Published online Mar 18, 2025. doi: 10.5500/wjt.v15.i1.99004
Revised: September 26, 2024
Accepted: October 21, 2024
Published online: March 18, 2025
Processing time: 139 Days and 5.7 Hours
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related deaths worldwide. Liver transplantation (LT) offers the most effective treatment. HCC recurrence is the strongest risk factor that decreases post-LT survival in patients transplanted for HCC. The rate of HCC recurrence is generally reported as 8%-20% in the literature. Many predictors of HCC have already been researched, however, to our knowledge there are no published studies on this topic using Australian data.
To determine the rate and identify predictors of HCC recurrence in a contemporary Western Australian LT cohort.
We performed a retrospective cohort study of all liver transplants in patients with HCC at Sir Charles Gairdner Hospital between 2006 and 2021. Data was collected from various health record databases and included recipient demographics, serum biochemistry, radiology, operation notes, explant histopathology and details of recurrence. Overall survival of HCC patients post-LT, stratified for recurrence, was calculated by Kaplan Meier analysis. Univariate and multivariate Cox regression was used to determine predictors of HCC recurrence post-LT.
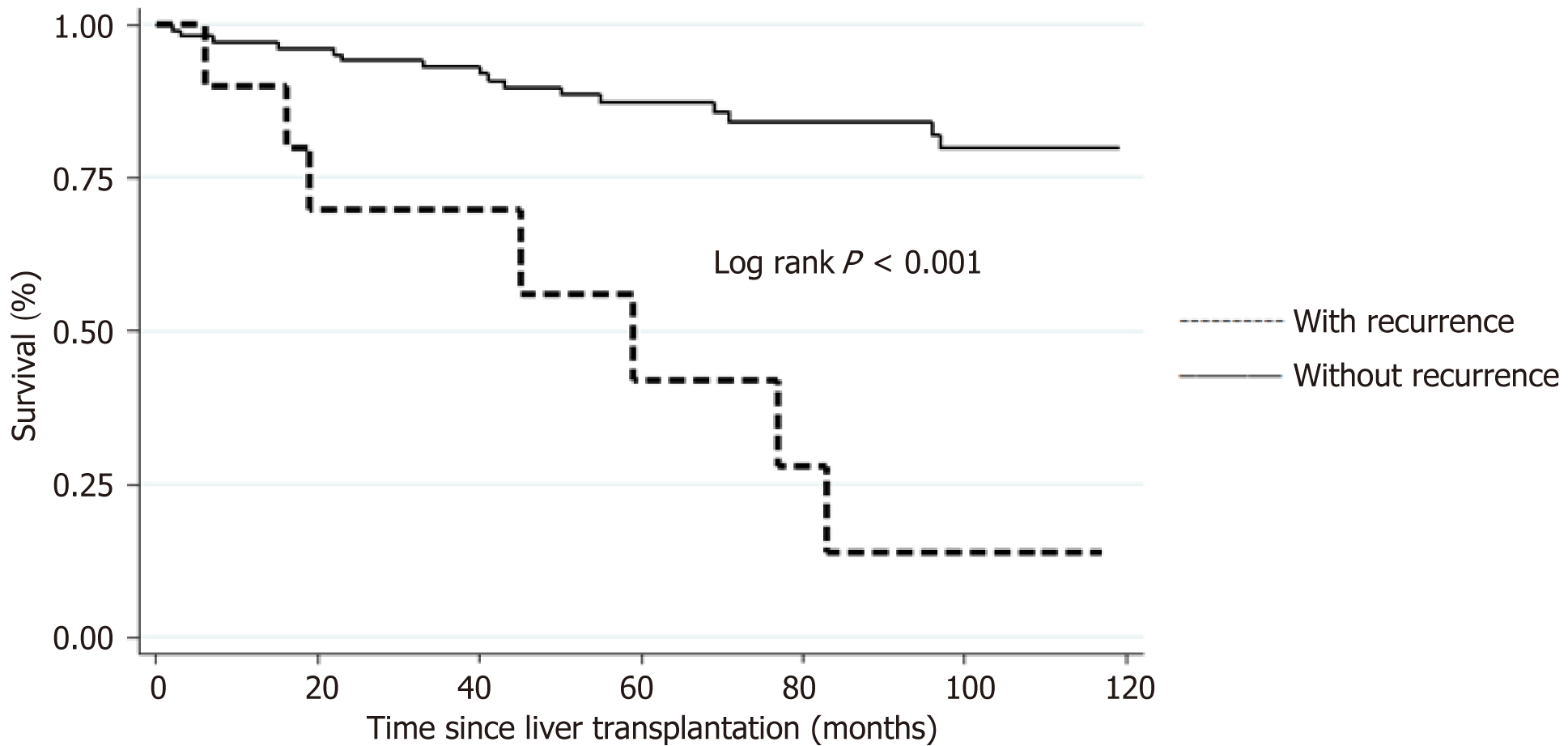
Between 1/1/2006 and 12/31/2021, 119 patients were transplanted with HCC. 8.4% of subjects developed recurrent HCC after LT with median follow-up time of 5.4 years. The median time to recurrence was 2.9 years ± 0.75 years. When comparing baseline characteristics, a greater proportion of subjects with recurrence had common characteristics on explant histopathology, including > 3 viable nodules (P = 0.001), vascular invasion (P = 0.003) and poorly differentiated HCC (P = 0.03). Unadjusted survival curves showed lower 1-year, 3-year, 5-year and 10-year survival rates in subjects with HCC recurrence compared to those without HCC recurrence (90% vs 92%, 70% vs 88%, 42% vs 80%, 14% vs 76%, respectively; log rank P < 0.001).
HCC recurrence was low at 8.4% in this contemporary Australian cohort, however it significantly impacted post-LT survival. Further studies are required to confirm predictors of recurrence and improve recipient outcomes.
Core Tip: This is the first study looking at hepatocellular carcinoma (HCC) recurrence using Australian data. It is well established that HCC recurrence significantly reduces survival post liver transplantation (LT). Optimal selection of candidates to minimize post-LT HCC recurrence remains controversial. The rate of HCC recurrence post-LT in Western Australia is 8.4%. A higher proportion of the patients with HCC recurrence had common baseline characteristics on explant; > 3 tumours, vascular invasion, and poorly differentiated disease. A larger multicentre study is required to investigate the predictive effects of these variables on post-LT HCC recurrence.
- Citation: Garas MG, Calzadilla-Bertot L, Smith BW, Delriviere L, Jaques B, Mou L, Adams LA, MacQuillan GC, Garas G, Jeffrey GP, Wallace MC. Hepatocellular carcinoma recurrence after liver transplant: An Australian single-centre study. World J Transplant 2025; 15(1): 99004
- URL: https://www.wjgnet.com/2220-3230/full/v15/i1/99004.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i1.99004
Hepatocellular carcinoma (HCC) is the 3rd leading cause of cancer deaths worldwide[1,2] and the 7th in Australia[3]. In carefully selected patients with nonresectable HCC, liver transplantation (LT) has been validated as the most effective treatment due to complete removal of the HCC and replacement of the diseased liver[2,4]. Historically, LT led to high rates of HCC recurrence, however, since the implementation of selective criteria, such as the Milan criteria, post-LT recurrence has significantly reduced[5]. In 2007, Australia and New Zealand moved to the University of California and San Francisco (UCSF) expanded criteria, and most recently, in 2020, the Metroticket 2.0 (MT2) model has been adopted.
HCC recurrence is the strongest predictor of worse survival in patients with HCC post-LT[6]. Whilst selection criteria have been refined and improved, HCC recurrence remains an issue with reported rates between 8% and 20%[7]. Recu
Given the numerous potential predictive factors for HCC recurrence and changes in transplant eligibility for patients with HCC over time, there is no clear understanding regarding the significance and interaction each factor has. It is important to identify any regional variations or novel trends in listing characteristics, management, and outcomes. To the best of our knowledge, no publications on this topic include Australian data. In this study we aim to calculate the rate of HCC recurrence in an Australia LT cohort. We additionally intend to analyse pre-transplant predictive factors of HCC recurrence, identify any new variables of significance and document the survival of these patient’s post-transplant.
We performed a single-centre, retrospective, cohort study of all liver transplants for HCC (including those in which HCC was found incidentally on explant analysis) undertaken at Sir Charles Gairdner Hospital (SCGH) between 2006 and 2021. Only liver transplants from deceased donors were performed. This study does not include any partial liver transplants or living-donor liver transplants. A minimum of 1 year follow-up time was required for all participants. Ethics approval was granted by the Sir Charles Gairdner Osborne Park Health Care Group Human Research Ethics Committee, approval No. 47721.
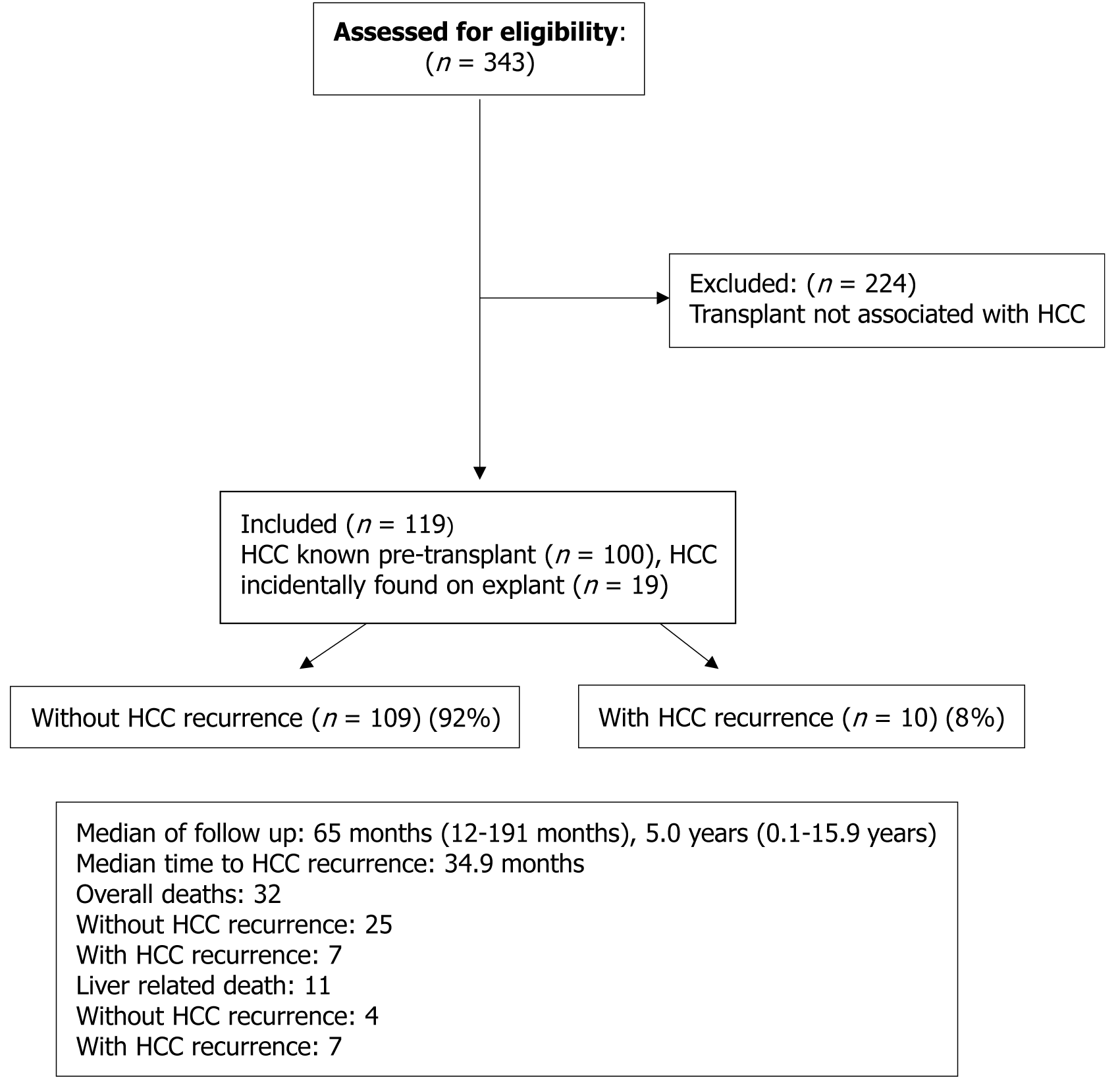
A total of 343 liver transplants were performed at SCGH during the study time. For inclusion to the study, patients were required to be adults (> 18 years of age), undergo a deceased-donor liver transplant for any cause with either: (1) A known diagnosis of HCC at the time of transplant (n = 100); or (2) Have a new diagnosis of HCC made on explant histology (n = 19). Patients who underwent multiple liver transplants (n = 4) were included in the study, provided that they only underwent a single liver transplant associated with HCC. Study participants were excluded if their transplant was not associated with HCC, if they were under the age of 18 years, or if they underwent multiple transplants associated with HCC. As per local management guidelines, patients underwent locoregional bridging therapy when indicated prior to their transplant. The protocol for downstaging at our centre was used when a patient did not meet the appropriate contemporary selection criteria for transplantation, for the majority of participants in this study that was the Milan criteria (defined below). The most appropriate modality of downstaging was selected based on availability of equipment and tumour characteristics. Following successful downstaging, patients were monitored for a minimum of 3 months to ensure they remained within transplant criteria, then they were activated on the liver transplant waiting list. Do
All data was collected from electronic medical records and clinical databases including the Western Australia liver transplant database. Data was deidentified and compiled onto a new database for the purpose of this study. The collected variables include: (1) Age at transplant; (2) Sex; (3) Body mass index (BMI) at transplant; (4) Aetiology of liver disease; (5) Child Pugh score; (6) Model for end-stage liver disease score; (7) Number of nodules identified on the most recent radiological imaging prior to transplant; (8) Serum AFP levels (immediately pre-transplant and highest level docu
The collected variables include an assessment of selection criteria eligibility. The criteria were defined as: (1) Milan criteria (single lesion ≤ 5 cm or up to three lesions all ≤ 3 cm, without vascular involvement or extrahepatic spread); (2) UCSF criteria (single lesion ≤ 6.5 cm or 2-3 Lesions ≤ 4.5 cm and total tumour diameter ≤ 8 cm); (3) MT2 criteria (group 1: Sum of number and size of lesions < 7 and AFP < 200 ng/mL; group 2: Sum of number and size of lesions < 6 and AFP 200-400 ng/mL; and group 3: Sum of number and size of lesions < 5 and AFP 400-1000 ng/mL).
The primary objective of our study was to calculate the rate of HCC recurrence post-LT in Western Australia, within our selected period. Secondary objectives included validating the established pre-transplant predictive factors of HCC recurrence post-LT, identifying any new variables of significance within our results, and comparing survival between those that did develop HCC recurrence and those that did not.
Baseline characteristics were summarized in percentages for categorical variables. Mean and standard deviation were calculated for continuous variables. Categorical variables were compared using χ² test. Continuous variables were compared using the t test for normally distributed variables and the nonparametric Wilcoxon rank-sum test for measures not normally distributed. Kaplan Meier analysis was used to calculate the overall survival curve of all patients post-LT and the overall survival stratified for recurrence. Univariate and multivariate Cox regression was used to determine predictors of HCC recurrence post-LT. Statistical analysis was based on STATA software, release 17.0, College Station, Texas: Stata Corp LP.
A total of 343 LT s for any indication took place at the SCGH liver transplant centre between January 1, 2006–December 31, 2021; 119 (34.7%) of those transplants were associated with HCC, yielding a sample size of 119 transplants (Figure 1). Of these transplants, 100 (84.0%) occurred following a known diagnosis of HCC, the remaining 19 (16.0%) transplants resulted in an incidental finding of HCC in explant histopathological analysis. Recurrent HCC post-LT was documented in 10 (8.4%) cases, with a median follow-up time of 5 years (1-15.9 years). The follow-up duration was consistent among both groups.
When comparing baseline characteristics to those without recurrence, a greater proportion of subjects with recurrence were female (20% vs 14%, P = 0.05), had underlying chronic hepatitis B virus infection (30% vs 4%, P = 0.001), had higher AFP levels at time of transplant (41 ng/mL ± 50 ng/mL vs 13.7 ng/mL ± 30 ng/mL, P = 0.01), and had common characteristics on explant histopathology, including > 3 viable nodules (40% vs 15%, P = 0.001), the presence of vascular invasion (40% vs 23%, P = 0.003) and poorly differentiated HCC (10% vs 3%, P = 0.03). All patients with underlying chronic hepatitis B at time of transplant were on antiviral treatment with an undetectable viral load. A full comparison of baseline characteristics of patients with and without recurrence after LT is represented in Table 1. The presence of macrovascular invasion in the explant was the only value to reach statistical significance on both univariate and multivariate analysis [hazard ratio (HR) = 12.2, 95%CI: 1.47-102, P = 0.02 and HR = 18.6, 95%CI: 1.98-174, P = 0.01, respectively] (Table 2).
| Variable | Without recurrence (n = 109) | With recurrence (n = 10) | P value |
| Age at transplant (years) | 56.8 | 55.8 | 0.68 |
| Female | 15 (14) | 2 (20) | 0.05 |
| Male | 94 (86) | 8 (80) | 0.58 |
| Aetiology of liver disease | |||
| HCV | 45 (41) | 5 (50) | 0.46 |
| Alcohol | 21 (19) | 1 (10) | 0.87 |
| Alcohol +HCV | 20 (18) | 0 (0) | 0.005 |
| Non-alcoholic fatty liver disease | 13 (12) | 1 (10) | 0.63 |
| Hepatitis B virus | 4 (4) | 3 (30) | 0.001 |
| Primary sclerosing cholangitis | 2(2) | 0 (0) | 0.74 |
| Other | 4 (4) | 0 (0) | 0.52 |
| Child Pugh | |||
| A | 32 (30) | 1 (10) | 0.78 |
| B | 40 (37) | 6 (60) | 0.25 |
| C | 37 (34) | 3 (30) | 0.94 |
| Model for End-stage liver disease | 15 ± 7.0 | 16 ± 7.4 | 0.64 |
| Within Milan Criteria | 102 (94) | 9 (90) | 0.81 |
| Within University of California and San Francisco | 105 (96) | 10 (100) | 0.82 |
| Within Metroticket 2.0 | |||
| Pre-transplant | 101 (92) | 9 (90) | 0.55 |
| Post-transplant | 75 (68) | 6 (60) | 0.57 |
| Comorbidities | |||
| Obesity | 35 (32) | 2 (20) | 0.34 |
| Overweight | 43 (40) | 6 (60) | 0.17 |
| Overweight or obese | 78 (71) | 8 (80) | 0.60 |
| Morphology at time of transplant | |||
| Number of nodules | |||
| 1 | 85 (78) | 7 (70) | 0.74 |
| 2-3 | 22 (20) | 3 (30) | 0.21 |
| > 3 | 2 (2) | 0 (0) | 0.78 |
| Serum markers pre-transplant | |||
| AFP at time of transplant (ng/mL) | 13.7 ± 30 | 41 ± 50 | 0.01 |
| Highest pre-op AFP (ng/mL) | 30.3 ± 64 | 41 ± 47 | 0.59 |
| neutrophil-lymphocyte ratio at time of transplant | 4.8 ± 13 | 2.9 ± 1.3 | 0.64 |
| Biopsy pre-transplant performed | 37 (34) | 5 (50) | 0.32 |
| HCC treatment pre-transplant | |||
| Transarterial chemoembolization | 25 (23) | 3 (30) | 0.56 |
| Ablative techniques | 19 (17) | 3 (30) | 0.78 |
| Surgery | 0 | 0 (0) | 0.25 |
| Radiology (selective internal radiation therapy/choline kinase) | 9 (8) | 0 (0) | 0.65 |
| Combined | 24 (22) | 4 (40) | 0.10 |
| Waiting time (days) | 166 ± 180 | 194 ± 153 | 0.63 |
| Explant histopathology findings | |||
| Number of viable nodules | |||
| 0 | 8 (8) | 1 (10) | 0.14 |
| 1 | 45 (43) | 3 (30) | 0.24 |
| 2-3 | 37 (35) | 2 (20) | 0.46 |
| > 3 | 16 (15) | 4 (40) | 0.001 |
| Vascular invasion | 25 (23) | 4 (40) | 0.003 |
| Type of vascular invasion | |||
| Microvascular | 25 (23) | 3 (30) | 0.001 |
| Macrovascular | 0 (0) | 1 (10) | 0.001 |
| Viable HCC on explant | 101 (92) | 9 (90) | 0.55 |
| Histology grading | |||
| Well differentiated | 44 (40) | 1 (10) | 0.001 |
| Moderately differentiated | 48 (44) | 6 (60) | 0.46 |
| Poor differentiated | 3 (3) | 1 (10) | 0.03 |
| Variable | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | 0.98 (0.91-1.05) | 0.62 | ||
| Male | 1.55 (0.32-7.32) | 0.57 | ||
| Female | 0.64 (0.13-3.03) | 0.58 | ||
| Obesity | 0.56 (0.11-2.65) | 0.46 | ||
| Overweight | 2.07 (0.58-7.35) | 0.25 | ||
| Histology pre-transplant performed | 2.10 (0.60-7.31) | 0.24 | ||
| Explant tumour characteristics | ||||
| Viable HCC on explant | 0.72 (0.09-5.7) | 0.76 | ||
| Number of viable lesions | ||||
| 1 | 0.55 (0.05-5.3) | 0.60 | ||
| 2-3 | 0.44 (0.04-4.8) | 0.50 | ||
| > 3 | 2.0 (0.22-17.9) | 0.53 | ||
| Max diameter of viable tumour (mm) | 1.02 (0.98-1.06) | 0.19 | ||
| Sum of diameters (mm) | 1.01 (0.96-1.0) | 0.64 | ||
| Macrovascular invasion | 12.2 (1.47-102) | 0.02 | 18.6 (1.98-174) | 0.01 |
| Microvascular invasion | 1.75 (0.43-7.04) | 0.42 | ||
| Tumour differentiation | ||||
| Well | 1.52 (0.17-13.1) | 0.58 | ||
| Moderate | 0.96 (0.10-8.6) | 0.47 | ||
| Poor | 1.87 (1.63-80.5) | 0.12 | ||
| Serum markers | ||||
| Highest Pre-op alpha-fetoprotein | 1.00 (0.64-1.00) | 0.64 | ||
| neutrophil-lymphocyte ratio | 0.90 (0.66-1.22) | 0.51 | ||
| Waiting time (days) | 1.00 (0.99-1.00) | 0.55 | ||
| Cold Ischemia time (minutes) | 1.00 (0.97-1.03) | 0.70 | ||
| Wait time from HCC diagnosis to liver transplantation | ||||
| < 6 months | 0.60 (0.17-2.15) | 0.44 | ||
| > 6 months | 1.64 (0.46-5.85) | 0.43 | ||
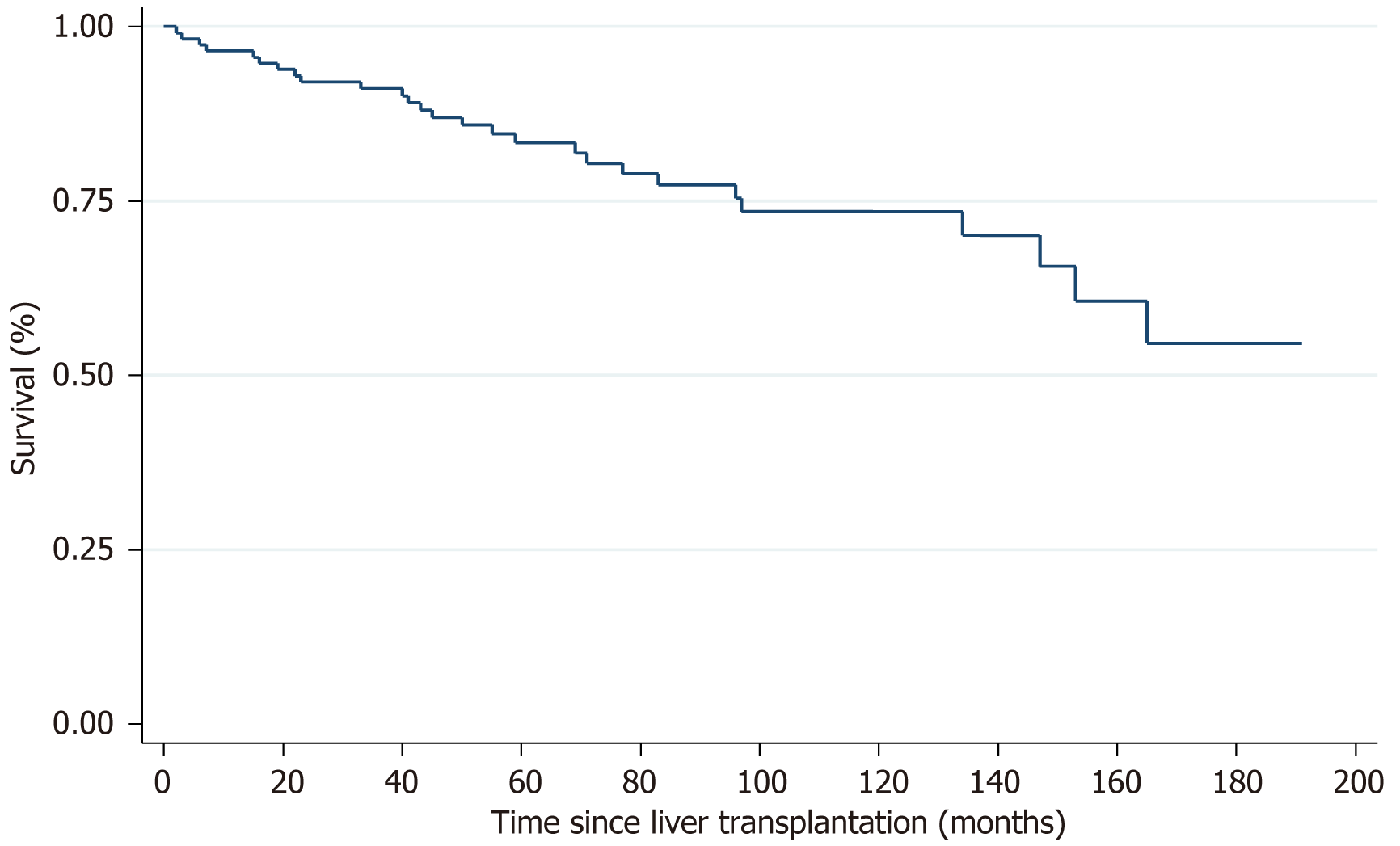
Overall survival of all patients who underwent a LT in association with HCC demonstrated a 1-year, 3-year, 5-year, 10-year and 15-year survival rate of 90.8%, 83.3%, 78.5%, 67.1%, and 52.3% respectively (Figure 2). Unadjusted survival curves showed lower 1-year, 3-year, 5-year and 10-year survival rates in subjects with HCC recurrence compared to those without HCC recurrence (90% vs 92%, 70% vs 88%, 42% vs 80%, 14% vs 76%, respectively; log rank P < 0.001) (Figure 3). The median time to recurrence was 2.9 years ± 0.75 years. Late recurrence (> 2 years) was more common than early recurrence (60% vs 40%) with the majority of recurrences (90%) being extrahepatic in location (Table 3). As of December 31, 2022, 32 of the patients included in the study had died. Seven of the deaths occurred in patients from the recurrence group, and all of those deaths were directly related to HCC recurrence. The median time to death following a diagnosis of HCC recurrence post-LT was 11 months. The remaining 25 deaths occurred in the non-recurrence group; 4 deaths were liver-related, 21 deaths were due to other causes.
| Variable | Overall (n = 10) |
| Time to recurrence (years) | 2.9 ± 0.75 |
| < 2 | 4 (40) |
| > 2 | 6 (60) |
| Location | |
| Hepatic | 1 (10) |
| Extrahepatic | 9 (90) |
| Alpha-fetoprotein at time of recurrence (ng/mL) | 4700 ± 9394 |
| Treatment of recurrence | |
| Yes | 6 (60) |
| No | 4 (40) |
| Type of treatment | |
| Systemic therapy | 1 (10) |
| Surgery | 0 (0) |
| Radiotherapy | 1 (10) |
| Combined | 2 (20) |
| Other | 2 (20) |
In this single centre cohort study, using novel Australian data, we found the rate of HCC recurrence post-LT to be 8.4%. The median time to recurrence was 2.9 years with 60% of the recurrence cases being diagnosed > 2 years post-transplant. The rate of HCC recurrence post-LT differs between centres, however, when collated, the generally accepted figure is between 8%-20%[2]. A recent 2022 study investigating a new composite tool in the prediction of HCC recurrence documented a 5-year recurrence rate of 19.6% in their European cohort and 16.9% in their Latin American cohort[12]. A separate American 2022 study analysing the relationship of downstaging and HCC reported a recurrence rate of 12.5%[13]. Our study yields a recurrence rate at the lower end of what has become the accepted norm.
It has been consistently reported that HCC recurrence is the strongest risk factor impeding long-term post-LT survival[9,12], which is reflected in our data (Figure 2). The further from the transplant, the greater the disparity in survival. We did not find any additional factors, such as waitlist time or cold ischemia time to have a significant impact on survival. A 2021 study by El-domiaty et al[8] reported a similar set of results with 3-year, 5-year and 10-year survival rates of 53% vs 88.7%, 31% vs 86%, 12.4% vs 72.8%, respectively; log rank P < 0.001. This study reported 61.3% (46/75) of patients to experience an early recurrence (< 2 years post-transplant). A similar finding was reported in 2019 by Chagas et al[7] with the majority of their patients being diagnosed with recurrent HCC within 12 months (85%, 73/86). They support the recommendation for high intensity HCC surveillance within the first 2 years post-LT. We documented the median time to recurrence to be 2.9 years, therefore, support the notion to continue intense HCC surveillance beyond the 2-year mark. We additionally found the majority of recurrences were extrahepatic in location, in keeping with other centres[7,8]. The implication we draw is that HCC surveillance must include imaging beyond the liver alone, such as computerised tomography (CT) chest/abdomen/pelvis. This recommendation is shared by a 2017 study by Fernandez-Sevilla et al[14]. However, they reported 70% of their cases of recurrence being diagnosed within 2 years, hence advocating for a high intensity 2-year surveillance program only.
Due to the relatively small sample size of our study, we were unable to prove any associations between pre-transplant variables and their predictive effect on HCC recurrence, however, a significant difference was that the HCC recurrence group had higher pre-transplant AFP levels compared to the group without recurrence (41 ng/mL ± 50 ng/mL vs 13.7 ng/mL ± 30 ng/mL, P = 0.01). Additionally, the AFP levels were measured at the time of diagnosis of recurrence and found to be highly variable (4700 ng/mL ± 9394 ng/mL). These findings are in keeping with a large multicentre study by Chagas et al[7] which found that AFP levels > 1000 ng/mL at the time of recurrence were associated with a poorer prognosis of survival. We conclude from these results that while AFP levels are important in the surveillance and diagnosis of HCC, they should not solely be relied upon.
Explant histopathological analysis provides the most reliable information regarding tumour characteristics; therefore, it can be used to predict recurrence. The presence of macrovascular invasion on explant was the only variable in our study to reach statistical significance on univariate and multivariate analysis, therefore, can be considered a predictor of recurrence. Macrovascular invasion is one of the most well-known independent risk factors of recurrence and is in fact an exclusion criterion for liver transplant under all selection criteria[15]. A single patient within our cohort was found to have macrovascular invasion on explant histology. There was no evidence of this on pre-transplant imaging. When comparing baseline characteristics in our study, a higher proportion of the recurrence group demonstrated > 3 viable nodules, the presence of microvascular invasion, macrovascular invasion, and poorly differentiated HCC. Additionally, a higher proportion of the non-recurrence group were documented to have well differentiated tumours. All of these findings are consistent with data reported at other centres. A recent publication by Tabrizian et al[13] reported variables independently associated with recurrence to be poor tumour differentiation [odds ratio (OR) = 3.37; 95%CI: 1.02-11.15, P = 0.05] and vascular invasion on explant pathologic findings (OR = 1.91; 95%CI: 1.03-3.50, P = 0.04).
To the best of our knowledge, this is the first published Australian series of HCC recurrence post-LT. A single liver transplant centre in Western Australia performs approximately 10% of all liver transplants in Australia per year. An Australia wide, multicentre study, collating data from all major transplant centres across Australia is currently being planned. Regional variation in data may reflect differences in screening and treatment of HCC and HCC recurrence, allowing for identification of novel predictors of recurrence.
The major limitation of this study is the small sample size and limited number of endpoints (post-transplant HCC recurrences). A second limitation is that our data is of a single centre, thus limiting our external validity. Thirdly, the minimum follow-up is 1 year, thus the only survival rate that includes the entire cohort is the 1-year survival rate.
In summary, the rate of HCC recurrence in Western Australia is 8.4%. Common baseline characteristics were observed among the patients who developed recurrent HCC, notably relating to the explant histopathological analysis. Our findings support the idea to continue intense HCC surveillance beyond the first 2 years post-LT. We propose 6 monthly chest/abdomen/pelvis CT scans for 2 years, then annually until 5 years post-transplant. A larger, nation-wide study is required to further investigate the predictors of HCC recurrence post-transplant.
The authors would like to acknowledge the contributions of Collins M, clinical nurse consultant for liver transplantation and hepatocellular carcinoma at Sir Charles Gairdner Hospital. Mrs Collins’s routine collection and organisation of transplant data within the Western Australia Liver Transplant Service allowed for an efficient data collection process for this project.
| 1. | World Health Organisation. Cancer. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer. |
| 2. | Bzeizi KI, Abdullah M, Vidyasagar K, Alqahthani SA, Broering D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers (Basel). 2022;14:5114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 3. | Australia Government Cancer Australia. Cancer Statistics. Cancer in Australia Statistics. 2022. Available from: https://www.canceraustralia.gov.au/impacted-cancer/what-cancer/cancer-australia-statistics. |
| 4. | Gabutti A, Bhoori S, Cascella T, Bongini M. Hepatocellular Carcinoma Recurrence After Liver Transplantation. Oncology (Williston Park). 2020;34:692516. [PubMed] |
| 5. | Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Lee KU. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 6. | Agarwal PD, Lucey MR. Management of hepatocellular carcinoma recurrence after liver transplantation. Ann Hepatol. 2022;27:100654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Chagas AL, Felga GEG, Diniz MA, Silva RF, Mattos AA, Silva RCMA, Boin IFSF, Garcia JHP, Lima AS, Coelho JCU, Bittencourt PL, Alves VAF, D'Albuquerque LAC, Carrilho FJ; Brazilian HCC Study Group. Hepatocellular carcinoma recurrence after liver transplantation in a Brazilian multicenter study: clinical profile and prognostic factors of survival. Eur J Gastroenterol Hepatol. 2019;31:1148-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | El-Domiaty N, Saliba F, Vibert E, Karam V, Sobesky R, Ibrahim W, Pittau G, Ciacio O, Salloum C, Amer K, Saeed MA, Shawky JA, Sa Cunha A, Rosmorduc O, Cherqui D, Adam R, Samuel D. Early Versus Late Hepatocellular Carcinoma Recurrence After Transplantation: Predictive Factors, Patterns, and Long-term Outcome. Transplantation. 2021;105:1778-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Filgueira N. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J Hepatol. 2019;11:261-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (3)] |
| 10. | Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, Kato T, Verna EC, Emond JC, Brown RS Jr. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 11. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 12. | Costentin C, Piñero F, Degroote H, Notarpaolo A, Boin IF, Boudjema K, Baccaro C, Podestá LG, Bachellier P, Ettorre GM, Poniachik J, Muscari F, Dibenedetto F, Duque SH, Salame E, Cillo U, Marciano S, Vanlemmens C, Fagiuoli S, Burra P, Van Vlierberghe H, Cherqui D, Lai Q, Silva M, Rubinstein F, Duvoux C; French-Italian-Belgium and Latin American collaborative group for HCC and liver transplantation. R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation. JHEP Rep. 2022;4:100445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 13. | Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian VG, Yao F, Busuttil RW, Roberts J, Emond JC, Samstein B, Brown RS Jr, Najjar M, Chapman WC, Doyle MM, Florman SS, Schwartz ME, Llovet JM. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022;157:779-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/