Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.101046
Revised: January 29, 2025
Accepted: February 27, 2025
Published online: September 18, 2025
Processing time: 227 Days and 8 Hours
Liver transplantation (LT) is the definitive treatment for end-stage liver disease, acute liver failure, and liver cancer. Although advancements in surgical tech
Core Tip: Liver transplantation (LT) is crucial for treating severe liver conditions, but it increases the risk of new cancers, especially non-melanoma skin cancers like squamous cell carcinoma. LT recipients also have a higher chance of developing solid organ cancers and lymphoproliferative disorders compared to the general population. Key risk factors include chronic immunosuppression, alcohol or tobacco use, and viral infections. Effective management involves regular cancer screenings, early detection, and adjusting immunosuppressive treatments, particularly for Kaposi's sarcoma and post-transplant lymphoproliferative disorders.
- Citation: Singh A, Singh C, Dhaliwal A, Singh N, Kumar V, Sohal A, Schneider J. Incidence, screening, and management of de novo malignancies in liver transplant patients: A review. World J Transplant 2025; 15(3): 101046
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/101046.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.101046
Liver transplant (LT) is the definitive treatment for decompensated end-stage liver disease, acute liver failure, and liver cancer. Advancements in LT techniques, postoperative care, and long-term immunosuppressive agents have significantly increased the number of recipients and markedly improved long-term survival. However, while long-term immunosuppression is required, it is associated with an increased risk of complications, such as infections, cardiovascular events, renal injury, and cancer. Notably, retrospective studies have highlighted that de novo malignancy (DNM) accounts for 20%-25% of deaths in patients who survive the early post-transplant period[1-3].
Compared to the general population, LT recipients face a two-fold increase in the incidence of solid organ malignancies and a dramatic 30-fold increase in the risk of lymphoproliferative disorders[4-8]. Risk factors for developing DNM in LT recipients include chronic immunosuppression, a history of alcohol or tobacco use, oncogenic viral infections, and underlying liver disease. This review aims to summarize the current literature on the incidence, risk factors, screening, and management of de novo malignancies following liver transplantation.
Non-melanoma skin cancers (NMSC) are the most prevalent type of cancer among Caucasian solid organ transplant recipients. The occurrence of NMSC in LT patients is more than 15 times higher than in the general population[9,10]. Squamous cell cancer (SCC) is the most prevalent type of skin cancer in LT recipients, whereas basal cell cancer (BCC) is more common in the general population. There have also been reports of Merkel cell carcinoma occurring following liver transplantation[11,12]. NMSCs often occur as a late complication following liver transplantation, with an average time from transplant to diagnosis of 50 months (Table 1)[13,14]. Fortunately, NMSCs generally do not impact mortality but can affect quality of life and require ongoing monitoring.
| Ref. | Country | Study period | No. of LT patients included in the study | Follow up period | Interval to DNM | Overall Incidence of DNM | Common malignancies (%) | Overall SIR |
| Frezza et al[115] | United States | Before 1992 | 1657 | - | - | 64 (3.8) | Basalioma 25%, SCC 20.3%, melanoma 6.2% | - |
| Krynitz et al[116] | Sweden | 1970-2008 | 1221 | 20 | - | (27) | - | All cancer: 3.4 (2.9-4.0); non-SCC: 2.3 (1.9-2.8); all skin cancer: 16 (12-20); SCC: 32 (24-42) |
| Haagsma et al[117] | The Netherlands | 1979-1996 | 174 | 5.9 | 5.1 | 23 (12) | Skin and lip cancers 52% | 4.3 |
| Oo et al[118] | United Kingdom | 1982-2004 | 1778 | 6.5 | - | 141 (7.9) | Skin cancer excluding melanoma 36.1%, lymphoma 12.7%, large bowel cancer 12.7%, lung cancer 10% | 2.1 |
| Aberg et al[5] | Finland | 1982-2005 | 540 | 6.3 | 5.1 | 39 (7.2) | Nonmelanoma skin cancer 25.6%, non-Hodgkin lymphoma 20.5% | 2.59 |
| Finkenstedt et al[51] | Austria | 1982-2007 | 779 | 4.1 | 4.4 | 105 (12.3) | Skin cancer 17%, lung cancer16%, oropharyngeal carcinoma 11% | 1.9 |
| Jiang et al[119] | Canada | 1983-1998 | 2034 | 3.5 ± 2.8 | 3.5 ± 2.8 | 113 (5.5) | Non-Hodgkins lymphoma 35.3, colorectal cancer 12.3, lung cancer 8.8% | 2.5 |
| Schrem et al[120] | Germany | 1983-2010 | 2000 | 7.3 | 6.8 | 120 (6) | PTLD 19.12%, lung cancer 11.6%, colorectal cancer 10.8%, gynecological cancer 8.03%, breast cancer 6.6%, gastric/esophageal cancer 5.8% | 2 |
| Galve et al[121] | Spain | 1984-1996 | 1827 | - | 2.5 ± 1.8 | 70 (3.83) | - | - |
| Mouchli et al[122] | United States | 1984-2012 | 293 | 11.5 | - | 73 (25) | PTLD 30.1%, SOT 63% | - |
| Sanchez et al[123] | United States | 1985-1999 | 1421 | 5.5 ± 3.7 | - | 123 (88) | Skin cancer 32.8%, lymphomas 25%, lung cancer 8%, colon cancer 5% | - |
| Wimmer et al[124] | Germany | 1985-2007 | 609 | 4.8 | 5.7 ± 3.7 | 87 (14.2) | SCC 15%, BCC 27.6% | - |
| Taborelli et al[125] | Italy | 1985-2014 | 2818 | NA | NA | 244 (8.6) | - | - |
| Shalaby et al[126] | Italy | 1985-2014 | 2653 | 3.6 for HCC, 6.6 non-HCC | 2.7 in HCC, 4.5 in non- HCC patients | 62 (6.6) in HCC patients, 127 (7.4) in non-HCC patients | - | - |
| Jiménez et al[127] | Spain | 1986-2000 | 505 | - | - | 62 (12.3) | Skin cancer and Kaposi sarcoma 25.8%, PTLD 21.6%, head and neck cancer 16% | - |
| Tjon et al[128] | Denmark | 1986-2007 | 385 | - | - | 66 (17.1) | non-melanoma skin cancer 55%, PTLD 21% | 2.2 |
| Engels et al[7] | United States | 1987-2008 | 37888 | - | - | 1563 (4.1) | PTLD- 23.3% | - |
| Jonas et al[129] | Germany | 1988-1994 | 458 | 4.2 | 3.6 | 33 (7.2) | Skin cancer 24%, PTLD 21%, gynecological cancer 21% | - |
| Kelly et al[130] | United Kingdom | 1988-1996 | 888 | - | 2 ± 1.5 | 31 (3.4) | Skin cancer and Kaposi sarcoma 25.8%, lung cancer 3% | - |
| Saigal et al[14] | United Kingdom | 1988-1999 | 1140 | - | 3.8 ± 2.8 | 30 (2.6) | Skin cancer 43%, oropharyngeal cancer 6%, bladder cancer 6%, acute leukemia 6% | - |
| Yao et al[131] | United States | 1988-2000 | 1043 | 6.7 | - | 53 (5) | Skin cancer 32%, GI cancer 21%, hematologic malignancies 17% | - |
| Rademacher et al[69] | Germany | 1988-2006 | 1616 | 14 | - | 322 (19.9) | Skin 25.7%, hematological 15.2%, solid organ tumors 60% | - |
| Watt et al[37] | United States | 1990-1994 | 798 | 10 | - | 271 (34) | Skin cancer 54.2%, hematologic malignancy 10.7%, solid organ cancer 35% | - |
| Herrero et al[15] | Spain | 1990-2001 | 187 | 5.5 | - | 63 (33) | Cutaneous neoplasia 55.5%, non-cutaneous neoplasia 44.4% | - |
| Ettorre et al[132] | Italy | 1990-2008 | 1675 | 5.2 | 3.2 | 98 (5.9) | PTLD 18.4%, head and neck cancer 19.3%, lung cancer 13.2%, colorectal cancer 11.2%, Kaposi's sarcoma 6.2% | - |
| Tajima et al[133] | Japan | 1990-2020 | 1781 | Approximately 12 years | - | 153 (8.6) | PTLD 53%, colorectal 9.1%, lung 7.8%, gastric 7.8% | SIR1993-1995: 8.12, SIR1996-1998: 3.11, SIR2005-2007: 1.31, SIR2008-2010: 1.34, SIR2014-2016: 2.27, SIR2017-2019: 2.07 |
| Benlloch et al[71] | Spain | 1991-2001 | 772 | 4.3 | - | 41 (5.3) | Solid organ tumours 75.6%, hematologic cancer 24.3% | - |
| Baccarani et al[8] | Italy | 1991-2005 | 417 | 6.7 | 4.2 | 43 (10.3) | Non-Hodgkin lymphoma 21%, head and neck cancer 18.6%, Kaposi sarcoma 14% | - |
| Sanaei et al[134] | Iran | 1992-2012 | 1700 | - | 5.5 | 38 (2.2) | PTLD 63%, GI cancer 10.5% | - |
| Sérée et al[101] | France | 1993-2012 | 11226 | - | - | 1200 (10.7) | Lung cancer 15.6%, esophagus, stomach, colorectal 11.8%, larynx 6.3%, oral/pharynx 6% | For all solid organ malignancies 2.2 |
| Jain et al[87] | United States | 1996-2006 | 1000 | 6.5 ± 1 | 3 | 57 (5.7) | Skin cancer including melanoma 38.6%, lung cancer14% oropharyngeal cancer 12.2% | - |
| Chatrath et al[1] | United States | 1997-2004 | 534 | 5.7 ± 3.2 | 4 ± 2.2 | 80 (15) | Solid tumors 50%, skin cancer 30%, hematologic malignancy 20% | 3.1 |
| Yeh et al[135] | Taiwan | 1997-2011 | 2127 | 4.2 | - | 111 (5.2) | - | 1.5 |
| Park et al[136] | South Korea | 1998-2008 | 1952 | 3.5 ± 2.8 | 3.4 ± 2.4 | 44 (2.3) | Stomach 25%, colorectal cancer 20.4%, breast cancer 9% | 7.7 for men and 7.3 for women |
| Egeli et al[137] | Türkiye | 1998-2016 | 429 | 8.6 | 5.3 | 9 (2) | Lung cancer 44.4% | - |
| Kobayashi et al[138] | Japan | 1999-2022 | 70 | 12.1 | - | 8 (11.4) | Lung cancer 50%, PTLD 37.5%, skin cancer 12.5% | - |
| Lucidi et al[139] | Italy | 2000-2015 | 789 | 6.75 | 4 | 67 (5.5) | Lung 16.4%, head and neck 16.4%, PTLD 13.4%, breast 7.4% | - |
| Mangus et al[140] | United States | 2001-2011 | 1275 | - | - | 180 (14) | Skin cancer 59.4%, GI malignancy 10.5% | - |
| Yu et al[141] | China | 2005-2011 | 569 | 3.5 ± 2.2 | - | 18 (3.2) | PTLD 16.7% | - |
| Antinucci et al[142] | Argentina | 2006-2014 | 159 | 1.1 | 1.3 | 12 (7.5) | Skin cancer 33.3% | - |
| Tiwari et al[143] | India | 2006-2017 | 2100 | 3.5 | - | 21 (1) | Oropharyngeal cancer 33.3%, lung cancer 19%, SCC 9.5% | - |
In a retrospective study the reported rates of cutaneous neoplasia to be 14% at 5 years and 24% at 10 years, while the rates for non-cutaneous neoplasia were noted to be 11% at 5 years and 22% at 10 years[15]. In a study with 151 LT patient, the overall incidence of skin cancers in LT recipients was 22.5%, with SCC being the most frequently observed neoplasm[16]. In a prospective cohort study of 161 adults, the prevalence of pre-malignant and neoplastic skin lesions was noted to increase over time (5% at 2-3 years, 12% at 3-5 years, and 28% beyond 5 years)[17]. Meanwhile, the frequency of malignant lesions was 0% at 2-3 years, 9% at 3-5 years, and 12% after more than 5 years of follow-up post-transplantation.
Kaposi's sarcoma (KS) is a multifocal antiproliferative neoplasm that affects the mucous membranes and skin, driven by human herpesvirus 8 (HHV-8) infection, and comprises approximately 4% of all tumors seen post-transplant[18,19]. It exclusively manifests in immunocompromised hosts[20]. A higher prevalence of KS in specific ethnic groups corresponds with regions where HHV-8 seroprevalence is elevated, such as Central and South Africa, the Mediterranean, the Caribbean, and the Middle East[21]. Solid organ transplant recipients have a 500-fold increased risk of developing KS compared to the general population[19]. Nearly all documented cases of KS are found on the skin, although some visceral cases with poor prognosis have also been reported[22].
Post-transplant lymphoproliferative disorders (PTLDs) are the next most common DNM in LT recipients. The overall incidence of PTLD in adults ranges from 1% to 5.5% in adults and up to 15% in children[4,23,24]. PTLD typically presents within one year of LT but may present as early as 20 days or as late as several decades after the transplant. Higher doses of immunosuppression after the LT are suspected to be the cause of higher incidence early on. Early-onset PTLDs are characterized by the outgrowth of Epstein-Barr Virus (EBV) infected B lymphocytes which occurs due to profound impairment of T lymphocytes applied to prevent graft rejection[25]. Late-onset PTLD usually presents as aggressive tumors with monoclonal proliferation and a lack of EBV genome tumor cells[26-28].
Approximately 1% of LT recipients develop solid organ cancers each year. The overall incidence of these malignancies ranges from 3% to 15% following transplantation[3,29]. Liver transplantation is associated with a two to five-fold increase in the risk of developing solid organ cancers compared to the general population[30]. In contrast to PTLD, solid organ cancers are more frequently observed after the first year following transplantation, do not particularly affect children, and are linked to older recipient age[3]. Higher risk has been noted in specific patient populations within the LT community, including those with a history of alcoholic liver disease (ALD) and primary sclerosing cholangitis (PSC)[31]. Recent studies show that solid organ tumors have become the most prevalent malignancies in LT recipients, surpassing skin cancers and PTLDs[32].
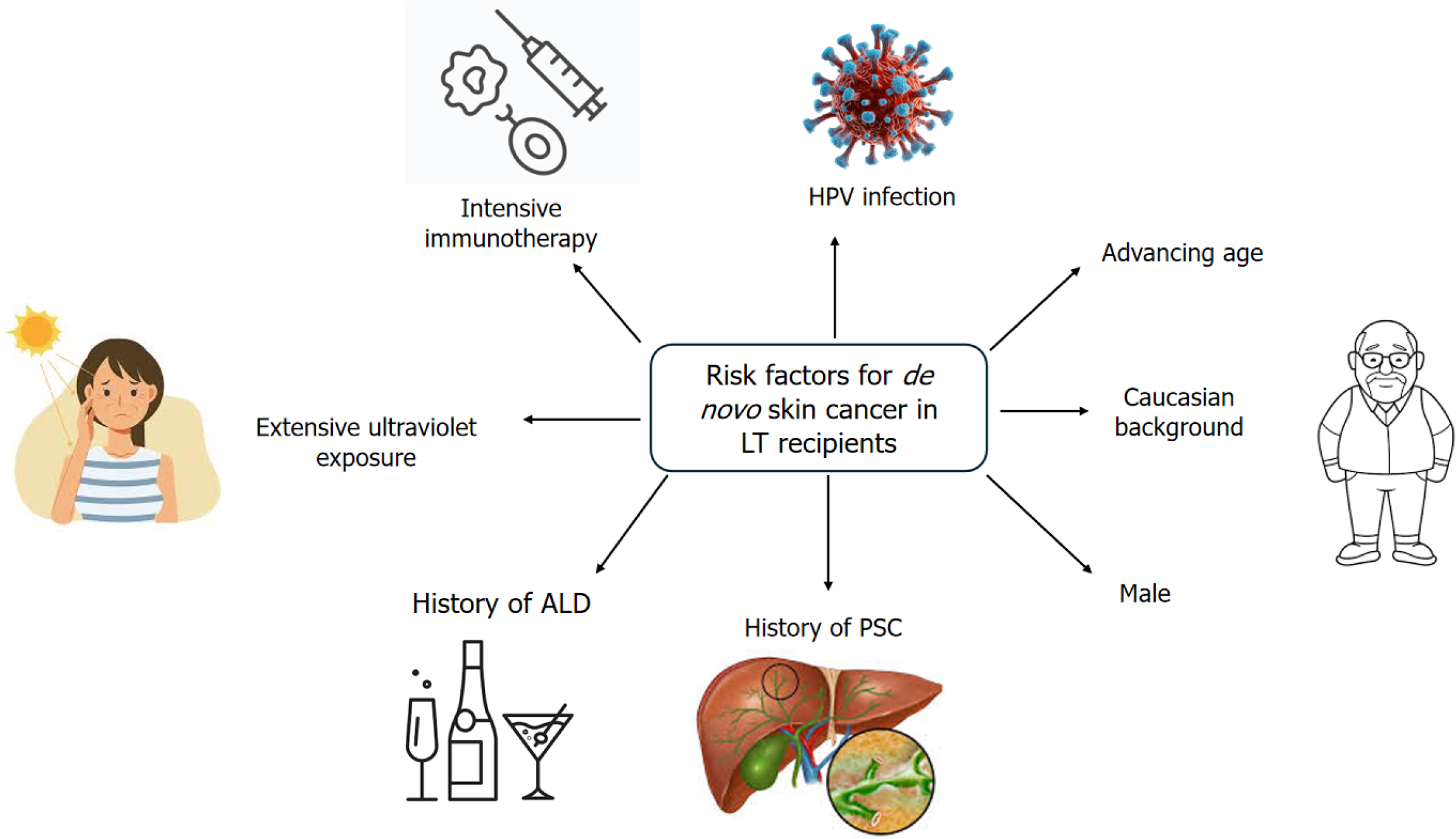
Risk factors for developing skin cancer include advancing age, prolonged and intensive immunosuppressive therapy, infection with human papillomavirus (HPV), extensive ultraviolet exposure, skin that burns easily (particularly skin phototypes II and III), a history of actinic keratosis, previous history of neoplasia [hepatocellular carcinoma (HCC) or other non-skin cancer], CD4 Lymphocytopenia, and having blue or hazel eyes (Figure 1)[15-17,33,34]. Male sex, red hair, Caucasian background, and monoclonal antibody induction therapy are additional risk factors noted in LT recipients[3,16,35]. A heightened risk of developing skin cancer is seen in patients who undergo liver transplantation for PSC, or ALD compared to those with other underlying conditions[14,16,34,36,37].
Studies have noted that cyclosporine A (CsA) is one of the most significant predictor of skin malignancy. Patients receiving CsA developed skin cancer more rapidly than those treated with tacrolimus[16]. Thus, CsA is considered an independent and specific risk factor for skin cancer in post-LT patients[34,38]. Similarly, azathioprine has been postulated to elevate the risk of developing squamous cell carcinoma of the skin in organ transplant recipients, including LT patients[39-41]. Although immunosuppression with CsA and azathioprine is a significant risk factor for NMSC, the extent of immunosuppression likely represents the main risk rather than the specific agent chosen[20,42].
Primary risk factors for KS include viral infections such as hepatitis B virus (HBV), cytomegalovirus, and EBV, but the most significant risk factor is HHV-8[43,44]. Also, there is a strong association between immunosuppression and the development of KS[18]. Therefore, customizing immunosuppressive therapy and carefully managing chemotherapy is essential to prevent the onset of KS[34]. Although ongoing trials are exploring new treatments for KS, current evidence indicates that changing the immunosuppressive regimen from CsA/tacrolimus to mTOR inhibitors is the most effective strategy for reducing the progression of KS[34,45,46].
Currently, there is limited data regarding the effectiveness of surveillance strategies for skin cancers following liver transplantation. It's essential to remain vigilant and implement surveillance strategies that target common DNMs in patients who are at higher risk[47]. Individuals with risk factors like alcohol consumption and smoking might require more frequent screenings. At the very least, standard cancer screening protocols used for the general population should be applied[47]. It is advised that all transplant recipients have regular dermatological check-ups with their healthcare providers, who should maintain a low threshold for performing biopsies or removing any suspicious skin lesions[3,48]. All LT recipients should undergo a full-body skin examination by a dermatologist once yearly[29,47,49,50]. Earlier examination, i.e., dermatological assessments every three to six months is recommended in patients with risk factors, particularly in LT recipients with a previous history of skin cancer, or as advised by their dermatologist[17,32,47-49]. All LT recipients should be informed that they have a higher risk of developing skin cancer compared to the general population. They should also be educated on how to protect their skin, including using sunscreen, and wearing hats, protective clothing, and sunglasses[13,29,47].
In a prospective cohort study, 779 consecutive LT recipients were followed, among which 96 patients (12.3%) de
Screening for KS is challenging because the HHV-8 viral load in affected individuals is often very low, detectable in less than half of the cases. Moreover, there is a lack of standardized serological tests for this condition[41,52-54].
Overall, the management of malignant neoplasms in LT recipients aligns with that of immunocompetent patients, utilizing surgery, chemotherapy, and radiotherapy. However, the management of these neoplasms differs as here the major focus is on modifying immunosuppressive therapy, particularly for tumors that are highly sensitive to immu
SCC: Superficial SCC can be managed using cryotherapy, electrocautery, and curettage, while invasive SCC requires wide local excision[55,56]. These invasive forms are typically aggressive and carry a high risk of recurrence and metastasis at the time of diagnosis.
BCC: The standard treatment for BCC continues to be surgical excision. Reducing immunosuppression in patients with recurrent basal cell carcinomas can help prevent additional recurrences[55]. In older patient populations radiotherapy is a suitable alternative to surgical methods. Electrodesiccation and curettage are frequently employed as primary treatments for low-risk lesions. Photodynamic therapy, along with topical treatments such as imiquimod and 5-fluorouracil, are also viable options[56].
Malignant melanoma: Treatment for malignant melanoma includes reducing immunosuppression and performing surgical excision, with or without sentinel lymphadenectomy[55,56].
Merkel-cell carcinoma: Localized Merkel cell cancer in transplant recipients is treated similarly to how it is in patients who are not immunosuppressed. If there is distant metastasis, temporarily discontinuing cyclosporine may elicit a response. However, the overall prognosis for Merkel cell carcinoma in immunosuppressed patients is poor[55,56].
KS: There is no established consensus on the optimal treatment for post-transplant KS. Treatment decisions are typically guided by clinical experience and medical expertise. Various treatment modalities have been employed, including surgical excision, radiation therapy, intralesional chemotherapy injections, reducing immunosuppressive therapy, and systemic chemotherapy[57]. In transplant patients, full recovery from KS has been observed after reducing immunosuppressive therapy or changing the immunosuppressive regimen to sirolimus[31,58,59]. Research indicates that using immunosuppressive treatments such as Sirolimus or Everolimus may lead to the regression of iatrogenic KS due to their antiangiogenic effects[60,61]. These properties are linked to reduced production of vascular endothelial growth factor (VEGF) and a constrained proliferative response of endothelial cells to VEGF[60]. Furthermore, Nichols et al[62] has suggested that mTOR inhibitors might help prevent KS in immunosuppressed individuals by inhibiting HHV-8 production through the modulation of replication and transcription activator expression.
If reducing or stopping immunosuppressive therapy does not yield results, conventional chemotherapy may be necessary. Pegylated liposomal doxorubicin is often the preferred first-line treatment, with around 70% of patients experiencing a complete or significant response[56,63]. Other cytotoxic drugs that have shown effectiveness in treating KS include vinblastine, bleomycin, taxanes, etoposide, and gemcitabine. Some data suggests that prophylactic treatment with ganciclovir or other antiviral therapy against HHV-8, along with other treatments may benefit patients who are at high risk, as noted in some observational studies[56,64].
Due to improvements in immunosuppression and supportive treatments, LT recipients have increased overall survival (OS), which increases the likelihood of developing de novo malignancies. Aggressive immunosuppression leads to stronger immune impairment and increases the risk of developing PTLD with cumulative incidence of PTLD over five years in LT recipients close to 1%-2%[65]. There is a significantly higher risk in the first year after transplantation. Moreover, the type of immunosuppression employed may play a role, with the use of tacrolimus resulting in a higher risk of PTLD compared to another calcineurin inhibitor (CNI), cyclosporine[66]. Agents suppressing T cell function have a greater likelihood of causing PTLD.
The majority of PTLDs have a B cell origin and are strongly associated with EBV infection. EBV-negative recipients receiving a LT from an EBV-positive donor have an exponentially greater risk of developing PTLD when compared to EBV-positive recipients due to no prior immunity to EBV[67]. Furthermore, CMV sero mismatch can potentially increase the risk. EBV-negative PTLD is less commonly encountered and found to be biologically different from EBV-positive disease. Some may arise from EBV infections which are undetectable now[68].
A single-center German study discovered a significantly higher incidence of hematological malignancies in recipients with hepatitis C virus (HCV) leading to LT[69]. Moreover, this relationship was observed in a systematic review, with the hypothesis being chronic stimulation of immune cells, primarily B cells, in patients with HCV infection[70,71]. Con
Most of the cases display constitutional symptoms of fevers, night sweats, weight loss, and lead to lymphadenopathy. Half of the cases present with extra-nodal involvement, affecting numerous organs including the liver, lungs, and central nervous system (CNS). Lab abnormalities can also aid in diagnosing PTLD which can present with unexplained cytopenias, elevated LDH, hyperuricemia, hypercalcemia, or the presence of monoclonal protein.
There are four different types of PTLD: Nondestructive (early stage), polymorphic, monomorphic, and classic Hodgkin lymphoma (cHL)[73]. Early-stage PTLD comprises benign polyclonal proliferation without destruction of the normal architecture of the involved tissue. The polymorphic type consists of lymphoid cells that do not fulfill the immunocompetent B cell or T/NK cell lymphoma criteria. The monomorphic type includes PTLD that fulfills the B cell or T/NK cell lymphomas criteria established in immunocompetent individuals. They include diffuse large B cell lymphoma, Burkitt lymphoma, Plasma cell myeloma, T cell lymphoma. cHL is the rarest PTLD type. Patients with high international prognostic index scores, bulky disease, EBV negativity, CNS and bone marrow involvement have a worse prognosis[74]. Moreover, earlier presentations after transplant confers a poor prognosis.
There are no screening guidelines in place for the detection of hematological malignancies after LT and the protocols are institution specific. Earlier tapering or withdrawal of immunosuppressants accompanied with anti-viral prophylaxis can reduce the risk of PTLD incidence. Lower targets of immunosuppressive agents’ serum concentrations without com
LT recipients presenting with constitutional symptoms without an explainable infectious etiology or without any concerns for organ rejection should have PTLD in the differential. Evaluation should involve LDH levels, positron emission tomography, and EBV viral load. Magnetic resonance imaging of the head and cerebrospinal fluid assessment should be performed if CNS PTLD is suspected.
The backbone of PTLD management rests on achieving complete remission with minimal toxicities. Antiviral therapy for EBV+ PTLD has not been shown to be effective. Most patients will respond to a reduction in immunosuppression, focusing on discontinuing antimetabolites such as azathioprine or mycophenolate, accompanied by decreasing the dose of CNIs like cyclosporine or tacrolimus, usually to 25%-50% of baseline. Close collaboration with the transplant team is vital as numerous factors, including transplant timing and previous rejection episodes, play a role in deciding the treatment modality. Moreover, different centers have different protocols in place for PTLD management. The subtype of PTLD will dictate the upfront treatment with early lesions responding to immunosuppression reduction alone. These lesions typically resolve in three to five weeks after immunosuppression reduction[76]. The polymorphic subtype re
Combining rituximab (anti-CD20 monoclonal antibody) with reduced immunosuppression has shown significantly improved OS in PTLD patients at 73% vs 33% when compared to reduced immunosuppression only[77]. Another study, called the PTLD-1 trial, evaluating sequential treatment with four cycles of Rituximab followed by four cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), demonstrated a 57% complete response (CR)[78]. This study was followed by a risk-stratified chemoimmunotherapy approach where PTLD patients with rituximab induction who did not achieve CR received R-CHOP, whereas patients achieving CR got rituximab maintenance instead[79]. The study reported about 25% of patients not requiring chemotherapy with a median OS of 6.6 years. This sequential approach has become the standard treatment modality in newly diagnosed PTLD patients. R-EPOCH administration is another approach that can be considered in high-grade PTLD cases and BCL2/MYC rearrangements[80].
Other therapies, including combinations of rituximab/ibrutinib and rituximab/brentuximab vedotin (anti-CD30 antibody-drug conjugate), have not shown any improvements in responses and are associated with significant side effects, especially with brentuximab vedotin[81,82]. Resection of PTLD lesions is rarely performed except in patients with gastrointestinal involvement at risk for obstruction and perforation.
Relapsed PTLD after exposure to chemoimmunotherapy is challenging. A retrospective analysis of EBV+ relapsed PTLD patients demonstrated a median OS of 4.1 months after relapse[83]. HLA-matched EBV-stimulated T cells, named tabelecleucel, were studied in the relapsed setting in the phase 3 ALLELE study, which resulted in CR in 12 out of 43 enrolled patients, leading to regulatory approval by the European Medicine Agency in 2022[84]. Brentuximab can also be used in the relapsed setting. Epigenetic therapy combining the histone deacetylase inhibitor Nanatinostat with valganciclovir has been studied in relapsed/refractory EBV+ PTLD, with 1 CR achieved in 4 patients[85]. Platinum-based salvage chemotherapy, CAR-T, and autologous stem cell transplant are other modalities that can be employed in both EBV+ and EBV- PTLD patients, depending on the disease biology and patient characteristics.
Head and neck cancers/upper aerodigestive cancers: These cancers are linked to smoking and alcohol consumption and develop from tissues within the aerodigestive tract which includes parts of both the respiratory and upper digestive systems, encompassing the lips, mouth, tongue, nose, throat, vocal cords, and portions of the esophagus and windpipe. Oropharyngeal cancer is more likely to develop in patients with alcoholic cirrhosis who undergo LT compared to those transplanted for other reasons[20,29]. A single-center study found that no cases of oropharyngeal cancer were reported in patients without a history of alcohol consumption or smoking[86]. Tongue and laryngeal cancers have been reported in smokers, and the carcinogenic effects of tobacco seen in the general population also apply to transplant recipients[87]. Determining the relative contribution of alcohol vs tobacco as risk factors for head and neck cancers is challenging, as alcohol is known to enhance the carcinogenic effects of smoking[18]. Additionally, individuals who smoke heavily are often also heavy drinkers.
Lung cancer: The primary risk factors for developing lung tumors after LT are a longer duration since the transplant, ALD as the indication for the transplant, and an extended history of tobacco use[44,88,89]. A 2 to 4 times increased risk of developing lung cancer is seen in patients with ALD who undergo LT[1,29]. In an analysis of the National Institute of Diabetes and Digestive and Kidney Disease’ LT database, patients with alcoholic cirrhosis had the highest risk of developing lung cancer, with 5- and 10-year risks of 2.0% and 4.8%, respectively, compared to non-alcoholic cirrhosis patients, whose risks were 0.15% and 1.3% over the same periods[37]. Similar to the link between smoking and lung cancer seen in the general population, smoking is associated with a higher risk of lung cancer in transplant recipients[1,18,44,87]. While this is likely due to an epidemiological correlation, as smokers are often heavy drinkers, a study found that patients who received LTs due to alcohol-related cirrhosis had higher rates of lung cancer compared to those who had LTs for other reasons[18,90].
Gastrointestinal cancers: The primary risk factors for gastrointestinal cancers after LT are duration, tobacco use/smoking, alcohol abuse, PSC [with/without inflammatory bowel disease (IBD)], obesity, diabetes and the presence of metabolic dysfunction-associated steatohepatitis (MASH). The colorectal cancer risk for PSC patients with LT was noted to rise above the 10-year general population rate for a 50-year-old with PSC at 5.75 years after LT for males and 3.25 years for females. Patients with IBD diagnosis were twice as likely to develop colorectal cancer as compared to those without IBD (hazard ratio 2.06, 95%CI: 1.03-4.15, P = 0.042)[91]. Diagnosis of PSC and MASH were also noted to be significant risk factors for pancreatic cancers. Additionally, it is suspected that the higher rates of gastric cancer in LT patients are related to immunosuppression and cirrhosis[92]. In a retrospective study of 9724 patients, it was noted that donor age > 60, donor history of diabetes, donor with body mass index of ≥ 35 kg/m2 and severe graft steatosis, and organs obtained after cardiac death with prolonged warm ischemia were associated with increased risk of HCC recurrence[93]. Other risk factors for HCC recurrence included active HBV/HCV infection and exposure to environmental carcinogens.
Genitourinary: Previous studies have indicated an increased risk of renal and bladder cancer in patients with LT compared to the general population[6,7,94]. Patients with a previous history of renal cell carcinomas (RCC), von Hippel-Lindau (VHL) disease, concurrent chronic kidney disease, and polycystic kidney disease are noted to be at increased risk. Bladder cancer risk is noted to be increased in all transplant patients; however, it is noted to be more aggressive with worse clinical outcomes[95]. Importantly, LT patients do not have an increased risk of prostate cancer-specific mortality[96].
Gynecological cancers: LT patients are at an increased risk of developing cervical and vulvar cancers. This elevated risk is primarily associated with a history of cervical dysplasia and the presence of an HPV infection. Immunosuppression can contribute to the reactivation of latent HPV infections, including oncogenic HPV types, thereby heightening cancer risk[97]. Additionally, immunosuppression impairs the body's ability to clear HPV infections, further exacerbating this vulnerability.
All LT recipients should receive education on the importance of smoking and alcohol cessation after their transplant. For lung cancer screening, it is advised to follow the general population guidelines, as there is no evidence supporting improved survival with more frequent screening in LT patients[98,99]. Annual oral examinations are recommended for all LT patients. However, those at higher risk, such as active smokers or those with active HPV infection, should undergo a comprehensive ear, nose, and throat examination annually[49,100]. Patients with IBD should undergo annual colonoscopies for surveillance for colon cancer, while those with PSC without IBD may undergo a colonoscopy every 3-5 years[101,102]. Patients with a history of HCC or MASH can be considered for a colonoscopy every 5 years if they are over the age of 50 years[49,91]. For RCC screening, the European Association of Urology recommends annual ultrasound for patients with previous RCC, VHL disease, or autosomal dominant polycystic kidney disease[103]. Guidelines for cervical and vulvar cancer screening in immunosuppressed women should also be followed for LT patients[104].
The initial management of de novo solid organ cancers in LT patients involves adjusting immunosuppressive regimens to the lowest effective dose that still preserves graft function. While various practice guidelines recommend initiating mTOR inhibitors, no randomized controlled trials have confirmed their benefit. However, retrospective studies suggest a reduction in mortality by approximately 55%-75% with CNI minimization or conversion to mTOR inhibitors[105,106]. Surgical approaches for solid organ de novo malignancies should follow the same principles as those for the general population, though it is important to note that LT patients are at higher risk for infections and increased 90-day postoperative mortality[107].
Early diagnosis of lung cancers allows for surgical resection in all transplant patients. If patients have significant comorbidity sublobar excisions with radiotherapy can be considered[108]. In advanced stages, the management is similar to that in the general population, with primary surgical resection in non-(small cell lung cancer) SCLC and che
Skin cancer, PTLD, and solid organ tumors are common DNM in post-LT recipients and are the leading causes of morbidity and mortality. The primary factors driving these malignancies are immunosuppression and latent oncogenic viruses. Additional major risk factors include age, sex, history of smoking or alcohol abuse, and the presence of PSC/IBD. Recent efforts to develop screening guidelines have seen reasonable success. To further improve outcomes, it is essential to implement reduced or alternative immunosuppressive therapies and adhere to guideline-directed screening protocols. Screening strategies should be tailored to each patient, taking into account their specific risk factors.
| 1. | Chatrath H, Berman K, Vuppalanchi R, Slaven J, Kwo P, Tector AJ, Chalasani N, Ghabril M. De novo malignancy post-liver transplantation: a single center, population controlled study. Clin Transplant. 2013;27:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Pruthi J, Medkiff KA, Esrason KT, Donovan JA, Yoshida EM, Erb SR, Steinbrecher UP, Fong TL. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001;7:811-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl. 2012;18:1277-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. 2005;56:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 292] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Aberg F, Pukkala E, Höckerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 521] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 7. | Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1139] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 8. | Baccarani U, Piselli P, Serraino D, Adani GL, Lorenzin D, Gambato M, Buda A, Zanus G, Vitale A, De Paoli A, Cimaglia C, Bresadola V, Toniutto P, Risaliti A, Cillo U, Bresadola F, Burra P. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Dig Liver Dis. 2010;42:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients-where do we stand today? Am J Transplant. 2008;8:2192-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Ciążyńska M, Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, Sławińska M, Pabianek M, Szczepaniak K, Hankiewicz A, Ułańska M, Morawiec J, Błasińska-Morawiec M, Morawiec Z, Piekarski J, Nejc D, Brodowski R, Zaryczańska A, Sobjanek M, Nowicki RJ, Owczarek W, Słowińska M, Wróbel K, Bieniek A, Woźniacka A, Skibińska M, Narbutt J, Niemczyk W, Ciążyński K, Lesiak A. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep. 2021;11:4337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | Modaresi Esfeh J, Hanouneh IA, Dalal D, Tabba A, Lopez R, Pagadala M, Eghtesad B, Zein NN. The incidence and risk factors of de novo skin cancer in the liver transplant recipients. Int J Organ Transplant Med. 2012;3:157-163. [PubMed] |
| 12. | Akdag D, Rasmussen A, Nielsen SD, Møller DL, Togsverd-Bo K, Wenande E, Haedersdal M, Pommergaard HC. Early Results of a Screening Program for Skin Cancer in Liver Transplant Recipients: A Cohort Study. Cancers (Basel). 2024;16:1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Desai R, Neuberger J. Donor transmitted and de novo cancer after liver transplantation. World J Gastroenterol. 2014;20:6170-6179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Saigal S, Norris S, Muiesan P, Rela M, Heaton N, O'Grady J. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transpl. 2002;8:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 15. | Herrero JI, Lorenzo M, Quiroga J, Sangro B, Pardo F, Rotellar F, Alvarez-Cienfuegos J, Prieto J. De Novo neoplasia after liver transplantation: an analysis of risk factors and influence on survival. Liver Transpl. 2005;11:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Mithoefer AB, Supran S, Freeman RB. Risk factors associated with the development of skin cancer after liver transplantation. Liver Transpl. 2002;8:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Belloni-Fortina A, Piaserico S, Bordignon M, Gambato M, Senzolo M, Russo FP, Peserico A, De Matteis G, Perissinotto E, Cillo U, Vitale A, Alaibac M, Burra P. Skin cancer and other cutaneous disorders in liver transplant recipients. Acta Derm Venereol. 2012;92:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Burra P, Rodriguez-Castro KI. Neoplastic disease after liver transplantation: Focus on de novo neoplasms. World J Gastroenterol. 2015;21:8753-8768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Euvrard S, Kanitakis J. Skin cancers after liver transplantation: what to do? J Hepatol. 2006;44:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Mukthinuthalapati PK, Gotur R, Ghabril M. Incidence, risk factors and outcomes of de novo malignancies post liver transplantation. World J Hepatol. 2016;8:533-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Penn I. Kaposi's sarcoma in transplant recipients. Transplantation. 1997;64:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | García-Sesma A, Jiménez C, Loinaz C, Meneu JC, Colina F, Marqués E, Gómez R, Abradelo M, Garcia JI, Moreno González E. Kaposi's visceral sarcoma in liver transplant recipients. Transplant Proc. 2003;35:1898-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 759] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 24. | Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, Brepoels L, Kuypers D, Vanhaecke J, Nevens F, Verleden G, Van Damme-Lombaerts R, Renard M, Pirenne J, De Wolf-Peeters C, Verhoef G. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54:2433-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Leblond V, Davi F, Charlotte F, Dorent R, Bitker MO, Sutton L, Gandjbakhch I, Binet JL, Raphael M. Posttransplant lymphoproliferative disorders not associated with Epstein-Barr virus: a distinct entity? J Clin Oncol. 1998;16:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 254] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Dotti G, Fiocchi R, Motta T, Gamba A, Gotti E, Gridelli B, Borleri G, Manzoni C, Viero P, Remuzzi G, Barbui T, Rambaldi A. Epstein-Barr virus-negative lymphoproliferate disorders in long-term survivors after heart, kidney, and liver transplant. Transplantation. 2000;69:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Knowles DM, Cesarman E, Chadburn A, Frizzera G, Chen J, Rose EA, Michler RE. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85:552-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 401] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Dotti G, Fiocchi R, Motta T, Mammana C, Gotti E, Riva S, Cornelli P, Gridelli B, Viero P, Oldani E, Ferrazzi P, Remuzzi G, Barbui T, Rambaldi A. Lymphomas occurring late after solid-organ transplantation: influence of treatment on the clinical outcome. Transplantation. 2002;74:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Singh A, De A, Singh V. Post-transplant malignancies in alcoholic liver disease. Transl Gastroenterol Hepatol. 2020;5:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Carenco C, Faure S, Ursic-Bedoya J, Herrero A, Pageaux GP. Solid, non-skin, post-liver transplant tumors: Key role of lifestyle and immunosuppression management. World J Gastroenterol. 2016;22:427-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Vallejo GH, Romero CJ, de Vicente JC. Incidence and risk factors for cancer after liver transplantation. Crit Rev Oncol Hematol. 2005;56:87-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Fuochi E, Anastasio L, Lynch EN, Campani C, Dragoni G, Milani S, Galli A, Innocenti T. Main factors influencing long-term outcomes of liver transplantation in 2022. World J Hepatol. 2023;15:321-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 33. | Doycheva I, Amer S, Watt KD. De Novo Malignancies After Transplantation: Risk and Surveillance Strategies. Med Clin North Am. 2016;100:551-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Manzia TM, Angelico R, Gazia C, Lenci I, Milana M, Ademoyero OT, Pedini D, Toti L, Spada M, Tisone G, Baiocchi L. De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature. World J Gastroenterol. 2019;25:5356-5375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1-17; quiz 18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 486] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Jiménez-Romero C, Manrique Municio A, Marqués Medina E, Colina F, Ortega Domene P, Gómez Sanz R, Meneu Diaz JC, Abradelo de Usera M, Moreno Elola A, Moreno Gonzalez E. Incidence of de novo nonmelanoma skin tumors after liver transplantation for alcoholic and nonalcoholic liver diseases. Transplant Proc. 2006;38:2505-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Sanchez W, Gores GJ. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Euvrard S, Ulrich C, Lefrancois N. Immunosuppressants and skin cancer in transplant patients: focus on rapamycin. Dermatol Surg. 2004;30:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | O'Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 442] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 40. | Jiyad Z, Olsen CM, Burke MT, Isbel NM, Green AC. Azathioprine and Risk of Skin Cancer in Organ Transplant Recipients: Systematic Review and Meta-Analysis. Am J Transplant. 2016;16:3490-3503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Burra P, Shalaby S, Zanetto A. Long-term care of transplant recipients: de novo neoplasms after liver transplantation. Curr Opin Organ Transplant. 2018;23:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Tessari G, Naldi L, Boschiero L, Nacchia F, Fior F, Forni A, Rugiu C, Faggian G, Sassi F, Gotti E, Fiocchi R, Talamini G, Girolomoni G. Incidence and clinical predictors of a subsequent nonmelanoma skin cancer in solid organ transplant recipients with a first nonmelanoma skin cancer: a multicenter cohort study. Arch Dermatol. 2010;146:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Aseni P, Vertemati M, Minola E, Arcieri K, Bonacina E, Camozzi M, Osio C, Forti D. Kaposi's sarcoma in liver transplant recipients: morphological and clinical description. Liver Transpl. 2001;7:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Jiménez-Romero C, Justo-Alonso I, Cambra-Molero F, Calvo-Pulido J, García-Sesma Á, Abradelo-Usera M, Caso-Maestro O, Manrique-Municio A. Incidence, risk factors and outcome of de novo tumors in liver transplant recipients focusing on alcoholic cirrhosis. World J Hepatol. 2015;7:942-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Piselli P, Busnach G, Citterio F, Frigerio M, Arbustini E, Burra P, Pinna AD, Bresadola V, Ettorre GM, Baccarani U, Buda A, Lauro A, Zanus G, Cimaglia C, Spagnoletti G, Lenardon A, Agozzino M, Gambato M, Zanfi C, Miglioresi L, Di Gioia P, Mei L, Ippolito G, Serraino D; Immunosuppression and Cancer Study Group. Risk of Kaposi sarcoma after solid-organ transplantation: multicenter study in 4,767 recipients in Italy, 1970-2006. Transplant Proc. 2009;41:1227-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 683] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 47. | Choudhary NS, Saigal S, Saraf N, Soin AS. Extrahepatic Malignancies and Liver Transplantation: Current Status. J Clin Exp Hepatol. 2021;11:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Pillai AA. Management of de novo malignancies after liver transplantation. Transplant Rev (Orlando). 2015;29:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Colmenero J, Tabrizian P, Bhangui P, Pinato DJ, Rodríguez-Perálvarez ML, Sapisochin G, Bhoori S, Pascual S, Senzolo M, Al-Adra D, Herrero JI, Petrowsky H, Dawson LA, Hosni A, Kutzke JL, Gastaca M, Watt KD. De Novo Malignancy After Liver Transplantation: Risk Assessment, Prevention, and Management-Guidelines From the ILTS-SETH Consensus Conference. Transplantation. 2022;106:e30-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 50. | Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 51. | Finkenstedt A, Graziadei IW, Oberaigner W, Hilbe W, Nachbaur K, Mark W, Margreiter R, Vogel W. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant. 2009;9:2355-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 52. | Haq IU, Dalla Pria A, Papanastasopoulos P, Stegmann K, Bradshaw D, Nelson M, Bower M. The clinical application of plasma Kaposi sarcoma herpesvirus viral load as a tumour biomarker: results from 704 patients. HIV Med. 2016;17:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Len O, Garzoni C, Lumbreras C, Molina I, Meije Y, Pahissa A, Grossi P; ESCMID Study Group of Infection in Compromised Hosts. Recommendations for screening of donor and recipient prior to solid organ transplantation and to minimize transmission of donor-derived infections. Clin Microbiol Infect. 2014;20 Suppl 7:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Chiereghin A, Barozzi P, Petrisli E, Piccirilli G, Gabrielli L, Riva G, Potenza L, Cappelli G, De Ruvo N, Libri I, Maggiore U, Morelli MC, Potena L, Todeschini P, Gibertoni D, Labanti M, Sangiorgi G, La Manna G, Pinna AD, Luppi M, Lazzarotto T. Multicenter Prospective Study for Laboratory Diagnosis of HHV8 Infection in Solid Organ Donors and Transplant Recipients and Evaluation of the Clinical Impact After Transplantation. Transplantation. 2017;101:1935-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Ajithkumar TV, Parkinson CA, Butler A, Hatcher HM. Management of solid tumours in organ-transplant recipients. Lancet Oncol. 2007;8:921-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Shalaby S, Burra P. De novo and recurrent malignancy. Best Pract Res Clin Gastroenterol. 2020;46-47:101680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Benhammane H, Mentha G, Tschanz E, El Mesbahi O, Dietrich PY. Visceral Kaposi's Sarcoma Related to Human Herpesvirus-8 in Liver Transplant Recipient: Case Report and Literature Review. Case Rep Oncol Med. 2012;2012:137291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Schneider JW, Dittmer DP. Diagnosis and Treatment of Kaposi Sarcoma. Am J Clin Dermatol. 2017;18:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Campistol JM, Gutierrez-Dalmau A, Torregrosa JV. Conversion to sirolimus: a successful treatment for posttransplantation Kaposi's sarcoma. Transplantation. 2004;77:760-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Lebbé C, Euvrard S, Barrou B, Pouteil-Noble C, Garnier JL, Glotz D, Legendre C, Francès C. Sirolimus conversion for patients with posttransplant Kaposi's sarcoma. Am J Transplant. 2006;6:2164-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Stallone G, Infante B, Grandaliano G, Schena FP, Gesualdo L. Kaposi's sarcoma and mTOR: a crossroad between viral infection neoangiogenesis and immunosuppression. Transpl Int. 2008;21:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Nichols LA, Adang LA, Kedes DH. Rapamycin blocks production of KSHV/HHV8: insights into the anti-tumor activity of an immunosuppressant drug. PLoS One. 2011;6:e14535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Di Trolio R, Di Lorenzo G, Delfino M, De Placido S. Role of pegylated lyposomal doxorubicin (PLD) in systemic Kaposi's sarcoma: a systematic review. Int J Immunopathol Pharmacol. 2006;19:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Verucchi G, Calza L, Trevisani F, Zambruni A, Tadolini M, Giuliani R, Manfredi R, Andreone P, Chiodo F, Bernardi M. Human herpesvirus-8-related Kaposi's sarcoma after liver transplantation successfully treated with cidofovir and liposomal daunorubicin. Transpl Infect Dis. 2005;7:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Penn I. Posttransplantation de novo tumors in liver allograft recipients. Liver Transpl Surg. 1996;2:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, Leichtman AB, Held PJ, Port FK. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 67. | Walker RC, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM, McGregor CG, Paya CV. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 265] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Craig FE, Johnson LR, Harvey SA, Nalesnik MA, Luo JH, Bhattacharya SD, Swerdlow SH. Gene expression profiling of Epstein-Barr virus-positive and -negative monomorphic B-cell posttransplant lymphoproliferative disorders. Diagn Mol Pathol. 2007;16:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Rademacher S, Seehofer D, Eurich D, Schoening W, Neuhaus R, Oellinger R, Denecke T, Pascher A, Schott E, Sinn M, Neuhaus P, Pratschke J. The 28-year incidence of de novo malignancies after liver transplantation: A single-center analysis of risk factors and mortality in 1616 patients. Liver Transpl. 2017;23:1404-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Chak E, Saab S. Risk factors and incidence of de novo malignancy in liver transplant recipients: a systematic review. Liver Int. 2010;30:1247-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Benlloch S, Berenguer M, Prieto M, Moreno R, San Juan F, Rayón M, Mir J, Segura A, Berenguer J. De novo internal neoplasms after liver transplantation: increased risk and aggressive behavior in recent years? Am J Transplant. 2004;4:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Altieri M, Sérée O, Lobbedez T, Segol P, Abergel A, Blaizot X, Boillot O, Boudjema K, Coilly A, Conti F, Chazouillères O, Debette-Gratien M, Dharancy S, Durand F, Duvoux C, Francoz C, Gugenheim J, Hardwigsen J, Houssel-Debry P, Kamar N, Latournerie M, Lebray P, Leroy V, Neau-Cransac M, Pageaux GP, Radenne S, Salamé E, Saliba F, Samuel D, Vanlemmens C, Besch C, Launoy G, Dumortier J. Risk factors of de novo malignancies after liver transplantation: a French national study on 11004 adult patients. Clin Res Hepatol Gastroenterol. 2021;45:101514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, Brousset P, Cerroni L, de Leval L, Dirnhofer S, Dogan A, Feldman AL, Fend F, Friedberg JW, Gaulard P, Ghia P, Horwitz SM, King RL, Salles G, San-Miguel J, Seymour JF, Treon SP, Vose JM, Zucca E, Advani R, Ansell S, Au WY, Barrionuevo C, Bergsagel L, Chan WC, Cohen JI, d'Amore F, Davies A, Falini B, Ghobrial IM, Goodlad JR, Gribben JG, Hsi ED, Kahl BS, Kim WS, Kumar S, LaCasce AS, Laurent C, Lenz G, Leonard JP, Link MP, Lopez-Guillermo A, Mateos MV, Macintyre E, Melnick AM, Morschhauser F, Nakamura S, Narbaitz M, Pavlovsky A, Pileri SA, Piris M, Pro B, Rajkumar V, Rosen ST, Sander B, Sehn L, Shipp MA, Smith SM, Staudt LM, Thieblemont C, Tousseyn T, Wilson WH, Yoshino T, Zinzani PL, Dreyling M, Scott DW, Winter JN, Zelenetz AD. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140:1229-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 1068] [Article Influence: 267.0] [Reference Citation Analysis (0)] |
| 74. | Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354-3362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 75. | San-Juan R, Manuel O, Hirsch HH, Fernández-Ruiz M, López-Medrano F, Comoli P, Caillard S, Grossi P, Aguado JM; ESGICH PTLD Survey Study Group; European Study Group of Infections in Compromised Hosts (ESGICH) from the European Society of Microbiology and Infectious Diseases (ESCMID). Current preventive strategies and management of Epstein-Barr virus-related post-transplant lymphoproliferative disease in solid organ transplantation in Europe. Results of the ESGICH Questionnaire-based Cross-sectional Survey. Clin Microbiol Infect. 2015;21:604.e1-604.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Tsai DE, Hardy CL, Tomaszewski JE, Kotloff RM, Oltoff KM, Somer BG, Schuster SJ, Porter DL, Montone KT, Stadtmauer EA. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 77. | Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, Gimelfarb A, Hattersley E, Mauro LA, Jovanovic B, Chadburn A, Stiff P, Winter JN, Mehta J, Van Besien K, Gregory S, Gordon LI, Shammo JM, Smith SE, Smith SM. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28:1038-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 78. | Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G, Morschhauser F, Jaccard A, Lamy T, Leithäuser M, Zimmermann H, Anagnostopoulos I, Raphael M, Riess H, Choquet S; German PTLD Study Group; European PTLD Network. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 79. | Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Salles G, Kliem V, Hauser IA, Tarella C, Van Den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S. Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification Into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. 2017;35:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 80. | Ford M, Orlando E, Jin Z, Lipsky AH, Sawas A, Pro B, Amengual JE. Treatment Modalities Effect on Outcome in Post-Transplant Lymphoproliferative Disorder. Blood. 2022;140:3794-3795. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 81. | Chaganti S, Maycock S, Mcilroy G, Iqbal WA, Mason J, Kanfer E, Kassam S, Cwynarski K, Wrench D, Arumainathan AK, Fox CP, Johnson R, Mckay P, Paneesha S, Rowntree CJ, Balotis C, Collins GP, Davies A, Wright J, Wheatley K, Menne TF. Risk-Stratified Sequential Treatment with Ibrutinib and Rituximab (IR) and IR-CHOP for De-Novo Post-Transplant Lymphoproliferative Disorder: Results of the Tidal Trial. Blood. 2021;138:2492-2492. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Pearse WB, Petrich AM, Gordon LI, Karmali R, Winter JN, Ma S, Kaplan JB, Behdad A, Klein A, Jovanovic B, Helenowski I, Smith SM, Evens AM, Pro B. A phase I/II trial of brentuximab vedotin plus rituximab as frontline therapy for patients with immunosuppression-associated CD30+ and/or EBV + lymphomas. Leuk Lymphoma. 2021;62:3493-3500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Dharnidharka V, Thirumalai D, Jaeger U, Zhao W, Dierickx D, Xun P, Minga P, Sawas A, Sadetsky N, Chauvet P, Sundaram E, Barlev A, Zimmermann H, Trappe RU. Clinical Outcomes of Solid Organ Transplant Patients with Epstein-Barr Virus-Driven (EBV +) Post-Transplant Lymphoproliferative Disorder (PTLD) Who Fail Rituximab Plus Chemotherapy: A Multinational, Retrospective Chart Review Study. Blood. 2021;138:2528-2528. [RCA] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 84. | Mahadeo KM, Baiocchi RA, Beitinjaneh A, Chaganti S, Choquet S, Dierickx D, Dinavahi R, Gamelin L, Ghobadi A, Guzman-becerra N, Joshi M, Mehta A, Nikiforow S, Reshef R, Ye W, Prockop S. New and Updated Results from a Multicenter, Open-Label, Global Phase 3 Study of Tabelecleucel (Tab-cel) for Epstein-Barr Virus-Positive Post-Transplant Lymphoproliferative Disease (EBV+ PTLD) Following Allogeneic Hematopoietic Cell (HCT) or Solid Organ Transplant (SOT) after Failure of Rituximab or Rituximab and Chemotherapy (ALLELE). Blood. 2022;140:10374-10376. [RCA] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 85. | Haverkos BM, Alpdogan O, Baiocchi R, Brammer JE, Feldman TA, Capra M, Brem EA, Nair SM, Scheinberg P, Pereira J, Shune L, Joffe E, Katkov A, Mcrae R, Royston I, Rojkjaer L, Porcu P. Nanatinostat (Nstat) and Valganciclovir (VGCV) in Relapsed/Refractory (R/R) Epstein-Barr Virus-Positive (EBV +) Lymphomas: Final Results from the Phase 1b/2 VT3996-201 Study. Blood. 2021;138:623-623. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Schmilovitz-Weiss H, Mor E, Sulkes J, Bar-Nathan N, Shaharabani E, Melzer E, Tur-Kaspa R, Ben-Ari Z. De novo tumors after liver transplantation: a single-center experience. Transplant Proc. 2003;35:665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Jain AB, Yee LD, Nalesnik MA, Youk A, Marsh G, Reyes J, Zak M, Rakela J, Irish W, Fung JJ. Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation. 1998;66:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Fung JJ, Jain A, Kwak EJ, Kusne S, Dvorchik I, Eghtesad B. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl. 2001;7:S109-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Herrero JI, Pardo F, D'Avola D, Alegre F, Rotellar F, Iñarrairaegui M, Martí P, Sangro B, Quiroga J. Risk factors of lung, head and neck, esophageal, and kidney and urinary tract carcinomas after liver transplantation: the effect of smoking withdrawal. Liver Transpl. 2011;17:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Jiménez C, Manrique A, Marqués E, Ortega P, Loinaz C, Gómez R, Meneu JC, Abradelo M, Moreno A, López A, Moreno E. Incidence and risk factors for the development of lung tumors after liver transplantation. Transpl Int. 2007;20:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Nasser-Ghodsi N, Mara K, Watt KD. De Novo Colorectal and Pancreatic Cancer in Liver-Transplant Recipients: Identifying the Higher-Risk Populations. Hepatology. 2021;74:1003-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Gong CS, Yoo MW, Kim BS, Hwang S, Kim KH, Yook JH, Kim BS, Lee SG. De Novo Gastric Cancer After Liver Transplantation. Ann Transplant. 2016;21:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Orci LA, Berney T, Majno PE, Lacotte S, Oldani G, Morel P, Mentha G, Toso C. Donor characteristics and risk of hepatocellular carcinoma recurrence after liver transplantation. Br J Surg. 2015;102:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 94. | Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 95. | Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR, Woodle ES. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87:1347-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 96. | Liauw SL, Ham SA, Das LC, Rudra S, Packiam VT, Koshy M, Weichselbaum RR, Becker YT, Bodzin AS, Eggener SE. Prostate Cancer Outcomes Following Solid-Organ Transplantation: A SEER-Medicare Analysis. J Natl Cancer Inst. 2020;112:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 97. | Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, Stanley MA, Franceschi S. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 644] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 98. | Herrero JI, Bastarrika G, D'Avola D, Montes U, Pueyo J, Iñarrairaegui M, Pardo F, Quiroga J, Zulueta J. Lung cancer screening with low-radiation dose computed tomography after liver transplantation. Ann Transplant. 2013;18:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, Smith JP. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1729] [Cited by in RCA: 1640] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 100. | Renaud L, Hilleret MN, Thimonier E, Guillaud O, Arbib F, Ferretti G, Jankowski A, Chambon-Augoyard C, Erard-Poinsot D, Decaens T, Boillot O, Leroy V, Dumortier J. De Novo Malignancies Screening After Liver Transplantation for Alcoholic Liver Disease: A Comparative Opportunistic Study. Liver Transpl. 2018;24:1690-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Sérée O, Altieri M, Guillaume E, De Mil R, Lobbedez T, Robinson P, Segol P, Salamé E, Abergel A, Boillot O, Conti F, Chazouillères O, Debette-Gratien M, Debray D, Hery G, Dharancy S, Durand F, Duvoux C, Francoz C, Gugenheim J, Hardwigsen J, Houssel-Debry P, Jacquemin E, Kamar N, Latournerie M, Lebray P, Leroy V, Mazzola A, Neau-Cransac M, Pageaux GP, Radenne S, Saliba F, Samuel D, Vanlemmens C, Woehl-Jaegle ML, Launoy G, Dumortier J. Longterm Risk of Solid Organ De Novo Malignancies After Liver Transplantation: A French National Study on 11,226 Patients. Liver Transpl. 2018;24:1425-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 102. | Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 103. | Kälble T, Lucan M, Nicita G, Sells R, Burgos Revilla FJ, Wiesel M; European Association of Urology. EAU guidelines on renal transplantation. Eur Urol. 2005;47:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 104. | Moscicki AB, Flowers L, Huchko MJ, Long ME, MacLaughlin KL, Murphy J, Spiryda LB, Gold MA. Guidelines for Cervical Cancer Screening in Immunosuppressed Women Without HIV Infection. J Low Genit Tract Dis. 2019;23:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 105. | Thimonier E, Guillaud O, Walter T, Decullier E, Vallin M, Boillot O, Dumortier J. Conversion to everolimus dramatically improves the prognosis of de novo malignancies after liver transplantation for alcoholic liver disease. Clin Transplant. 2014;28:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 106. | Rousseau B, Guillemin A, Duvoux C, Neuzillet C, Tlemsani C, Compagnon P, Azoulay D, Salloum C, Laurent A, de la Taille A, Salomon L, Cholley I, Haioun C, Dupuis J, Wolkenstein P, Matignon MB, Grimbert P, Tournigand C. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Drevet G, Duruisseaux M, Maury JM, Riche B, Grima R, Ginoux M, Mornex JF, Tronc F. Lung cancer surgical treatment after solid organ transplantation: A single center 30-year experience. Lung Cancer. 2020;139:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Anyanwu AC, Townsend ER, Banner NR, Burke M, Khaghani A, Yacoub MH. Primary lung carcinoma after heart or lung transplantation: management and outcome. J Thorac Cardiovasc Surg. 2002;124:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Sigel K, Veluswamy R, Krauskopf K, Mehrotra A, Mhango G, Sigel C, Wisnivesky J. Lung Cancer Prognosis in Elderly Solid Organ Transplant Recipients. Transplantation. 2015;99:2181-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Na S, Lee GH, Song JH, Ahn JY, Kim SO, Park SJ, Park SE, Kim MY, Lee J, Choi KS, Kim DH, Song HJ, Choi KD, Jung HY, Kim JH. Endoscopic resection of gastric neoplasm in solid-organ transplant recipients. Transplantation. 2014;97:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Dobrindt EM, Biebl M, Rademacher S, Denecke C, Andreou A, Raakow J, Kröll D, Öllinger R, Pratschke J, Chopra SS. De-novo Upper Gastrointestinal Tract Cancer after Liver Transplantation: A Demographic Report. Int J Organ Transplant Med. 2020;11:71-80. [PubMed] |
| 112. | Merchea A, Shahjehan F, Croome KP, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Colorectal Cancer Characteristics and Outcomes after Solid Organ Transplantation. J Oncol. 2019;2019:5796108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Rompianesi G, Ravikumar R, Jose S, Allison M, Athale A, Creamer F, Gunson B, Manas D, Monaco A, Mirza D, Owen N, Roberts K, Sen G, Srinivasan P, Wigmore S, Fusai G, Fernando B, Burroughs A, Tsochatzis E. Incidence and outcome of colorectal cancer in liver transplant recipients: A national, multicentre analysis on 8115 patients. Liver Int. 2019;39:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Coordes A, Albers AE, Lenarz M, Seehofer D, Puhl G, Pascher A, Neuhaus R, Neuhaus P, Pratschke J, Andreou A. Incidence and long-term survival of patients with de novo head and neck carcinoma after liver transplantation. Head Neck. 2016;38:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 115. | Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology. 1997;44:1172-1181. [PubMed] |
| 116. | Krynitz B, Edgren G, Lindelöf B, Baecklund E, Brattström C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008-a Swedish population-based study. Int J Cancer. 2013;132:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 117. | Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, Slooff MJ, Jansen PL. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 250] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 118. | Oo YH, Gunson BK, Lancashire RJ, Cheng KK, Neuberger JM. Incidence of cancers following orthotopic liver transplantation in a single center: comparison with national cancer incidence rates for England and Wales. Transplantation. 2005;80:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | Jiang Y, Villeneuve PJ, Fenton SS, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008;14:1588-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 120. | Schrem H, Kurok M, Kaltenborn A, Vogel A, Walter U, Zachau L, Manns MP, Klempnauer J, Kleine M. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 2013;19:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 121. | Galve ML, Cuervas-Mons V, Figueras J, Herrero I, Mata M, Clemente G, Prieto M, Margarit C, Bernardos A, Casafont F. Incidence and outcome of de novo malignancies after liver transplantation. Transplant Proc. 1999;31:1275-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Mouchli MA, Singh S, Loftus EV Jr, Boardman L, Talwalkar J, Rosen CB, Heimbach JK, Wiesner RH, Hasan B, Poterucha JJ, Kymberly WD. Risk Factors and Outcomes of De Novo Cancers (Excluding Nonmelanoma Skin Cancer) After Liver Transplantation for Primary Sclerosing Cholangitis. Transplantation. 2017;101:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Sanchez EQ, Marubashi S, Jung G, Levy MF, Goldstein RM, Molmenti EP, Fasola CG, Gonwa TA, Jennings LW, Brooks BK, Klintmalm GB. De novo tumors after liver transplantation: a single-institution experience. Liver Transpl. 2002;8:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 124. | Wimmer CD, Angele MK, Schwarz B, Pratschke S, Rentsch M, Khandoga A, Guba M, Jauch KW, Bruns C, Graeb C. Impact of cyclosporine versus tacrolimus on the incidence of de novo malignancy following liver transplantation: a single center experience with 609 patients. Transpl Int. 2013;26:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 125. | Taborelli M, Piselli P, Ettorre GM, Baccarani U, Burra P, Lauro A, Galatioto L, Rendina M, Shalaby S, Petrara R, Nudo F, Toti L, Fantola G, Cimaglia C, Agresta A, Vennarecci G, Pinna AD, Gruttadauria S, Risaliti A, Di Leo A, Rossi M, Tisone G, Zamboni F, Serraino D; Italian Transplant and Cancer Cohort Study. Survival after the diagnosis of de novo malignancy in liver transplant recipients. Int J Cancer. 2019;144:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Shalaby S, Taborelli M, Zanetto A, Ferrarese A, D'Arcangelo F, Gambato M, Senzolo M, Russo FP, Germani G, Boccagni P, Ettorre GM, Baccarani U, Lauro A, Galatioto L, Rendina M, Petrara R, De Rossi A, Nudo F, Toti L, Fantola G, Vennarecci G, Risaliti A, Pinna AD, Gruttadauria S, Di Leo A, Rossi M, Tisone G, Zamboni F, Cillo U, Piselli P, Serraino D, Burra P; Italian Transplant and Cancer Cohort Study. Hepatocellular carcinoma and the risk of de novo malignancies after liver transplantation - a multicenter cohort study. Transpl Int. 2021;34:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 127. | Jiménez C, Rodríguez D, Marqués E, Loinaz C, Alonso O, Hernández-Vallejo G, Marín L, Rodríguez F, García I, Moreno E. De novo tumors after orthotopic liver transplantation. Transplant Proc. 2002;34:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Tjon AS, Sint Nicolaas J, Kwekkeboom J, de Man RA, Kazemier G, Tilanus HW, Hansen BE, van der Laan LJ, Tha-In T, Metselaar HJ. Increased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with C2 monitoring and recipient age. Liver Transpl. 2010;16:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 129. | Jonas S, Rayes N, Neumann U, Neuhaus R, Bechstein WO, Guckelberger O, Tullius SG, Serke S, Neuhaus P. De novo malignancies after liver transplantation using tacrolimus-based protocols or cyclosporine-based quadruple immunosuppression with an interleukin-2 receptor antibody or antithymocyte globulin. Cancer. 1997;80:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 130. | Kelly DM, Emre S, Guy SR, Miller CM, Schwartz ME, Sheiner PA. Liver transplant recipients are not at increased risk for nonlymphoid solid organ tumors. Cancer. 1998;83:1237-1243. [PubMed] |
| 131. | Yao FY, Gautam M, Palese C, Rebres R, Terrault N, Roberts JP, Peters MG. De novo malignancies following liver transplantation: a case-control study with long-term follow-up. Clin Transplant. 2006;20:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Ettorre GM, Piselli P, Galatioto L, Rendina M, Nudo F, Sforza D, Miglioresi L, Fantola G, Cimaglia C, Vennarecci G, Vizzini GB, Di Leo A, Rossi M, Tisone G, Zamboni F, Santoro R, Agresta A, Puro V, Serraino D. De novo malignancies following liver transplantation: results from a multicentric study in central and southern Italy, 1990-2008. Transplant Proc. 2013;45:2729-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Tajima T, Hata K, Tanaka K, Iyama N, Kusakabe J, Kageyama S, Ogawa E, Okamoto T, Haga H, Uemoto S, Hatano E. Chronological alterations in de novo malignancies after living-donor liver transplantation: A cohort study of 1781 recipients using annual comparisons of standardized incidence ratios. J Hepatobiliary Pancreat Sci. 2024;31:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 134. | Sanaei AK, Aliakbarian M, Kazemi K, Nikeghbalian S, Shamsaeefar A, Mehdi SH, Bahreini A, Dehghani SM, Geramizadeh B, Malekhosseini SA. De novo malignancy after liver transplant. Exp Clin Transplant. 2015;13:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 135. | Yeh CC, Khan A, Muo CH, Yang HR, Li PC, Chang CH, Chen TL, Jeng LB, Liao CC. De Novo Malignancy After Heart, Kidney, and Liver Transplant: A Nationwide Study in Taiwan. Exp Clin Transplant. 2020;18:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 136. | Park HW, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Namgoong JM, Yoon SY, Park CS, Park YH, Lee HJ, Lee SG. De novo malignancies after liver transplantation: incidence comparison with the Korean cancer registry. Transplant Proc. 2012;44:802-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 137. | Egeli T, Unek T, Ozbilgin M, Agalar C, Derici S, Akarsu M, Unek IT, Aysin M, Bacakoglu A, Astarcıoglu I. De Novo Malignancies After Liver Transplantation: A Single Institution Experience. Exp Clin Transplant. 2019;17:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 138. | Kobayashi T, Miura K, Ishikawa H, Sakata J, Takizawa K, Hirose Y, Toge K, Saito S, Abe S, Kawachi Y, Ichikawa H, Shimada Y, Takahashi Y, Wakai T, Kinoshita Y. Malignancy After Living Donor Liver Transplantation. Transplant Proc. 2024;56:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 139. | Lucidi C, Biolato M, Lai Q, Lattanzi B, Lenci I, Milana M, Lionetti R, Liguori A, Angelico M, Tisone G, Avolio AW, Agnes S, Rossi M, Grieco A, Merli M. Cumulative incidence of solid and hematological De novo malignancy after liver transplantation in a multicentre cohort. Ann Hepatol. 2021;24:100309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Mangus RS, Fridell JA, Kubal CA, Loeffler AL, Krause AA, Bell JA, Tiwari S, Tector J. Worse Long-term Patient Survival and Higher Cancer Rates in Liver Transplant Recipients With a History of Smoking. Transplantation. 2015;99:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 141. | Yu S, Gao F, Yu J, Yan S, Wu J, Zhang M, Wang W, Zheng S. De novo cancers following liver transplantation: a single center experience in China. PLoS One. 2014;9:e85651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 142. | Antinucci F, Anders M, Orozco F, Mella J, Cobos M, McCormack L, Mastai R. [De novo malignant tumors following liver transplantation. A single-center experience in Argentina]. Medicina (B Aires). 2015;75:18-22. [PubMed] |
| 143. | Tiwari A, Saigal S, Choudhary NS, Saha S, Rastogi A, Bhangui P, Saraf N, Srinivasan T, Yadav SK, Gautam D, Nundy S, Soin AS. De Novo Malignancy After Living Donor Liver Transplantation: A Large Volume Experience. J Clin Exp Hepatol. 2020;10:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/