain regarding their optimal utilization and impact on patient short- and long-term outcomes. This systematic review and meta-analysis aim to elucidate the established facts and unsolved questions surrounding the role of AHCs in LT. Specifically, it seeks to consolidate existing evidence on the selection of inflow sites (SC vs IR), post-operative complications related to conduit, and overall graft/patient survival.
The study design of systematic review and meta-analysis was chosen to define the role of published evidence on different AHCs. The study followed the preferred reporting items for systematic review and meta-analysis (PRISMA) statement standards[20,21]. Our review was registered at the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42024545810).
A search strategy in line with the Meta-analysis Of Observational Studies in Epidemiology guidelines and previous recommendations for the conduction of systematic reviews of prognostic variables was developed. An electronic search of MEDLINE, EMBASE, PubMed, Cochrane Library, CINAHL, and Google Scholar was conducted independently by authors BU and SG. The databases were searched to identify studies reporting on AHCs in LT in the last 10 years (January 2014 onwards). Full literature search strategy in MEDLINE (OVID) was: (Liver transplantation OR hepatic graft OR ("Liver Transplantation/adverse effects"[Mesh] OR "Liver Transplantation/classification"[Mesh]) AND (y_10[Filter])) AND (Hepatic artery OR ("Blood Vessel Prosthesis/adverse effects"[Mesh] OR "Blood Vessel Prosthesis/classification"[Mesh] OR "Blood Vessel Prosthesis/history"[Mesh] OR "Blood Vessel Prosthesis/supply and distribution"[Mesh]) AND (y_10[Filter])).
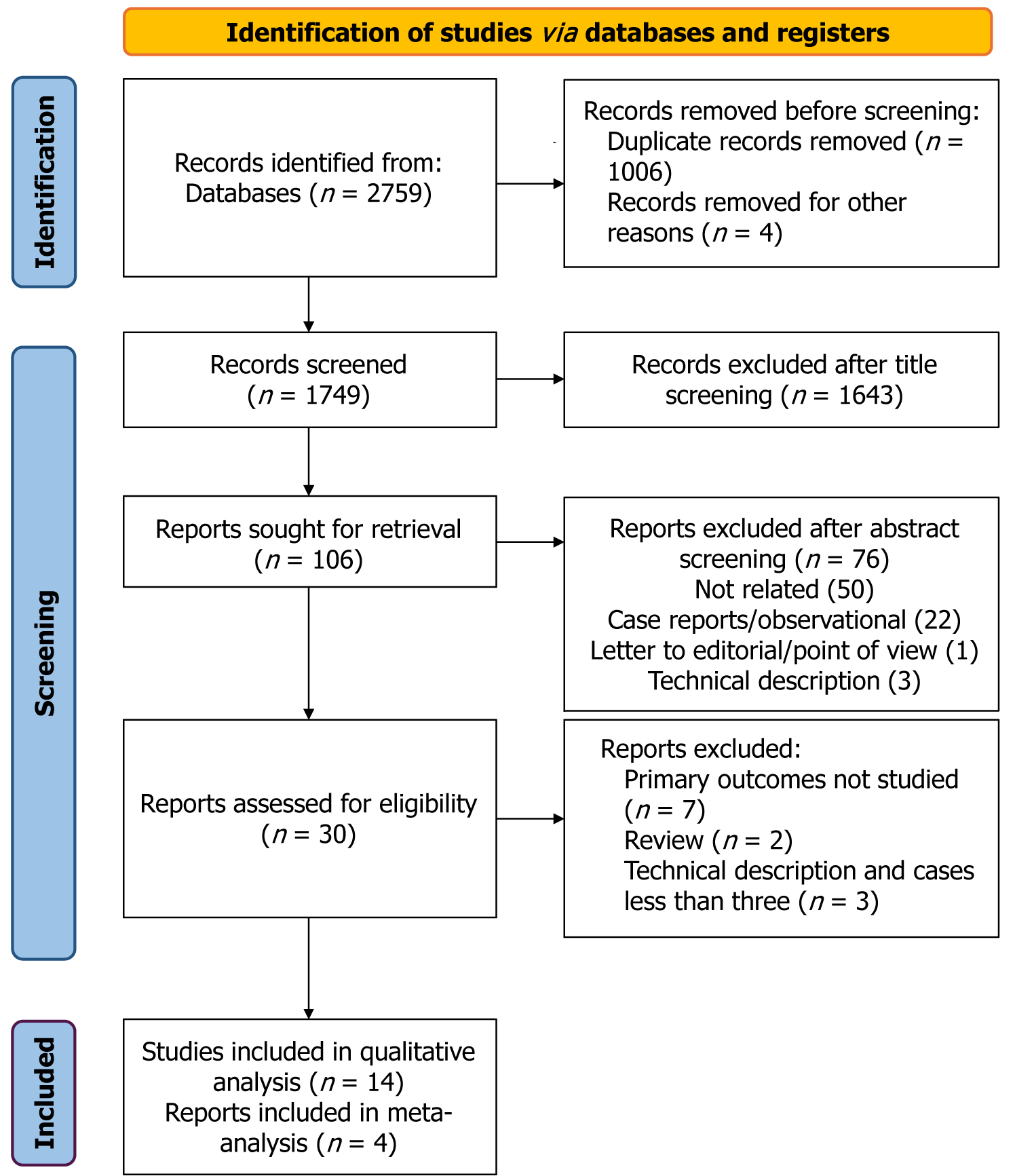
Only studies involving human participants were included. No language restrictions were applied. Additional relevant studies were identified through manual screening of reference lists of included articles and by using the “related articles” feature in PubMed. Studies available solely in abstract form or unpublished reports were excluded. The final literature search was conducted on 19 May 2024. The PRISMA chart depicts the search strategy (Figure 1).
We included studies that analyzed the outcome of AHCs. Observational and comparative studies enrolling more than three patients were considered for inclusion. All studies were rigorously assessed for potential duplication or overlapping data. When multiple studies originated from the same institution, the study of superior methodological quality—judged by the primary outcomes assessed—or the most recent publication was selected for inclusion. If a study described different aspects of the overlapping populations, both studies were included. However, only one study, which provided the largest sample size, was counted in the total number calculation.
Studies were excluded if they consisted of case reports or if they involved overlapping patient cohorts or institutional data already presented in higher-quality publications. Review articles, studies involving animal models, and experimental (non-clinical) models were also excluded. Studies with extra-anatomic inflow without a conduit were excluded.
Two authors (Appuhamilage BU and Gupta S) independently conducted the literature search and screened all identified abstracts for eligibility based on predefined inclusion criteria. In cases where abstracts were unavailable or insufficiently informative, full-text articles were retrieved and assessed. Discrepancies in study selection between the two reviewers were resolved through consensus with a third (Hakeem AR) and fourth author (Parente A), both of whom independently reviewed all retrieved articles to ensure the robustness and consistency of the selection process.
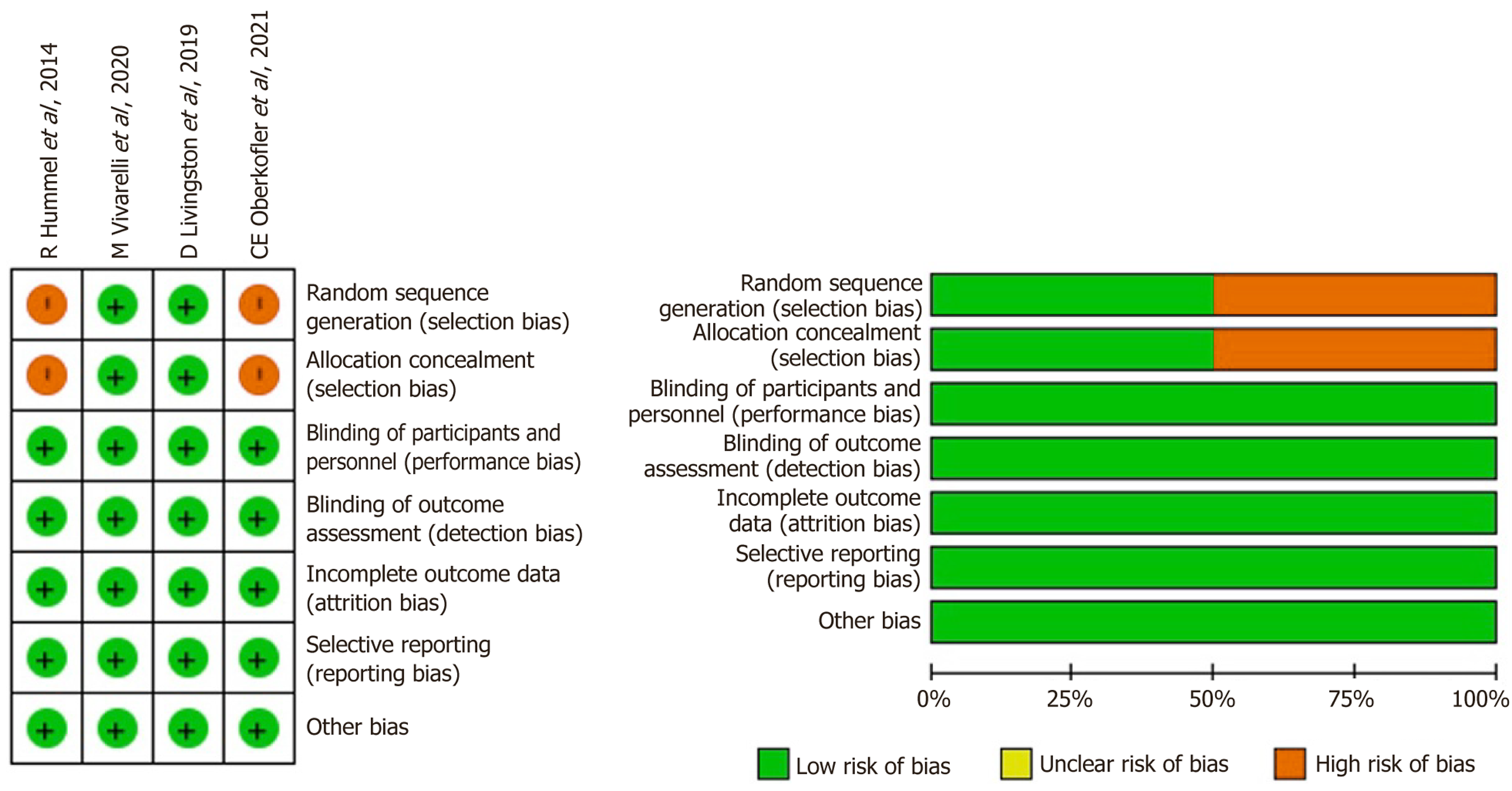
The methodological quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS). Two authors (Hakeem AR and Appuhamilage BU) independently evaluated the level of evidence based on three core domains: Selection of study groups, comparability between groups, and ascertainment of exposure or outcome. For cohort studies reporting solely on AHC outcomes, or those comparing SC-AHCs with IR-AHCs, each domain comprised one to four items, with individual items assigned a maximum score of one or two. The total NOS score was used to categorize the overall quality of each study, as summarized in Table 1.
The pooled mean across included studies was calculated by the following formula: Pooled mean of included studies = (N1 × M1 + N2 × M2 + N3 × M3)/(N1 + N2 + N3), where M1, M2 and M3 represent the means of individual studies, and N1, N2, and N3 denote the corresponding sample sizes. For studies that did not report mean and variance, these values were estimated from the reported median, range, and sample size, following methods described in previously published literature[22,23].
Categorical variables were analysed by calculating odds ratios (ORs), while continuous variables were assessed using standardized mean differences (MD). A random-effects meta-analysis was conducted using the DerSimonian-Laird method to account for between-study variability in outcome measures. Funnel plots were used to visually evaluate potential publication bias among the included studies. Study heterogeneity was quantified using the I² to identify the extent of variation beyond chance. I² values were interpreted as indicating low (< 25%), moderate (25%-75%), or high (> 75%) heterogeneity. The Egger test was used to assess funnel plot asymmetry. Statistical significance was defined as a P-value less than 0.05. All statistical analyses were conducted using Review Manager (RevMan) version 5.4.1 (The Cochrane Collaboration, Copenhagen, 2020).
Excluding duplicates, the search strategy identified a total of 1749 publications from 2014 to 19th May 2024. After title screening, 106 articles were included for the abstract review. From this, 30 full papers were assessed for eligibility. Ultimately, 14 papers were included in the systematic review. All 14 papers were retrospective, observational studies[8,11-14,18,24-30]. Three papers focused on living donor LT (LDLT)[15,19,30], while the remainder addressed deceased donor LT (DDLT). Four manuscripts, which analyzed SC and IR conduit groups, were included in the meta-analysis[11,13,14,25]. Of the remaining 10 papers, two compared AHC with other extra-anatomical inflow sites[8,12], and two compared AHC characteristics with non-conduits[15,24]. Bhatti et al[17,22] had two publications, from 2019 and 2023. Given the infrequent utilization of AHCs in LDLT and the evolution of techniques over the last three years of the study period, both papers were included in the systematic review[17,22]. Oberkofler et al[21] reported their outcome data in 2018 and 2023, with the latter being a multicenter study. For the calculation of the total number of transplants and total AHCs, only the multicenter study was considered[13]. Among the four papers included in the meta-analysis, two provided data from single-center studies[11,25], while the remaining two reported multicenter data[13,14].
The risk of bias for all included studies was evaluated using the NOS. The outcomes of this quality assessment are presented in Table 1 and Figure 2, indicating that all studies received NOS scores exceeding 7 (high quality). Con
The studies included data from the period between 1990 to 2020. Table 2 summarizes the demographic characteristics of the included studies. Pooled data revealed a total of 32486 DDLTs, of which 1136 (3.5%) required AHCs. Among these, 207 (18.2%) were SC AHC, 738 (65.0%) were IR AHC, and 25 (2.2%) utilized iliac artery inflow conduits. The remaining 166 (14.6%) DDLTs did not specify the type of conduit used.
| Ref. | Year | Country | Study period | Study design | Conduit material | Total No. of LT | Aorto-hepatic conduits | Supra-coeliac/infra-renal | Median follow up (months) |
| Hummel et al[11] | 2014 | Germany | 2005-2008 | Retrospective | IA | 114 | 15 | 8/7 | NA |
| Denecke et al[12] | 2016 | Austria | 1990-2012 | Retrospective | IA | 947 | 43 | 1/42 | NA |
| Li et al[13] | 2017 | Taiwan | 2002-2015 | Retrospective | GSV | NA | 11 | 11/0 | NA |
| Kazemi et al[14] | 2017 | Iran | 2011-2016 | Retrospective | IA | 2135 | 76 | 0/76 | NA |
| Jung et al[15] | 2018 | Korea | 2011-2016 | Retrospective | IA | 1928 | 25 | 0/25 | 3 |
| Oberkofler et al[16] | 2018 | Switzerland | 2007-2016 | Retrospective | IA | 361 | 29 | 2/27 | 46 |
| Bhatti et al[17] | 2019 | Pakistan | 2012-2017 | Retrospective | GSV | 452 | 19 | 3/16 | 18 |
| Livingston et al[18] | 2019 | United States | 2000-2016 | Retrospective | IA | 3125 | 104 | 22/82 | 52.8 |
| Vivarelli et al[19] | 2020 | Italy (multicentre) | 2003-2018 | Retrospective | IA/Axillary | 4255 | 120 | 64/56 | SC: 47.5; IR: 62.5 |
| Devcic et al[20] | 2020 | USA | 2000-2016 | Retrospective | IA | 3125 | 104 | NA | NA |
| Oberkofler et al[21] | 2021 | International multicentre (14 centres) | 2007-2016 | Retrospective | IA/Prosthetic | 11133 | 565 | 111/42 (Iliac = 14) | 36.5 |
| Beaurepaire et al[8] | 2022 | France | 2002-2017 | Retrospective | IA/Prosthetic | 1677 | 68 | Iliac artery: SC + IR = 50, Iliac = 6. Prosthesis: SC = 1, IR = 6, Iliac = 5 | 61 |
| Bhatti et al[22] | 2023 | Pakistan | 2017-2020 | Retrospective | GSV | 498 | 14 | 4/10 | 34.9 |
| Sohrabi et al[23] | 2024 | Iran | 2009-2020 | Retrospective | IA | 4010 | 16 | 0/16 | 35 |
Pooled analysis revealed comparable demographic characteristics. The OR and MD analysis confirmed the findings of no difference in age [MD -0.57 (-2.48, 1.34); P = 0.56], male gender [OR = 0.86 (0.61, 1.22); P = 0.40], recipient BMI [MD -0.92
| Ref. | Year | No. of Patients | Recipient age, median (SD) | Recipient gender (male%) | Recipient BMI (kg/m2) | Redo transplant | Indications for Transplant (V-Viral, A- Alcohol, M-MASLD, O-Other, NA-not available) | MELD | Previous TACE | ||||||||
| SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | ||
| Hummel et al[11] | 2014 | 8 | 7 | 50.9 (12.2) | 46.6 (14.3) | 6 (75) | 7 (100) | 23.5 (3) | 27.4 (3.7) | 3 (37.5) | 3 (42.9) | NA | NA | 22.7 (11.7) | 16.5(9.5) | NA | NA |
| Livingston et al[18] | 2019 | 22 | 82 | 51.5 (13.3) | 52.7 (10.7) | 14 (63.6) | 57 (69.5) | 25.7 (5.5) | 26.3 (5.2) | 10 (45.5) | 45 (54.9) | NA | NA | 19.6 (9.7) | 19.0 (10.4) | NA | NA |
| Vivarelli et al[19] | 2020 | 64 | 56 | 49.5 (14.4) | 49.5 (11.8) | 45 (70.3) | 41 (73.2) | 24.2(3.6) | 25.5 (4.9) | 16 (25.0) | 14 (25.0) | V-32 (50.0%) A-8 (12.5%) M-8 (12.5%) O-3 (4.7%) | V-30 (53.6%) A-6 (10.7%),M-2 (3.6%),O-4 (7.1%) | 22.7 (10.7) | 18.7 (7.8) | 13 (20.3) | 12 (21.4) |
| Oberkofler et al[21] | 2021 | 111 | 428 | 52.5 (11.9) | 53.2 (4.9) | 65 (59.0) | 261 (61.0) | 25.1(1.4) | 25.5 (2.0) | 54 (49.0) | 147 (34.0) | NA | NA | 25.0 (5.2) | 22.7 (6.3) | 20.0 (20.0) | 61 (15.0) |
Out of 14 included studies, 10 (71.0%) specified the indication for AHCs. The most frequent indications for AHC use included; poor arterial flow, intimal dissections, and hepatic artery thrombosis (HAT). Other notable reasons were insufficient length, size discrepancy of the artery, challenging dissection, and severe atherosclerosis of the hepatic artery[8,12,13,24-26,30]. Sohrabi et al[23] reported on 16 patients who underwent AHCs for HAT, although it is unclear if other indications were also considered. In the context of LDLTs, Bhatti et al[17] indicated that 85% of cases were due to arterial dissections, whereas Li et al[13] identify failure of the primary anastomosis (due to poor caliber) as the reason for AHCs.
Of the 14 papers reviewed, only 7 (50.0%) provided a rationale for selecting a specific inflow site over alternative options. Three papers cited 'surgeon preference' as the determining factor, while three adhered to predetermined inflow sites based on institutional policy[8,11,17]. Notably, two of these studies favored the splenic artery and coeliac artery before considering the aorta for conduit[15,24]. One study uniquely reported that the choice was made intraoperatively, based on the assessment of the aortic vasculature and the patient's history of previous abdominal surgeries[25].
In DDLTs, 11 studies identify the iliac artery as the primary conduit material[8,11,12,14,17,24-26,28]. Additionally, the axillary artery is mentioned as an alternative conduit option[14]. Prosthetic grafts were rarely utilized[13]. In LDLT settings, the GSV was employed as the conduit material in all three studies[15,19,30]. Two were published by the same author with varying study periods[15,30]. GSV is the conduit of choice in all patients (100%). Bleeding has been the most common complication after GSV, and conduit and it ranges between 9% to 14%. Both series do not demonstrate any early or late occlusion events.
Among the 14 studies, nine reported their anticoagulation protocols[8,11,13,14,15,24,26,30]. Aspirin was used as the preferred antiplatelet agent in 66% of these studies, primarily due to conduit use. Intravenous heparin was included in 33% of the protocols, with durations ranging from 5 days to a maximum of 3 months[15,24]. One study described the concurrent use of both aspirin and heparin[24], while only one center specified lifelong use of aspirin[15].
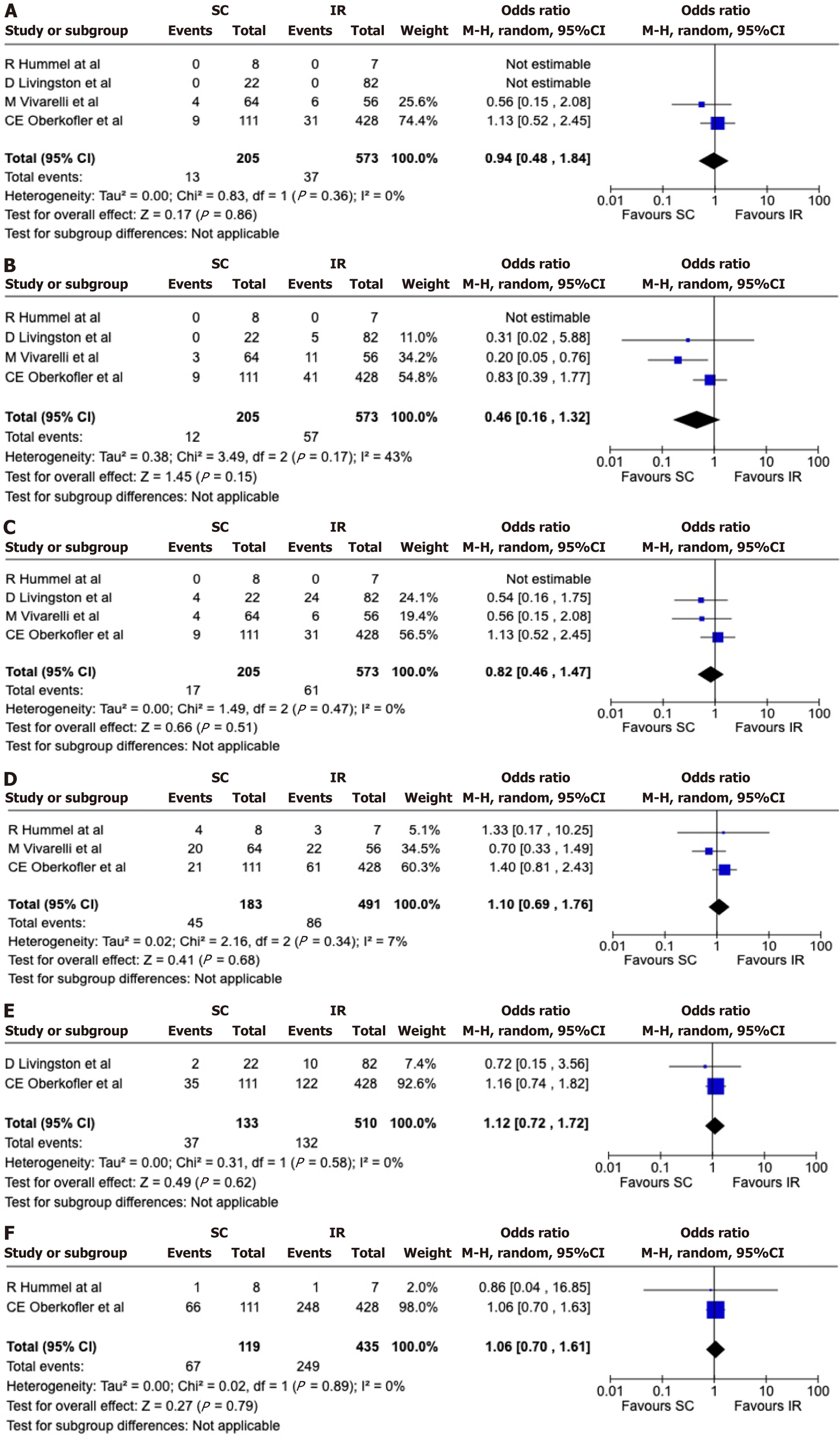
The median follow-up duration ranged from 18 to 52 months. Complication rates did not significantly differ between the SC and IR groups (Tables 4 and 5). Forest plot analysis confirmed no significant differences in early occlusions of AHCs [OR = 0.94 (0.48, 1.84); P = 0.86] (Figure 3A), and late occlusions of AHCs [OR = 0.46 (0.16, 1.32); P = 0.15) (Figure 3B), early allograft dysfunction [OR = 0.82 (0.46, 1.47); P = 0.51] (Figure 3C), biliary complications [OR = 1.10 (0.69, 1.76); P = 0.68] (Figure 3D), post-transplant renal replacement therapy (RRT) requirement [OR = 1.12 (0.72, 1.72); P = 0.62] (Figure 3E), and major surgical complications (Clavien-Dindo > 3b) [OR = 1.06 (0.70, 1.61); P = 0.79] (Figure 3F and Table 6). The median duration for graft occlusion was approximately 142 days, ranging from 13 to 3313 days (Table 4).
| Ref. | Year | Number of patients | Early occlusions | Late occlusion | Bile duct complications | Graft survival 1-year | Graft survival 5-year | Patient survival 1-year | Patient survival 5-year | ||||||||
| SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | ||
| Hummel et al[11] | 2014 | 8 | 7 | 0 | 0 | 0 | 0 | 4 (50.0) | 3 (42.9) | NA | NA | NA | NA | 57.1% | 85.7% | NA | NA |
| Livingston et al[18] | 2019 | 22 | 82 | 0 | 0 | 0 | 5 | NA | NA | 85.1% | 79.1% | 53.9% | 56.0% | 85.1% | 88.3% | 67.8% | 64.9% |
| Vivarelli et al[19] | 2020 | 64 | 56 | 4 (6.2) | 6 (10.7) | 3 (4.7) | 11 (19.6) | 20 (31.2) | 22 (39.3) | 77.0% | 66.0% | 67.0% | 50.0% | 80.0% | 73.0% | 74.0% | 56.0% |
| Oberkofler et al[21] | 2021 | 111 | 428 | 9 (8.1) | 31 (7.2) | 9 (8.0) | 41 (9.8) | 21 (19.0) | 61 (14.0) | NA | NA | NA | NA | NA | NA | NA | NA |
| Ref. | Year | No. of AHCs | Total occlusions | Surgical interventions for oocclusions | Endovascular intervensions for occlusions | Success rate of surgery without retransplantation | Success rate of endovascular | |||||
| Re-anastomosis | Re-conduit formation | Retransplants | Stent | PTA | Stent/PTA | CDTL | ||||||
| Hummel et al[11] | 2014 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| Livingston et al[18] | 2019 | 104 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Vivarelli et al[19] | 2020 | 120 | 24 (20.0) | 3 (12.5) | NA | 8 (33.0) | 8 (33.0) | NA | NA | NA | NA | |
| Oberkofler et al[21] | 2021 | 565 | 96 (17.0) | 8 (8.3) | 4 (4.2) | 22 (23.0) | 4 (4.2) | 12 (13.0) | 7 (7.3) | 6 (11.0) | 7 (27.0) | 18 (82.0) |
| Sohrabi et al[23] | 2024 | 16 | 2 (12.5) | 0 | 0 | 2 (100) | 0 | 0 | 0 | 0 | NA | NA |
| Devcic et al[20] | 2020 | 104 | 7 (6.7.0) | 0 | 0 | 0 | 0 | 7 (100) | 0 | 0 | NA | NA |
| Ref. | Year | Number of patients | Pre-Cr at LT (mg/dL) | Post-Cr maximum (mg/dL) | Post-transplant CRRT | Early allograft dysfunction | Major surgical interventions (Clavien 3b>0) | ||||||
| SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | SC | IR | ||
| Hummel et al[11] | 2014 | 8 | 7 | 0.56 (0.33) | 0.97 (0.37) | 0.8 | 1.47 | NA | NA | 0 | 0 | 1 (12.5) | 1 (14.3) |
| Livingston et al[18] | 2019 | 22 | 82 | 1.15 (0.71) | 1.20 (0.72) | 1.17 (0.22) | 1.2 (0.26) | 2 (10.5) | 10 (12.2) | 4 (18.2) | 24 (29.3) | NA | NA |
| Vivarelli et al[19] | 2020 | 64 | 56 | 1.35 (0.79) | 1.82 (1.1) | NA | NA | NA | NA | 4 (6.2) | 6 (10.7) | NA | NA |
| Oberkofler et al[21] | 2021 | 111 | 428 | NA | NA | NA | NA | 35 (32.0) | 122 (29.0) | 9 (8.1) | 31 (7.2) | 66 (60.0) | 248 (58.0) |
Pooled data revealed one-year graft and patient survival rates for SC conduits were 77% to 81.1% and 80% to 85.1%, respectively. For IR conduits, one-year graft and patient survival rates were 66% to 79.1% and 73% to 88.3%, respectively. Five-year graft and patient survival rates for SC conduits were 53.9% to 67% and 67.8% to 74%, respectively. For IR conduits, five-year graft and patient survival rates were 50% to 56% and 56% to 64.9%, respectively.
Except for two studies, all others reported doppler ultrasound scan (USS) as the post-transplant imaging patency surveillance method[8,11,14,24-26,30]. However, the frequency of doppler USS application was inconsistent among the studies. Some studies performed daily USS in the first week post-transplant[12,14,24-26]. One study conducted doppler USS every 12 hours, while others performed it two or three times per week[24]. Angiography was the next reported imaging modality, utilized on demand based on suspicion of conduit occlusion.
Out of 14 studies, 5 reported interventions for stenosis[13,17,24,25,28]. The systematic review identified four options for managing AHC occlusion: Endovascular dilation with or without stenting, catheter-directed thrombolysis, conduit revision, and retransplantation. Among these, angioplasty and stenting emerged as the preferred treatments, with retransplantation being the last resort. The re-anastomosis rate ranged from 0 to 12.5%, while the re-conduit rate was 0 to 4.2%[13,17].
This study primarily focused on the use of AHCs in LT, specifically comparing the outcomes between the two common inflow sites, namely SC and IR. When the native recipient’s hepatic artery is unusable, some series have shown an increasing preference for splenic artery transposition over the formation of AHCs[7,8]. However, in cases where the coeliac axis is involved by the same pathology, AHC becomes the only option for revascularizing the liver graft. This study showed that IR conduit is the most preferred inflow site over SC, however this systematic review identified choice was mostly “predetermined” rather than an intra-operative decision[31]. The absence of notable differences in early outcomes between SC and IR conduits indicates that surgeons often base their choice on personal expertise and familiarity with the technique. These results provide reassurance that variations in surgical preference do not compromise patient outcomes, affirming that both methods are equally effective when performed by experienced hands. This study revealed a utilization rate of only 3.5% for AHCs in DDLT, which is lower than the 9% conduit rate reported in a previous meta-analysis conducted by Reese et al[32]. In this meta-analysis, 12 of the 13 studies included the patient populations from after 2000, whereas Reese et al[32] included patients from 1989 onwards. The decline in the rate of AHC in LT may be attributed to advancements in pre-transplant imaging and surgical planning over the past two decades. Currently, patients with complex arterial issues and associated vascular and metabolic comorbidities are likely excluded due to more stringent and improved patient selection criteria.
This meta-analysis also identified the donor iliac artery as the main AHC in the DDLT setting. The use of prosthetic grafts has been very rare. In LDLT settings, the GSV serves as the main conduit material. Although data are limited and the patient cohort is small (Table 7), outcomes with GSV appear encouraging, demonstrating a low occlusion rate[15,30]. However, this systematic review did not identify any use of GSV in the DDLT setting. Although the GSV shows promising outcomes as an AHC in LDLT, it is well reported that the arterial conduits generally provide the best results in cardiac bypass surgery[33]. In the context of high-pressure aortic flow and the long length of AHC grafts, particularly in IR aortic conduits, the use of the GSV conduit instead of an arterial conduit, such as the radial artery, requires justi
| Aorto-hepatic conduit details | Number of studies that have reported (n = 14) |
| Infow sites | 14 |
| Conduit material | 14 |
| Second party vs third party donor | 1 |
| Storage of the conduits | 0 |
| Reason for conduit | 9 |
| Choice of inflow site | 12 |
| Post-operative doppler monitoring | 12 |
| Post-operative anticoagulation | 9 |
| Early occlusions | 8 |
| Late occlusions | 8 |
| Endovascular Interventions for occlusions | 3 |
| Surgical interventions (revisions/reconduits/retransplants) | 4 |
Conduit occlusion is primarily driven by endothelial injury and thrombosis, with chronic inflammation—such as that associated with rejection or graft arteriopathy—also playing a key role in its development[13]. The rate of conduit occlusion in the included studies ranged from 0% to 20%[13,14]. The meta-analysis shows no difference between SC and IR conduits in relation to short-term occlusion rates. However, there is a discernible tendency towards higher late occlusion in the IR group. These findings are consistent with the multicenter study conducted by Oberkofler et al[21]. The SC group had similar or slightly superior outcomes despite higher MELD scores, suggesting that the SC conduit may give the best results in the long-term compared to the IR group. The increased late occlusion rates observed in IR conduits may be due to their greater length, which is associated with turbulence-induced arteriopathy, and luminal narrowing. In contrast, SC conduits—being shorter and offering more physiological blood flow—may provide a protective effect, contributing to improved long-term patency. The role of anticoagulation and antiplatelet therapy, aimed at preventing thrombotic events, has become crucial, particularly for IR conduits, to reduce the incidence of late occlusions. Of note, Sohrabi et al[23] identified significantly fewer biliary complications in AHCs compared to HA anastomosis revision (47.4% vs 31.2%), in the context of early HAT which is likely to be attributable to multiple re-explorations after the HA revision[24]. Vivarelli et al's multicenter European study supports our findings, indicating no significant differences in early outcomes between SC and IR AHCs[19]. However, while both studies underscore the long-term occlusion risks associated with the IR group, our review places greater emphasis on the clinical implications of anticoagulation protocols and postoperative monitoring, whereas the referenced study is more focused on procedural differences in conduit placement.
In LDLT, Li et al[13] performed all their conduit placements using the SC approach. In contrast, Bhatti et al[17] utilized both the SC and IR aorta, with a predominance of conduits being placed in the IR aorta. According to Bhatti et al[17], the majority of conduits are utilized for managing arterial dissections, which are typically identified after reperfusion. This post-reperfusion identification of dissection can make access to the SC aorta challenging, prompting the preference for the IR aorta.
Another finding of this systematic review reveals a notable trend towards endovascular interventions and a decreased preference for re-anastomosis or re-conduit in cases of conduit occlusion. Given the limited availability of donor organs, it is understandable that most centers are focusing on salvaging the existing graft whenever possible, reserving retransplantation as a last resort. According to Devcic et al[20], angioplasty achieved a 100% technical success rate in the seven patients who required this intervention. Only two patients needed reintervention and there were no procedure-related adverse events[28].
This systematic review has highlighted the variable use of anticoagulation following acute AHC thrombosis. All studies reviewed employed additional pharmacological anticoagulation methods, such as aspirin, heparin, or a combination of both. However, the specific duration of these treatments was not clearly defined. Considering the limited data and the reported median graft occlusion duration of 142 days, it can be suggested that a pharmacological prophylaxis regimen lasting at least six months could provide beneficial outcomes, potentially enhancing results with AHCs[28]. However, this should be further investigated in a large multicenter study. To date, the value of lifelong anticoagulation as a preventive strategy for AHC occlusion remains contentious, although it is commonly practiced in some centers[15]. Oberkofler et al[21] in their multicentre study identified the use of aspirin as an effective preventive method for conduit occlusion[12]. Due to the great variability in Doppler scan surveillance in graft inflow and outflow, the review is unable to evaluate the validity or effectiveness of these surveillance practices[32,33]. The absence of standardized protocols, particularly during the early postoperative period, can have serious consequences, including graft ischaemia and failure. With endovascular intervention on board, it is of utmost importance to detect AHC occlusions early, as a more proactive approach can salvage the conduit and the graft[12,32].
The main limitation of this study is the potential overlap between the two large multicenter studies. Additionally, the limited LDLT data make it challenging to draw firm conclusions. The higher MELD scores in the SC group may introduce bias in inflow site selection due to issues like suboptimal aortic vessels and bowel adhesions from recurrent SBP. There's also considerable variability in patient characteristics, management strategies, and study designs. While subgroup analyses and I² statistics were used to assess variability, these reflect real-world clinical practice and improve generalizability. However, further subgroup analyses or meta-regression are limited by the small number of studies and the potential for unreliable results.
The study highlights several key areas for future research related to AHC. These include investigating the use of larger, high-pressure conduits in pediatric LT, exploring the impact of novel perfusion technologies, and examining how preoperative handling of the donor hepatic artery might influence outcomes. The lack of consensus on post-transplant anticoagulation also underscores the need for further studies and the development of guidelines. Addressing these gaps could improve clinical practices. In the meantime, this review provides valuable data that can serve as a basis for future research
| 1. | Mourad MM, Liossis C, Gunson BK, Mergental H, Isaac J, Muiesan P, Mirza DF, Perera MT, Bramhall SR. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2014;20:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208:896-903; discussion 903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Warner P, Fusai G, Glantzounis GK, Sabin CA, Rolando N, Patch D, Sharma D, Davidson BR, Rolles K, Burroughs AK. Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation - univariable and multivariable analysis. Transpl Int. 2011;24:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003;9:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Vivarelli M, Cucchetti A, La Barba G, Bellusci R, De Vivo A, Nardo B, Cavallari A, Pinna AD. Ischemic arterial complications after liver transplantation in the adult: multivariate analysis of risk factors. Arch Surg. 2004;139:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Silva MA, Jambulingam PS, Gunson BK, Mayer D, Buckels JA, Mirza DF, Bramhall SR. Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl. 2006;12:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Ullah K, Dogar AW, Shams-Ud-Din, Bilal H. Splenic artery transposition for hepatic arterial supply in living donor liver transplantation. Pak J Med Sci. 2023;39:154-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Beaurepaire JM, Orlando F, Levi Sandri GB, Jezequel C, Bardou-Jacquet E, Camus C, Lakehal M, Desfourneaux V, Merdrignac A, Gaignard E, Thobie A, Bergeat D, Meunier B, Rayar M. Comparison of alternative arterial anastomosis site during liver transplantation when the recipient's hepatic artery is unusable. Hepatobiliary Surg Nutr. 2022;11:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Muralidharan V, Imber C, Leelaudomlipi S, Gunson BK, Buckels JA, Mirza DF, Mayer AD, Bramhall SR. Arterial conduits for hepatic artery revascularisation in adult liver transplantation. Transpl Int. 2004;17:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Dokmak S, Aussilhou B, Landi F, Dondéro F, Termos S, Paugam-Burtz C, Durand F, Belghiti J. The recipient celiac trunk as an alternative to the native hepatic artery for arterial reconstruction in adult liver transplantation. Liver Transpl. 2015;21:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Hummel R, Irmscher S, Schleicher C, Senninger N, Brockmann JG, Wolters HH. Aorto-hepatic bypass in liver transplantation in the MELD-era: outcomes after supraceliac and infrarenal bypasses. Surg Today. 2014;44:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Denecke C, Weiss S, Biebl M, Fritz J, Dziodzio T, Aigner F, Sucher R, Brandl A, Bösmüller C, Pratschke J, Öllinger R. An Arterial Conduit is Not a Risk Factor for Survival Following Orthotopic Liver Transplantation: An Analysis of 20 Years of Liver Transplantation in Innsbruck. Ann Transplant. 2016;21:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Li PC, Thorat A, Jeng LB, Yang HR, Li ML, Yeh CC, Chen TH, Hsu SC, Poon KS. Successful application of supraceliac aortohepatic conduit using saphenous venous graft in right Lobe living donor liver transplantation. Liver Transpl. 2017;23:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Kazemi K, Samidoost P, Deilami HN, Malek Hosseini SA, Nikeghbalian S, Shamsaeefar A, Dehghani M, Mansoorian M, Gholami S, Khosravi B. A New Consideration in Hepatic Artery Reconstruction in Adult Liver Transplant: Arterial Transposition Versus Extra-Anatomic Jump Grafts. Exp Clin Transplant. 2017;15:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Jung DH, Park CS, Ha TY, Song GW, Park GC, Cho YP, Lee SG. Placement of an Aortohepatic Conduit as an Alternative to Standard Arterial Anastomosis in Liver Transplantation. Ann Transplant. 2018;23:61-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Oberkofler CE, Reese T, Raptis DA, Kuemmerli C, de Rougemont O, De Oliveira ML, Schlegel A, Dutkowski P, Clavien PA, Petrowsky H. Hepatic artery occlusion in liver transplantation: What counts more, the type of reconstruction or the severity of the recipient's disease? Liver Transpl. 2018;24:790-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Bhatti ABH, Dar FS, Qureshi AI, Haider S, Khan NA. Saphenous vein conduits for hepatic arterial reconstruction in living donor liver transplantation. Langenbecks Arch Surg. 2019;404:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Livingston D, Lee DD, Croome S, Burcin Taner C, Croome KP. Comparison of Supraceliac and Infrarenal Aortic Conduits in Liver Transplantation: Is There a Difference in Patency and Postoperative Renal Dysfunction? Transplant Direct. 2019;5:e499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Vivarelli M, Benedetti Cacciaguerra A, Lerut J, Lanari J, Conte G, Pravisani R, Lambrechts J, Iesari S, Ackenine K, Nicolini D, Cillo U, Zanus G, Colledan M, Risaliti A, Baccarani U, Rogiers X, Troisi RI, Montalti R, Mocchegiani F. Infrarenal versus supraceliac aorto-hepatic arterial revascularisation in adult liver transplantation: multicentre retrospective study. Updates Surg. 2020;72:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Devcic Z, Toskich BB, Livingston D, Croome KP, Lewis AR, Ritchie C, Frey G, McKinney JM, Paz-Fumagalli R. Endovascular Treatment of Aortohepatic Conduit Stenosis Following Liver Transplant. Transplant Proc. 2020;52:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Oberkofler CE, Raptis DA, DiNorcia J, Kaldas FM, Müller PC, Pita A, Genyk Y, Schlegel A, Muiesan P, Tun Abraham ME, Dokus K, Hernandez-Alejandro R, Rayar M, Boudjema K, Mohkam K, Lesurtel M, Esser H, Maglione M, Vijayanand D, Lodge JPA, Owen T, Malagó M, Mittler J, Lang H, Khajeh E, Mehrabi A, Ravaioli M, Pinna AD, Dutkowski P, Clavien PA, Busuttil RW, Petrowsky H. How to Handle Arterial Conduits in Liver Transplantation? Evidence From the First Multicenter Risk Analysis. Ann Surg. 2021;274:1032-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Bhatti ABH, Khan S, Farooq MH, Ishtiaq W, Khan NY. Liver transplantation with interposition saphenous vein conduits for arterial reconstruction: Impact of morbidity and arterial ischemia time. Surgery. 2023;174:1263-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Sohrabi Nazari S, Eslamian M, Sheikhbahaei E, Zefreh H, Lashkarizadeh MM, Shamsaeefar A, Kazemi K, Nikoupour H, Nikeghbalian S, Vatankhah P. Early hepatic artery thrombosis treatments and outcomes: aorto-hepatic arterial conduit interposition or revision of anastomosis? BMC Surg. 2024;24:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Ali MA, Yong CC, Eng HL, Wang CC, Lin TL, Li WF, Wang SH, Lin CC, Yap A, Chen CL. Cryopreserved arterial grafts as a conduit in outflow reconstruction in living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2015;22:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Park GC, Hwang S, Jung DH, Ha TY, Song GW, Ahn CS, Moon DB, Kim KH, Yoon YI, Cho HD, Choi JU, Lee SG. Refined surgical techniques to improve the patency of cryopreserved iliac artery homografts for middle hepatic vein reconstruction during living donor liver transplantation. Ann Surg Treat Res. 2020;99:294-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48658] [Article Influence: 2862.2] [Reference Citation Analysis (3)] |
| 27. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13811] [Article Influence: 812.4] [Reference Citation Analysis (3)] |
| 28. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 13631] [Article Influence: 851.9] [Reference Citation Analysis (1)] |
| 29. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 7286] [Article Influence: 347.0] [Reference Citation Analysis (1)] |
| 30. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 8054] [Article Influence: 671.2] [Reference Citation Analysis (0)] |
| 31. | Muiesan P, Rela M, Nodari F, Melendez HV, Smyrniotis V, Vougas V, Heaton N. Use of infrarenal conduits for arterial revascularization in orthotopic liver transplantation. Liver Transpl Surg. 1998;4:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Reese T, Raptis DA, Oberkofler CE, de Rougemont O, Györi GP, Gosteli-Peter M, Dutkowski P, Clavien PA, Petrowsky H. A systematic review and meta-analysis of rescue revascularization with arterial conduits in liver transplantation. Am J Transplant. 2019;19:551-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Gaudino M, Benedetto U, Fremes S, Biondi-Zoccai G, Sedrakyan A, Puskas JD, Angelini GD, Buxton B, Frati G, Hare DL, Hayward P, Nasso G, Moat N, Peric M, Yoo KJ, Speziale G, Girardi LN, Taggart DP; RADIAL Investigators. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N Engl J Med. 2018;378:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/