Published online Mar 18, 2025. doi: 10.5500/wjt.v15.i1.97458
Revised: September 4, 2024
Accepted: November 4, 2024
Published online: March 18, 2025
Processing time: 180 Days and 17.6 Hours
The endothelium modulates vascular homeostasis owing to a variety of vasoconstrictors and vasodilators. Endothelial dysfunction (ED), characterized by im
Core Tip: Endothelium promotes vascular homeostasis by regulating vascular permeability, leucocyte diapedesis, hemostatic response, and vascular tone. Endothelial dysfunction (ED) plays a key role in the genesis of cardiovascular (CV) and kidney outcomes seen in the chronic kidney disease and kidney transplant population. There is increased CV morbidity and mortality imparted due to ED in transplant recipients. Among various biomarkers and diagnostic tests available to detect endothelial function, flow-mediated dilation is a widely recognized and reproducible test available in clinical settings. Though overlooked in kidney transplant settings, promoting CV and graft health by monitoring endothelial functions and mitigating risk factors of ED can improve overall outcomes.
- Citation: Prabhahar A, Batta A, Hatwal J, Kumar V, Ramachandran R, Batta A. Endothelial dysfunction in the kidney transplant population: Current evidence and management strategies. World J Transplant 2025; 15(1): 97458
- URL: https://www.wjgnet.com/2220-3230/full/v15/i1/97458.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i1.97458
The endothelium, which forms the inner layer of the blood vessels, actively regulates vascular functions by sensing and responding to mechanical and biochemical signals. This vasoregulation happens through modulating vascular tone, mediating cellular adhesion, influencing the migration of inflammatory cells, and providing resistance to the action of pro-thrombotic and pro-inflammatory mediators. Under a homeostatic state, endothelial cells release nitric oxide (NO) under the influence of endothelial NO synthase (eNOS)[1]. NO is one of the major mediators of endothelial functions that prevents platelet aggregation and causes vasodilation by modulating the tone of vascular smooth muscle cells. In addition to NO, fibrinolytic and antithrombotic properties of the endothelium are also mediated through other mediators such as tissue plasminogen activator, antithrombin III, and prostacyclin[2]. Bone marrow-derived endothelial progenitor cells are responsible for the renewal of endothelial cells during repair.
Endothelial dysfunction (ED) is marked by a decrease in NO production/availability and an inflammatory endothelial cell phenotype (activated endothelial cells). There is poor expression of eNOS following damage to the endothelial glycocalyx, a gel-like layer of glycoproteins and extracellular matrix components involved in trans-endothelial transport at the blood-endothelial interface[3]. Additionally, there is eNOS uncoupling, leading to the generation of reactive oxygen species (ROS) instead of NO[4]. Thus, there is decreased NO availability and production. Activation of endothelial cells (novel immune cells) by various inflammatory signals results in the upregulation of cellular adhesion molecules, including e-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell-adhesion molecule-1 (VCAM-1), which promote inflammation. Subsequently there is leukocyte diapedesis in conjunction with increased endothelial permeability[2]. Activated endothelial cells behave like macrophages, secreting cytokines. The disturbed and inflamed endothelium favors permeation and trapping of lipoprotein particles beneath the endothelial cell layer, which can subsequently get oxidized[2]. The deposited lipoproteins are internalized by macrophages and smooth muscle cells, resulting in the formation of foam cells and the accumulation of fatty streaks. Altered efferocytosis (removal of apoptotic endothelial cells) leads to vascular stiffening[5]. Some endothelial cells may undergo endo-mesenchymal transition, facilitating deposition of the extracellular matrix, thereby further aggravating vascular stiffness[6]. Arterial constrictions due to lipid accumulation in conjunction with poor vasodilation increases regions of turbulent or oscillatory blood flow, which further exacerbates endothelial cell activation and denudation. Lipid-rich plaques with a thin fibrous cap in these areas are at high risk for rupture, causing thrombotic occlusions. This risk is exacerbated by the expression of pro-coagulant tissue factor, anti-fibrinolytic plasminogen activator inhibitor-1 (PAI-1), and von Willebrand factor (vWF)[2,7]. Thus, ED is a forerunner for atherosclerosis and thrombosis.
In this review, we examine cardiovascular (CV) diseases prevalent in chronic kidney disease (CKD) and transplant settings, focusing on their pathogenesis as it relates to ED. We emphasize the inadequacies in the prevention, diagnosis, and management of ED in real-world scenarios. By correlating the pathogenesis of ED with its diagnostic and therapeutic approaches, we aim to provide valuable insights for clinicians. We hope that this review will inspire researchers to undertake collaborative studies with clinicians, thereby advancing our understanding of ED in the context of kidney transplantation.
The decline in glomerular filtration rate in CKD leads to a fast-tracked CV aging trajectory. Patients with advanced CKD (stage 4 and 5) have the highest risk of dying from CV diseases. Almost 50% of all deaths in advanced CKD are due to CV diseases[8]. In addition to coronary artery diseases, heart failure (with both reduced and preserved ejection fraction), arrhythmias, valvular abnormalities, peripheral arterial disease, and sudden cardiac death are also commonly observed in the CKD population[8]. ED contributes significantly to increased CV risk in patients with CKD. CKD is characterized by persistent low-grade inflammation due to oxidative stress, cardio-metabolic risk factors, infections, uremic toxins, acidosis, and mineral-bone alterations, all of which damage the endothelium[9,10]. Concrete evidence suggests the role of inflammation in causing CV diseases through the activation of endothelial cells in patients with CKD[11]. Levels of pro-inflammatory cytokines such as interleukins (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), and high-sensitivity C-reactive protein (hs-CRP) positively correlate with increased CV risk in CKD[12]. Anti-inflammatory therapy with canakinumab (targets IL-1β) has shown an 18% reduction in the primary endpoint (non-fatal myocardial infarction, non-fatal stroke, or CV death) in patients with moderate CKD[13]. Oxidative stress and uremic toxins of CKD state induce pro-inflammatory signals and cause post-translational modification (oxidation and carbonylation) of both low-density lipoprotein (LDL) and high-density lipoprotein (HDL), which are CV risk factors[12]. Thus, alterations in lipoprotein particle profiles in CKD exacerbate the atherogenicity of LDL, and transform HDL from a protective to a potentially harmful entity. In addition to the above risk factors, metabolic acidosis, decreased levels of klotho (an anti-vascular aging protein), vascular calcification induced by phosphate alterations, and altered gut microbiome contribute to ED in CKD[12]. Dialysis modalities are also implicated in causing CV diseases like pulmonary hypertension through ED[14,15]. Hemodialysis (HD) patients have higher endothelial microparticles and pro-inflammatory monocytes [cluster of differentiation (CD) 14 + CD16 + monocytes] than patients on peritoneal dialysis (PD)[16].
In the clinical setting, a cohort study from India by Prasad et al[17] analyzing practice patterns in mild to moderate CKD, showed that renin-angiotensin-aldosterone system (RAAS) blockers were prescribed to 47.9% of CKD patients overall and 58.8% of those with proteinuria, while only 40.4% were on statins. Both the drug classes are protective to the endothelium. This highlights a missed opportunity in CKD to safeguard endothelial function due to non-compliance with guideline recommendations. Statins lower plasma cholesterol levels and decrease the endothelial trapping of modified lipoproteins. Statins also exhibit “pleiotropic” vascular benefits, including enhancing endothelial function by increasing NO bioavailability by upregulating eNOS, endothelial repair, reducing oxidative stress, and inhibiting inflammation. Statins also decrease the levels of endothelin-1, a potent vasoconstrictor[18]. In adults with dialysis-dependent CKD (unlike CKD patients not initiated on dialysis), the Kidney Disease: Improving Global Outcomes (KDIGO) recommend against starting statins or statin/ezetimibe combinations[19]. Although CV risk is elevated in this subgroup of CKD, the effectiveness of statins is uncertain due to significant competing mortality risks. RAAS activation promotes vascular stiffness by promoting pro-fibrotic and inflammatory pathways. RAAS activation causes smooth muscle cell proliferation, extracellular matrix deposition, and transforming growth factor β (TGF-β) secretion (pro-fibrotic)[20]. Despite the benefits, many patients with CKD are discontinued from RAAS inhibitors because of adverse events[21]. There is less inclusion of CKD patients in CV clinical trials despite calls for better representation[22]. Thus, in CKD, there is an unchecked and progressive endothelial damage. This culminates in increased CV risk as the patient prepares for a kidney transplant.
Kidney transplant candidates have a notable prevalence of both pre-existing (risk factors acquired during CKD state) and de novo transplant-related CV risk factors, which contribute to adverse CV outcomes[23]. The traditional CV risk factors in the post-transplant period include hypertension (40%-90%), diabetes (24%-42%), dyslipidemia (50%), smoking (25%), and obesity. The non-traditional CV risk factors specific to kidney transplant settings include adverse effects of immunosuppressive medications, persistent secondary hyperparathyroidism after transplant, anemia, chronic inflammation, and graft dysfunction[23,24]. The spectrum of CV diseases in kidney transplant settings does not differ greatly compared to the CKD population, and include coronary artery disease, valvopathy, heart failure, stroke, cardiac arrhythmias, pulmonary artery hypertension, and peripheral arterial disease.
Despite comorbidities, patients with CKD after transplantation exhibit improved CV health compared to those who are waitlisted for transplant[25]. Kidney transplantation significantly improves maximal oxygen consumption (from 20.7 to 22.5 mL/kg/minute, P < 0.001) compared to those who remain on dialysis (declines from 18.9 to 17.7 mL/kg/minute, P < 0.001) at 1 year of follow-up[26]. Similarly, improvements were seen with ejection fraction in a kidney transplant group (improved from 60.1% to 63.2% P = 0.02) compared to a waitlisted group (61.4% to 59.3%)[26]. However, not all risk factors that are acquired in the pre-transplant period are mitigated. The leading cause of death with a functioning graft in the long-term remains due to CV causes[27]. There is a high incidence of myocardial infarction, heart failure, and stroke in the 1st year after kidney transplant [cumulative incidence in the 1st year is 2.6%, 95% confidence interval (CI): 1.9-3.2], and the risk continues to increase in the subsequent post-transplant years (cumulative incidence between the years 2-5 is 8.3; 95%CI: 7.1-9.5)[28].
There is a significant spillover of CV risk factors acquired from the pre-transplant period. Among 62706 kidney transplant recipients, 6.4% had atrial fibrillation before transplant. Over a mean follow-up of 4.9 years, those with atrial fibrillation experienced higher mortality (40.6% vs 24.9%), greater all-cause graft failure (46.8% vs 36.4%), and higher rates of ischemic stroke (2.8% vs 1.6%) after transplantation[29]. In a cohort of 1063 kidney transplant recipients, left ventricular hypertrophy [hazard ratio (HR) = 1.58; P = 0.022] and increased relative wall thickness (HR = 1.44; P = 0.041) before transplant independently predicted CV events after transplant. Persistent brachiocephalic fistula is also a risk factor for pulmonary arterial hypertension. In addition to the spillover of CV diseases, de novo CV diseases after transplantation do occur. The cumulative incidence of de novo congestive heart failure after kidney transplantation is 10.2% at 12 months and 18.3% at 36 months after transplant[30].
A recent review article by Cardinal et al[31] briefly addresses the immunopathogenesis of endothelial injury following kidney transplantation, with a primary focus on renal endothelium. ED extends beyond the kidneys and is a precursor to various CV diseases. Although direct evidence linking ED to different CV diseases in kidney transplant settings is sparse due to a limited number of studies, recent data suggest that a significant proportion of CV diseases in these settings may be attributable to ED. Also, as pointed out by Cardinal et al[31], ED damages the graft endothelium and causes graft failure.
The cumulative incidence of post-transplant myocardial infarction (PTMI) was 4.3% at 6 months, 5.6% at 12 months, and 11.1% at 36 months[32]. The role of ED in atherogenesis has already been explained. The high incidence of PTMI early post-transplant may be attributed to the high levels of inflammatory markers such as vWF, PAI-I, and homocysteine levels[33]. A hospital-based registry from the United States[34] showed notable increases in admissions among transplant recipients between 2005 and 2011 for congestive heart failure and cardiac arrhythmias (P = 0.01), which accounted for 16% to 17% of all hospital admissions. Coronary ED leads to heart failure with preserved ejection fraction[35]. Pre-emptive transplantation may reduce the risk of heart failure, and there is a lesser risk of coronary ED by avoiding HD[36]. The incidence of stroke after kidney transplantation varies from 1.1% to 8% and ischemic stroke is the most common variant. The risk of rupture of intracranial aneurysms may theoretically be increased in patients with autosomal dominant polycystic kidney disease (ADPKD). ADPKD is notably associated with RAAS activation and ED[37]. However, data show favorable CV outcomes in patients with ADPKD, including stroke[38]. In Cox regression analysis, modeling kidney transplant as a time-dependent covariate showed an adjusted HR (aHR) of 0.77 (95%CI: 0.67-0.89) for intracranial hemorrhage in ADPKD patients during the post-transplant period compared to the pre-transplant period[39]. The 3-year cumulative incidence of de novo peripheral arterial disease after kidney transplant is 20% among CKD patients with diabetes[40]. Ankle brachial index before transplant was an independent predictor of graft failure [odds ratio (OR) = 2.77; 95%CI: 1.68-4.58; P < 0.001] and secondary CV endpoints, namely myocardial infarction; cerebrovascular accident; and limb ischemia, gangrene, or amputation (HR = 1.39; 95%CI: 0.97-1.99; P = 0.076), and death (HR = 1.84; 95%CI: 1.26-2.68; P = 0.002)[41]. Important regulators of pulmonary circulation include endothelin-1 and NO, which are therapeutic targets in treating pulmonary hypertension[42]. One etiopathogenesis of pulmonary arterial hypertension is due to ED caused by arterio-venous fistula created for dialysis access[43]. Fistula ligation may be a reasonable option in patients with pulmonary hypertension after transplant.
Obesity: The protective impact that obesity might have on patients undergoing dialysis disappears following a transplant. In obesity, ED is primarily driven by adipose tissue inflammation (increased IL-6, TNF-α), decreased expression of eNOS, insulin resistance, and oxidized LDL (oxLDL)[44]. Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of eNOS, is elevated in obesity[44]. Weight gain happens in the 1st year after transplant, especially in children[45]. Steroid minimization is associated with lesser weight gain[46]. Obesity in transplant candidates is also a risk factor for diabetes mellitus[47], and sleeve gastrectomy in obese kidney transplant patients improved blood pressure and glucose control[48]. Pre-transplant obesity is a risk factor for delayed graft function[49]. Obstructive sleep apnea (OSA) associated with obesity may re-emerge long-term in transplant recipients, even if symptoms of OSA initially improved after the transplant[50]. OSA decreases NO production due to repeated episodes of hypoxia, and is a risk factor for pulmonary arterial hypertension[51,52].
Clinical vignette: The role of novel anti-obesity and anti-diabetic agents, particularly glucagon-like peptide-1 (GLP-1) analogs, in modulating the effects of ADMA represents a promising area of research.
Smoking: Smoking in kidney transplant candidates is associated with high rates of coronary artery disease[53], graft loss, and non-CV mortality[54]. Almost 50% of the kidney transplant population in a cohort study were current or former smokers. Smoking promotes ED by the generation of ROS, increased oxLDL, inactivation of eNOS, and increased expression of cell-adhesion molecules. Smoking cessation has been shown to improve endothelial functions. Among 1504 smokers, flow-mediated dilatation (FMD) (described in detail below) improved by 1% (P = 0.005) after 1 year in those who quit smoking, while remaining unchanged in those who continued (P = 0.643)[55].
Clinical vignette: Increased public awareness of the health risks associated with tobacco has driven many to use electronic cigarettes (e-cigarettes) as a potentially less harmful alternative to smoking. On the contrary, e-cigarettes also cause ED. E-cigarette users and smokers showed lower FMD compared to non-users[56]. They demonstrated higher concentrations of receptors for advanced glycation end products (RAGE) than smokers[56]. RAGE ligands amplify pro-inflammatory responses in the endothelial cells.
Dyslipidemia: In the initial year after kidney transplantation, dyslipidemia is seen in 60%-88% of both adult and pediatric patients, surpassing the prevalence seen in the general population[57]. Most of the commonly used immunosuppressives are associated with abnormal lipid metabolism, especially cyclosporine and mammalian target of rapamycin (mTOR) inhibitors[57]. Cyclosporine and corticosteroids have an additive effect on raising cholesterol, with partial improvement upon steroid withdrawal[58]. Conversely, mTOR inhibitors significantly increase both cholesterol and triglycerides in a dose-dependent manner[58]. Belatacept-based immunosuppression is associated with improved LDL levels[59]. The role of oxLDL and carbamylated LDL in atherogenesis has already been mentioned. OxLDL levels correlated with increased proteinuria and graft fibrosis in a porcine renal transplantation model[60].
Statin use was linked to lower mortality in kidney transplant recipients (aHR = 0.95; 95%CI: 0.90-0.99)[61]. A trial showed that atorvastatin was more effective than switching from cyclosporine to tacrolimus in reducing total cholesterol and LDL, with additional LDL reduction when combined with tacrolimus[62]. Atorvastatin increased FMD from 4.0% to 6.5% (P < 0.05), while tacrolimus slightly improved FMD to 5.1% (P < 0.05), but adding atorvastatin to tacrolimus had no significant effect on FMD[62]. The role of statins (both LDL lowering and pleiotropic functions) in preventing ED has already been discussed. According to the KDIGO, kidney transplant recipients are advised to receive statin therapy irrespective of their LDL concentrations. The usage of statins in kidney transplant recipients is still 69.1% only as per the 2021 United States renal data system annual report[63].
Clinical vignette: The role of proprotein convertase subtilisin/kexin type 9 antibodies on lipid abnormalities and endothelial functions is still unexplored in the transplant recipients[64].
Hypertension: Hypertension is present in 50%-80% of kidney transplant recipients[65,66]. In the post hoc analysis of folic acid for vascular outcome reduction in transplantation trial, each 20-mmHg rise in baseline systolic blood pressure correlates with a 32% elevation in subsequent CV risk (HR = 1.32; 95%CI: 1.19-1.46) and a 13% increase in the risk of mortality (HR = 1.13; 95%CI: 1.01-1.27)[67]. The chances of graft failure in poorly controlled hypertensives are twice that of controlled hypertensives[68]. Inflammation is believed to play an important role in the pathogenesis of hypertension[69]. Chronic sympathetic nervous system activation, RAAS activation, obesity, high salt intake, immunosuppressive drugs, graft dysfunction, infections, and oxidative stress promote the release of pro-inflammatory cytokines causing endothelial damage leading to hypertension and further target organ damage[69]. Donor risk factors (age, hypertensive donors, graft renal artery stenosis, donor smoking, dyslipidemia, atherosclerosis) also play an important role[70]. Drugs such as calcineurin inhibitors (CNI), which includes both cyclosporine and tacrolimus, are hypothesized to enhance the expression of angiotensin II type 1 receptors and reduce NO production, leading to renal vasoconstriction[66]. Calcium channel blockers are theoretically beneficial in patients on CNI by facilitating calcium entry into vascular smooth muscles, leading to vasodilatation[66]. Posterior reversible encephalopathy syndrome in kidney transplant recipients associated with CNI usage is attributable to ED[71]. Spironolactone, a mineralocorticoid antagonist, has been shown to improve ED in experimental rat models[72]. However, a randomized controlled trial among kidney transplant recipients failed to show the effect of spironolactone in mitigating ED[73]. Despite increased plasma aldosterone levels with spironolactone treatment, there were no significant improvements in markers of ED such as nitrite, nitrate, cyclic guanosine mono
Higher salt intake (6%) in kidney transplant candidates often contributes to poor control of hypertension[74]. Higher salt is associated with post-prandial ED measured through FMD[75]. Hence, salt intake should be restricted for kidney transplant candidates. ED in hypertensive renal transplant recipients is linked to elevated ambulatory blood pressure and heightened BP variability (BPV), irrespective of mean blood pressure levels[76]. Notably, increased BPV in 24-hour systolic and diastolic readings is associated with ED measured through FMD[77]. Thus, ambulatory blood pressure monitoring in kidney transplant individuals has a significant diagnostic utility in preventing ED-related target organ damage[76].
Clinical vignette: Promising novel approaches for hypertension include cyclic guanosine monophosphate aug
Diabetes mellitus: Other than those who have pre-existing diabetes mellitus before transplantation, new-onset diabetes after kidney transplant (NODAT) develops in 10%-40% of kidney transplant recipients[79]. Though kidney trans
Clinical vignette: Recommended measures to improve endothelial functions include reduced steroid/steroid-free immunosuppression in patients with low immunological risk, early post-operative basal insulin therapy for hyper
Role of inflammation, toxins, infections, and oxidative stress: CKD is associated with retention of uremic solutes, inflammation, and oxidative stress. Factors causing inflammation in CKD include altered lipoprotein profiles, the gut microbiome, biomarkers of accelerated aging, oxidative stress, RAAS, and calcium-phosphate homeostasis. CKD and inflammation are interconnected in a bidirectional loop, where both exacerbate each other. The inflammatory state induces post-translational modifications of proteins in vascular biology, including the formation of glycated albumin, oxLDL, carbamylated sortilins, and AGEs[12,93]. They cause vascular calcification[94]. The inflammatory state leads to platelet activation, resulting in adherence to the endothelium and subsequent pathological thrombosis, which increases the risk of bleeding[95]. In CKD, dysbiosis of the gut microbiome results in the accumulation of bacterial-derived toxins such as endotoxins, indoxyl sulfate, p-cresyl sulfate, trimethylamine N-oxide, and phenylacetylglutamine[11]. These toxins can cause ED. A distinct population of monocytes, human leukocyte antigen (HLA)-DRhiIMs isolated from blood, produce higher amounts of TNF-α and IL-1β and is elevated in CKD[96].
Though kidney transplantation is associated with improved clinical outcomes, the inflammatory milieu of CKD continues to exist even after kidney transplant. Uremic toxins like ADMA (their role has been previously described) have been shown to persist in kidney transplant settings, and correlate with excess mortality and graft failure rates[97,98]. Hyperuricemia, hyperglycemia, hypertension, obesity, and dyslipidemia, which are common in the post-transplant state, cause ED[99]. Further, infections by immunomodulatory viruses such as cytomegalovirus, which are common in kidney transplant recipients, are known to cause expansion of pro-inflammatory and pro-thrombotic cell lines[100,101]. Coronavirus disease 2019 (COVID-19) is also known to cause similar immuno-thrombotic states in kidney transplant recipients[95]. Sepsis-induced acute kidney injury affects renal microvascular endothelial responses[102]. The gut microbiome in kidney transplant recipients is altered due to immunosuppressants and graft function[103].
Clinical vignette: Anti-inflammatory therapies like canakinumab and colchicine targeting atherosclerosis have never been tried in transplant settings. As transplantation is a risk factor for malignancy, the usage of immune checkpoint inhibitors for treating cancers can cause ED through the production of cytokines, and CV risk prevention is advised[104].
Uric acid: Purine metabolism through xanthine oxidase (XO) generates ROS, in addition to the production of uric acid[105]. ROS decreases NO availability through eNOS uncoupling. Uric acid, by itself, causes ED by entering the endo
Clinical vignette: The mechanism behind elevated blood pressure in hyperuricemic individuals involves both oxidative stress and intracellular urate activity, suggesting that anti-hyperuricemic drugs could be effective in controlling blood pressure[112].
Immunosuppressive drugs: Cyclosporine induces the release of endothelial microparticles, markers of endothelial activation[113]. Endothelial microparticles activate components of alternate complement pathways, leading to complement-mediated CV inflammation[114]. CNIs, both tacrolimus and cyclosporine, induce TGF-β production, which promotes endothelial-to-mesenchymal transition[115]. A small study of 31 renal transplant patients on cyclosporine and tacrolimus showed that the FMD in the cyclosporine group was significantly lower compared to healthy controls (6.3% vs 11.9%; P = 0.024). In contrast, no difference existed between the tacrolimus group and controls (8.8% vs 11.9%)[116]. CNI cause thrombotic microangiopathy (TMA) and chronic allograft nephropathy through ED.
Mycophenolate mofetil (MMF) has been shown to inhibit T-lymphocyte adhesion and penetration, indicating its potential as a therapeutic agent for mitigating ED in transplant recipients[117].
The “mTOR inhibitors” refer to medications that target the mTOR, a critical regulatory protein kinase involved in various cellular processes such as cell growth, proliferation, and metabolism. Sirolimus and everolimus are drugs used in both transplant settings and cardiology. mTOR inhibitors are associated with hyperuricemia, dyslipidemia, rejection, and proteinuria, which can cause ED[118]. In a prospective parallel-group study with a 7-month follow-up, sirolimus combined with MMF demonstrated superior FMD compared to MMF combined with cyclosporine[116].
Belatacept, a co-stimulation blocker of T cells used in CNI minimization strategy, has been associated with better metabolic and CV side effects than CNIs[119,120]. In the BENEFIT-EXT trial, which included elderly patients with comorbidities, the rate of serious CV events was 5.2 per 100 patient-years with cyclosporine and 4.1 per 100 patient-years with belatacept, representing a 21% relative risk reduction with belatacept[121].
Clinical vignette: Belatacept has been tried successfully as a rescue agent in de novo drug-induced TMA, which is caused due to endothelial activation[122].
Ischemia-reperfusion injury: Ischemia-reperfusion injury (IRI) is a complex process occurring when blood supply returns to tissues after a period of ischemia, leading to tissue damage and delayed graft function, especially in deceased donor kidney transplants. ED is a key component of IRI as reperfusion leads to endothelial cell death by releasing ROS[123]. There is consequent inflammation and thrombosis due to the activation of complement and coagulation pathways. Delayed graft function is associated with increased CV mortality and death-censored graft failure[124,125].
Clinical vignette: To prevent delayed graft function due to IRI strategies, include thymoglobulin administration, shorter cold ischemia time, hypothermic machine perfusion, and improving outcomes of higher kidney donor profile index organs[125,126].
Allograft dysfunction and rejection: Allograft dysfunction caused by both chronic T-cell-mediated and antibody-mediated rejection is characterized by endothelial damage[127]. Usage of CNIs causes allograft vasculopathy due to ED. Also, graft dysfunction is associated with poorly controlled hypertension with resultant downstream adverse CV outcomes[128].
HLA antigens are expressed on the endothelial surfaces[129]. Both complement and non-complement binding HLA antibodies, responsible for antibody-mediated rejection damage the endothelium[130,131]. Non-HLA antibodies like anti-angiotensin-1 receptor antibodies can also damage the endothelium[132]. Accelerated arteriosclerosis in kidney allografts is 2.9 times more common when circulating antibodies are present[133].
The therapies available to treat antibody-mediated endothelial damage are limited. Mineralocorticoid antagonists and aldosterone synthase inhibitors were unable to prevent chronic allograft injury in clinical trials[134]. Therefore, the prevention and effective management of rejection may offer prospects for long-term improvement in endothelial function.
Clinical vignette: Donor-derived cell-free DNA generated from donor-cell damage or death is an emerging non-invasive biomarker employed to study endothelial injury in the allograft[135].
Risk factors implicated due to dialysis modality: Microcirculatory dysfunction is a well-described phenomenon with dialysis, more with HD than with PD. Circulatory stress during HD can cause acute, reversible segmental myocardial hypoperfusion and regional systolic contractile dysfunction (myocardial stunning). Repeated episodes result in cumulative injury, leading to heart failure[136]. Dialysis membranes and tubing have been shown to activate complement and the factor XII-driven contact system of coagulation[137]. This activation stimulates the intrinsic coagulation pathway and the kallikrein/kinin system, contributing to CV injury through pro-coagulant and pro-inflammatory effects[137]. Endothelial glycocalyx components, including hyaluronan, syndecan-1, and hyaluronidase activity are elevated in patients with HD[138]. HD is commonly associated with increased oxidative stress[139]. Compared to PD, HD arteries exhibit reduced contractility and endothelium-dependent relaxation[140]. ED has also been demonstrated with PD. Brachial FMD was significantly lower in PD patients (2.9%) compared to controls (6.2%; P < 0.001) in a prospective study[141].
Clinical vignette: Microcirculatory protection during HD includes maintenance of BPs within an optimal range, avoiding large-volume ultra-filtration rate, daily dialysis, dialysate cooling, ischemic pre-conditioning, and combining PD with HD[142]. Hemodiafiltration, which removes middle molecular proteins, caused improvement in FMD compared to HD[143].
Native kidney diseases: Patients with nephrotic syndrome demonstrate poor FMD when their disease is active[144]. Nephrotic syndrome broadly includes minimal change disease, focal segmental glomerulosclerosis (FSGS), and membranous nephropathy. The pathophysiological processes underlying the development of atherosclerotic lesions and FSGS appear to overlap, involving shared mechanisms such as endothelial cell injury, macrophage infiltration, dyslipidemia, and hypertension[145]. Soluble urokinase-type plasminogen activator receptor, a novel biomarker released from endothelial cells and leukocytes, is associated with FSGS recurrence and the development of coronary ED[146,147]. Other markers of endothelial activation such as vWF, VCAM, syndecan-1, and e-selectin are also elevated in nephrotic syndrome[148]. FSGS recurrence after kidney transplant is also associated with the production of angiotensin-receptor-1 antibodies, which are known to damage the renal endothelial cells[149]. FSGS recurrence is estimated to occur in 30%-50% of patients with transplantation. Hence, ED can be assumed to be significant in patients who had nephrotic syndrome before transplant and those who had recurrences in their allograft.
The disease process in ADPKD is primarily influenced by the RAAS and endothelial cell dysfunction associated with polycystin. Alongside these, the sympathetic nervous system activation, NO, endothelin-1, and ADMA are likely to exert pivotal deleterious effects on the endothelium[150,151].
TMA encompasses a clinical and pathological syndrome where endothelial damage precipitates manifestations such as thrombocytopenia, microangiopathic hemolytic anemia, and renal injury. Complement-mediated endothelial damage has been implicated as the primary pathogenesis of TMA[152]. Complement inhibitor therapies such as eculizumab have been shown to reduce the levels of markers of ED such as thrombomodulin, VCAM-1, and soluble tumor necrosis factor receptor 1[153].
Lupus nephritis patients after kidney transplant have been shown to have pre-mature CV events, which stem from ED[154]. ED in lupus nephritis is not localized to the kidney, but is systemic. The pathophysiology of ED in this condition is due to autoantibodies, inflammatory mediators, immune complex deposition in the vasculature, complement activation, oxidative stress, hypertension, and usage of immunosuppressive drugs such as corticosteroids and CNIs[155]. Newer biological agents and reduced steroid usage can improve endothelial functions in lupus nephritis patients[156].
ED has also been demonstrated in IgA nephropathy[157], anti-neutrophilic cytoplasmic antibody related vasculitis[158], and reflux nephropathy[158].
Clinical vignette: Statin therapy improves both dyslipidemia and FMD in patients with nephrotic syndrome[159].
Bone mineral disease: CKD-mineral and bone disorder (CKD-MBD) emerges due to disturbances in calcium and phosphorus regulation, accompanied by elevated parathyroid hormone and fibroblast growth factor 23 (FGF23) levels, and reduced levels of 1,25-dihydroxyvitamin D. These disruptions lead to aberrant bone restructuring and calcification beyond the skeletal system, contributing significantly to mortality rates among affected patients[160]. Phosphate retention due to low estimated glomerular filtration rate exacerbates vascular calcification and oxidative stress, leading to ED. Elevated phosphate levels disrupt vascular calcification regulation, inducing arterial wall thickening and stiffness, while triggering the transformation of vascular smooth muscle cells into osteoblast-like cells[161]. Diabetic patients with CKD develop a typical MBD, namely, adynamic bone disease (low bone turnover state), known for accelerated vascular calcification[162]. However, the mechanisms of ED in a low turnover state are not clearly defined[161].
Kidney transplant recipients frequently encounter disruptions in phosphate homeostasis immediately following transplantation. This spectrum encompasses early-onset hypophosphatemia within the initial 3 months post-tran
Clinical vignette: Routine vitamin D supplementation to enhance endothelial function represents a promising area of research. The authors are part of a randomized controlled trial that looks at the change in FMD and endothelial biomarkers with vitamin D (cholecalciferol) supplementation in stable kidney transplant recipients.
Diet and nutrients: Diet is an important parameter that affects vascular aging[168]. A healthy diet is protective of the endothelium. The Mediterranean diet reduces oxidative stress and improves ED. A recent study showed that during a median follow-up of 5.4 years, higher adherence to the Mediterranean diet was associated with significantly lower risks of graft failure (HR = 0.68; 95%CI: 0.50-0.91), kidney function decline (HR = 0.68; 95%CI: 0.55-0.85), and graft loss (HR = 0.74; 95%CI: 0.63-0.88)[169]. The Mediterranean diet reduces the risk of developing NODAT[170]. In a randomized crossover trial of 22 stable kidney transplant recipients on RAAS blockade, a 6-week low-sodium diet (target: 50 mmol/24 h) significantly reduced systolic BP (from 140 to 129 mmHg) and diastolic BP (from 86 to 79 mmHg) compared to a regular-sodium diet (target: 150 mmol/24 h), without affecting urinary albumin excretion or estimated glomerular filtration rate[171]. Sodium restriction is thus effective for BP management in this population. The dietary approaches to stop hypertension (DASH) diet is characterized by a high intake of fruits, vegetables, legumes, nuts, and whole grains, along with low-fat dairy products. It emphasizes reduced consumption of sodium, red and processed meats, and sugar-sweetened beverages. In a prospective cohort study of 632 stable renal transplant recipients[172], a higher DASH score was associated with a reduced risk of renal function decline (HR = 0.57; 95%CI: 0.33-0.96; P = 0.03) and all-cause mortality (HR = 0.52; 95%CI: 0.32-0.83; P = 0.006) over a median follow-up of 5.3 years. Thus, both the Mediterranean and DASH diets may confer protective effects on endothelial function.
Isoflavone-containing soy products are protective of the endothelium. A small study of 20 patients with stable renal functions demonstrated improved FMD (3.2 ± 1.8% vs 6.3 ± 1.9%, respectively; P < 0.001) with 5 weeks of soy protein diet[173].
Clinical vignette: Intermittent fasting improves lipid profiles, decreases white adipose tissue, and enhances gut microbiome health. This leads to lower inflammation and a decreased risk of CV disease. Implementing intermittent fasting represents a compelling area of research in transplant settings for enhancing CV health.
Genomics: Personalized medicine for preventing CV events in kidney transplant recipients involves tailoring preventive and therapeutic strategies based on individual genetic, physiological, and clinical profiles. This approach integrates genetic risk assessments, such as the genetic risk score (GRS) derived from specific single nucleotide polymorphisms (SNPs), to identify patients at higher risk for CV complications. A composite GRS based on 27 SNPs, previously shown to predict CV events in the general population, demonstrated significant predictive value for major CV events in kidney transplant recipients (HR = 1.81; P = 0.006)[174]. This suggests that the GRS is applicable in renal transplant populations, with the potential for further refinement to enhance its predictive accuracy in this context. Polygenic risk scores from donors and recipients were able to predict the occurrence of NODAT[175]. The eNOS gene polymorphism identifies allograft outcomes related with atherosclerosis such as interstitial fibrosis and tubular atrophy[176].
Clinical vignette: Pharmacogenomics helps in precision prescribing and can avoid unnecessary CV side effects of immunosuppressants[177].
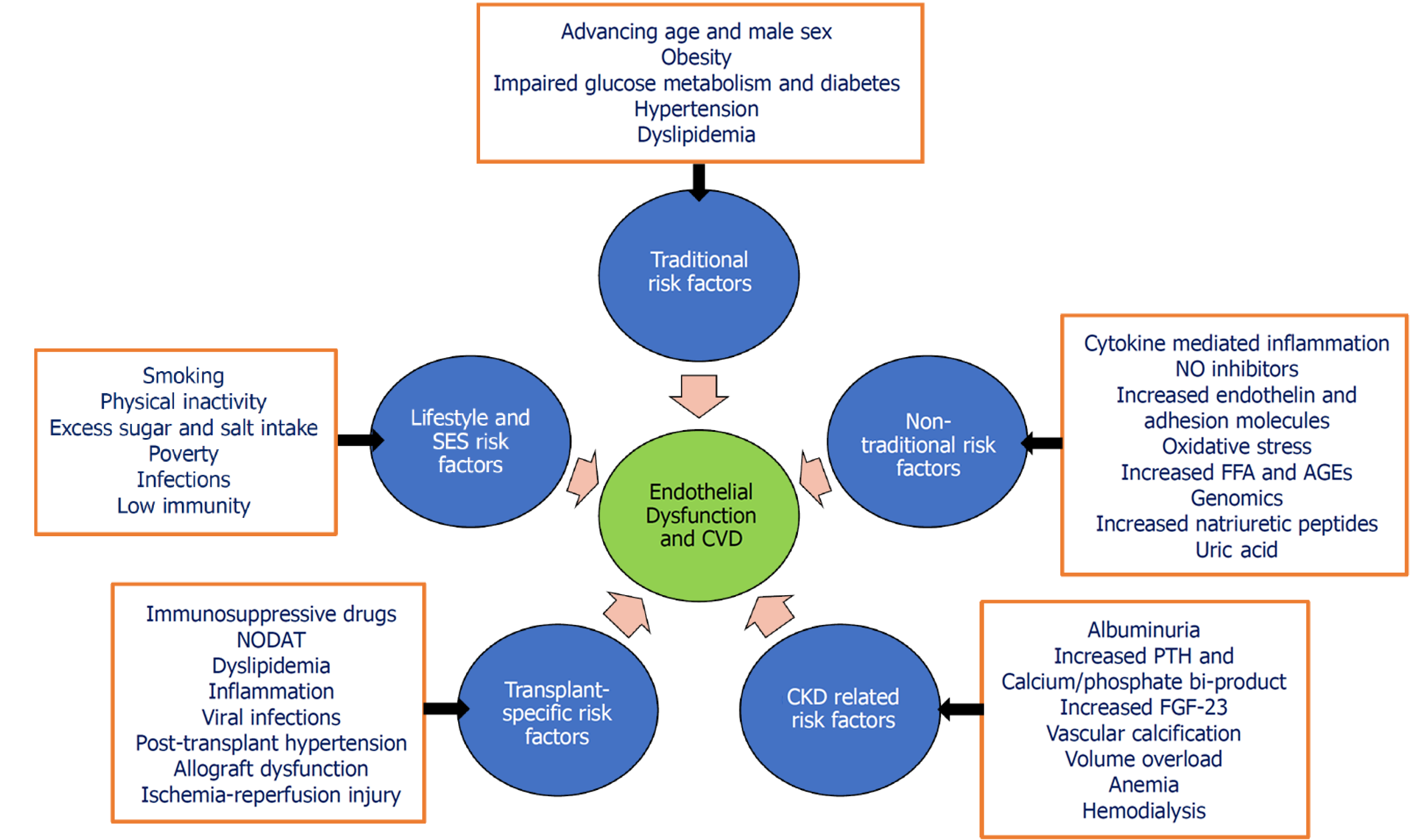
Figure 1 highlights the common pathways and risk factors leading to ED and major CV adverse events among post-kidney transplant recipients.
Biomarkers provide diagnostic information and insights into newer diagnostics, pathogenesis, and therapeutic targets. Biomarkers of endothelial activation and dysfunction are valuable for diagnosing vascular pathologies. These biomarkers often lack specificity and sensitivity in assessing CV disease in humans. Clinical utility is hindered by factors like small sample sizes, conflicting data, and poor understanding of some of the functions of biomarkers. Despite these challenges, biomarkers offer insights into disease mechanisms and are promising research tools for CV diseases. A massive surge in the endothelial biomarkers research occurred with the emergence of COVID-19 infection, where identifying these biomarkers helped us to study endothelial biology in inflammatory conditions and better risk stratify patients based on CV health[178,179]. The European Society of Cardiology is advocating for the integration of biomarkers alongside FMD for better prediction of CV events[180,181]. Detailed reviews of biomarkers have previously been published[182-184]. Table 1 lists a few important biomarkers belonging to various functional groups.
| Functional group | Biomarkers |
| Oxidative stress | NADPH oxidase, ROS |
| Acute phase proteins | IL-1β, IL-6, TNF-α and its receptors, MCP-1, CRP, and SAA |
| Cell-adhesion molecules | ICAM-1, VCAM-1, e-selectin, p-selectin |
| Proteins modified by post-translational modification | OxLDL, OxHDL, carbamylated LDL, AGE, RAGE |
| Glycocalyx damage | Syndexan-1, endocan |
| Vascular tone | Endothelin-1, angiotensin II, resistin |
| Metabolizes catecholamines | Renalase |
| eNOS uncoupling | ADMA, SDMA |
| Thrombosis | PAI-1, angiopoietin-2, vWF, TF, soluble thrombomodulin, thromboxane A2, d-dimer, factor VIII, homocysteine |
| Marker of angiogenesis and hyperpermeability | VEGF-A, TGF-β1, PlGF |
| Marker of endothelial proliferation and apoptosis | Circulating endothelial cells |
| Membranous particles released from endothelial cells | Endothelial microvesicles |
| Endothelial-protective molecules | NO, klotho, sirtuins, Nrf2, PGI2 |
| Others | miRNA, gut microbiota-derived metabolites, suPAR |
Arterial health is often assessed by examining the vessel’s capacity for dilation in response to various stimuli. Physiologically, a key marker of arterial health is the ability of arteries to undergo endothelium-dependent vasodilation in reaction to reactive hyperemia. This phenomenon occurs due to increased shear stress resulting from elevated blood flow. In healthy arteries, this dilatory response is mediated by the endothelium through the release of vasodilatory factors such as NO, which counteracts the elevated shear stress by promoting vessel relaxation. Thus, impaired or blunted vaso
Invasive methods: Coronary ED is characterized by the lack of expected vasodilation and the inadequate increase in blood flow within the epicardial coronary arteries following acetylcholine administration. These changes are commonly assessed using two main techniques: Quantitative coronary angiography and intravascular ultrasound[185]. While definitions of an abnormal angiographic response may vary across sources, a practical criterion is any degree of constriction of the epicardial arteries exceeding 0%, but not exceeding 90% following acetylcholine administration[188]. Agents other than acetylcholine that can be used include bradykinin, papaverine, and substance P[189]. Changes in coronary blood flow (CBF), a surrogate of coronary microvascular functions, are assessed through coronary flow reserve (CFR), calculated as maximal CBF during induced hyperemia divided by resting CBF. A CFR below 2.0 indicates an abnormality. Invasive methods like doppler flow velocity or thermodilution are used, albeit with technical challenges and reproducibility concerns[185].
Non-invasive methods to study coronary microvascular functions include the following[190]: (1) Contrast echocardiography; (2) Transthoracic doppler echocardiography; (3) Contrast tomography (CT); (4) Positron emission tomography (PET); (5) Single-photon emission computed tomography; and (6) Cardiac magnetic resonance imaging. Among these imaging modalities, PET offers precise global and regional myocardial perfusion (MP), blood flow, and function measurements, with advanced software enabling routine assessment of myocardial blood flow and viability using tracers like 18F-fluoro deoxy-glucose and 82-rubidium[191]. Enhanced PET/CT scanners facilitate accurate coronary micro
For asymptomatic patients, invasive techniques, as mentioned before, may not be suitable. Therefore, non-invasive methods to detect endothelial functions have become increasingly popular[192]. Assessment by some of the techniques has been shown to replicate the endothelial functions in the coronary circulation[193-195].
FMD: Skeletal muscle arteries respond rapidly to reactive hyperemia of the supplying muscles[196]. FMD of conduit arteries like the brachial artery is a commonly used test to measure endothelial function[197]. Brachial artery FMD, a marker of endothelium-dependent vasodilation in response to vascular hyperemia (created by deflating an already inflated cuff at supra systolic blood pressure), mirrors NO production in the endothelium and aligns with coronary artery endothelial function[198]. It has good reproducibility. A small study[199] evaluated the reliability and reproducibility of FMD and peripheral arterial tonometry (PAT) for assessing endothelial function. Simultaneous FMD (brachial artery ultrasound) and PAT (EndoPAT 2000) assessments were performed twice with a 30-minute interval and repeated after 2 days. Within-day variability was lower for FMD (10%) compared to PAT (18%; P < 0.05), while between-day variability was similar (11%). A significant correlation between PAT and FMD was observed (r = 0.57, P < 0.001). FMD showed better reliability for both within- and between-day measurements compared to PAT. Systematic reviews demonstrate that even a modest increase of 1% in FMD value is linked to a significant 12% to 13% reduction in the risk of CV events, regardless of traditional CV risk factors[200,201]. This underscores the potential of FMD as a valuable vascular biomarker for stratifying CV risk. FMD is measured by real-time computer-based software, which will detect changes in the vessel wall diameters in response to hyperemia compared to baseline, i.e., %FMD[202]. If not performed and analyzed consistently, FMD measurements can have high intra- and inter-observer variability. Standard reference ranges are available, which help identify and monitor at-risk individuals[198,203]. In 1579 healthy individuals, FMD was inversely correlated with age, body mass index, glucose, cholesterol, blood pressure, and brachial artery diameter. Receiver operating characteristic analysis indicated that FMD > 6.5% excludes coronary artery disease (95% sensitivity, 60% specificity), while FMD < 3.1% excludes 95% of healthy individuals[203]. A meta-analysis of 82 studies (n = 3509) found an average FMD of 6.4% (95%CI: 6.2%-6.7%) with a significant age-related decline (-0.3%/decade)[203].
Nitroglycerin-mediated dilatation: Nitroglycerine-induced vasodilation (NMD), a marker of endothelium-independent vasodilation, is used as a control test for FMD to confirm that impaired FMD is not due to vascular smooth muscle dysfunction or structural alterations[204]. Instead, it confirms that the observed FMD impairment genuinely stems from ED. Combined FMD and NMD can better predict CV events than NMD alone[205]. NMD is usually performed by administering sublingual isosorbide dinitrite[144]. Again, the reference values for NMD are available for the general population[198].
Venous occlusion plethysmography: Forearm blood flow changes are measured via plethysmography in response to vasoactive substances introduced through a cannulated brachial artery, allowing quantification of both endothelial-dependent and independent vasodilation. Because of its semi-invasiveness, time-consuming, and impracticality for serial measurements, it has limited usage in the current era[206].
PAT: PAT is an innovative approach for evaluating endothelial function, utilizing finger plethysmography to measure pulse wave amplitude (PWA) at rest and during shear stress[207]. The reactive hyperemia-PAT index quantifies the ratio of PWA signal post-cuff release to baseline via computerized algorithms[208]. PAT offers advantages like using the subject’s contralateral arm as an internal control and requiring minimal training. It correlates well with endothelial function across various populations and has predictive value for CV events[209,210].
Retinal artery diameters and reactivity: Evidence supports retinal microvascular analysis as a predictor of systemic CV risk and disease; however, this association is not yet strongly established[185]. Some studies highlight its clinical utility and predictive value for CV outcomes by assessing endothelial function, supporting its use in the primary and secondary prevention of chronic non-communicable diseases such as hypertension and diabetes mellitus[211,212]. Retinal vessel dimensions and flicker light-induced dilation are employed to detect endothelial functions[212]. Continuous retinal vessel diameter measurements during flicker light provocation reveal significant differences between those with CV disease and those with both CV disease + diabetes mellitus[212]. The distinct dilation and constriction patterns in diabetes mellitus (P < 0.030) could serve as a marker for monitoring CV disease progression[212]. The lack of randomized controlled studies and the non-availability of standard values hinder further progress in this field[185].
Studies on endothelial function testing in kidney transplant recipients: Kidney transplantation has been demonstrated to improve ED immediately after transplant, attributed to the reduction of uremia-related and traditional risk factors that cause endothelial damage[213-215]. The positive correlation measured in the above studies was seen until 90 days after the kidney transplant, as evidenced by improvement in FMD and biomarkers. However, their values were still significantly lower than in a healthy population[213]. In stable kidney transplant patients > 6 months after transplant, there was a significant decrease in FMD (-1.52% ± 2.74%; P = 0.03) at 3-6 months compared to baseline[216]. However, the decline in NMD was not significant. In a 6.5-year follow-up study of 152 kidney transplant recipients, significant post-transplant ED (defined as FMD ≤ 5.36%) was found to be associated with increased mortality (HR = 9.80; 95%CI: 1.29-74.62; P = 0.03) and higher risk of uncensored graft loss (HR = 7.80; 95%CI: 1.83-33.30; P = 0.01)[217]. Mortality risk increased with diminishing FMD levels until approximately 5% dilation, beyond which further reduction did not significantly affect the risk, suggesting a critical threshold for adverse outcomes in kidney transplant recipients[217]. Hence, a decline in FMD following an initial improvement is evident.
In a pilot study[218], MP reserve (MPR) was lower in kidney transplant patients (4.1 ± 1.1) compared to healthy controls (4.3 ± 1.6), but after adjusting for basal MP (1.3 ± 0.4 vs 1.0 ± 0.2 mL/minute/g) and cardiac workload, differences in MPR and stress MP (3.8 ± 1.0 vs 4.0 ± 0.9 mL/minute/g) were not significant. Increased resting MP in transplant patients is due to higher cardiac workload.
A study[219] analyzing endothelial biomarkers tried to delineate correlations between renalase and markers of inflammation and coagulation within a cohort of 62 kidney allograft recipients. Notably, renalase concentrations correlated with key endothelial markers such as vWF, thrombomodulin, ICAM-1, and VCAM-1[219]. Also, renalase was higher in hypertensive subjects compared to normotensive subjects[219]. ADMA (decreased eNOS) levels were associated with increased all-cause mortality (HR = 1.52; 95%CI: 1.26-1.83) in kidney transplant recipients[220].
ED in kidney transplant recipients is often under-recognized and inadequately treated, despite its significant CV implications. Increased CV mortality in this population is associated with ED. Many traditional and non-traditional risk factors for ED are modifiable, and optimal use of endothelial-protective medications is warranted. There is a critical need to include kidney transplant patients in CV clinical trials focusing on ED. Both FMD and biomarkers offer promise for early diagnosis of CV diseases compared to conventional diagnostic methods. As the number of kidney transplants continues to rise annually, emphasis should be placed on the prevention, early recognition, and treatment of ED to improve graft longevity and overall survival.
| 1. | Bauer V, Sotníková R. Nitric oxide--the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys. 2010;29:319-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Mussbacher M, Schossleitner K, Kral-Pointner JB, Salzmann M, Schrammel A, Schmid JA. More than Just a Monolayer: the Multifaceted Role of Endothelial Cells in the Pathophysiology of Atherosclerosis. Curr Atheroscler Rep. 2022;24:483-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Suzuki A, Tomita H, Okada H. Form follows function: The endothelial glycocalyx. Transl Res. 2022;247:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Łuczak A, Madej M, Kasprzyk A, Doroszko A. Role of the eNOS Uncoupling and the Nitric Oxide Metabolic Pathway in the Pathogenesis of Autoimmune Rheumatic Diseases. Oxid Med Cell Longev. 2020;2020:1417981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Cabrera JTO, Makino A. Efferocytosis of vascular cells in cardiovascular disease. Pharmacol Ther. 2022;229:107919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 6. | Huang Q, Gan Y, Yu Z, Wu H, Zhong Z. Endothelial to Mesenchymal Transition: An Insight in Atherosclerosis. Front Cardiovasc Med. 2021;8:734550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Shao Y, Saredy J, Yang WY, Sun Y, Lu Y, Saaoud F, Drummer C 4th, Johnson C, Xu K, Jiang X, Wang H, Yang X. Vascular Endothelial Cells and Innate Immunity. Arterioscler Thromb Vasc Biol. 2020;40:e138-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 8. | Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 1306] [Article Influence: 261.2] [Reference Citation Analysis (2)] |
| 9. | Roumeliotis S, Mallamaci F, Zoccali C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 10. | Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, Mambet C, Anton G, Tanase C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J Immunol Res. 2018;2018:2180373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 463] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 11. | Speer T, Dimmeler S, Schunk SJ, Fliser D, Ridker PM. Targeting innate immunity-driven inflammation in CKD and cardiovascular disease. Nat Rev Nephrol. 2022;18:762-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 147] [Reference Citation Analysis (0)] |
| 12. | Baaten CCFMJ, Vondenhoff S, Noels H. Endothelial Cell Dysfunction and Increased Cardiovascular Risk in Patients With Chronic Kidney Disease. Circ Res. 2023;132:970-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 13. | Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4997] [Cited by in RCA: 7147] [Article Influence: 794.1] [Reference Citation Analysis (0)] |
| 14. | Choi HY, Lee JE, Han SH, Yoo TH, Kim BS, Park HC, Kang SW, Choi KH, Ha SK, Lee HY, Han DS. Association of inflammation and protein-energy wasting with endothelial dysfunction in peritoneal dialysis patients. Nephrol Dial Transplant. 2010;25:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, Wiecek A, Martinez-Castelao A, Covic A, Goldsmith D, Suleymanlar G, Lindholm B, Parati G, Sicari R, Gargani L, Mallamaci F, London G, Zoccali C. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Merino A, Portolés J, Selgas R, Ojeda R, Buendia P, Ocaña J, Bajo MA, del Peso G, Carracedo J, Ramírez R, Martín-Malo A, Aljama P. Effect of different dialysis modalities on microinflammatory status and endothelial damage. Clin J Am Soc Nephrol. 2010;5:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Prasad N, Yadav AK, Kundu M, Sethi J, Jaryal A, Sircar D, Modi GK, Kamboj K, Sahay M, Gopalakrishnan N, Kaur P, Vikrant S, Varughese S, Baid-Agrawal S, Singh S, Gang S, Parameswaran S, Kumar V, Ghosh A, Jha V. Prescription Practices in Patients With Mild to Moderate CKD in India. Kidney Int Rep. 2021;6:2455-2462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 440] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 20. | Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl (2011). 2016;6:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Colombijn JMT, Idema DL, van Beem S, Blokland AM, van der Braak K, Handoko ML, In 't Veld LFH, Kaul T, Kolagasigil-Akdemir N, Kusters MPT, Meijvis SCA, Oosting IJ, Spijker R, Bots ML, Hooft L, Verhaar MC, Vernooij RWM. Representation of Patients With Chronic Kidney Disease in Clinical Trials of Cardiovascular Disease Medications: A Systematic Review. JAMA Netw Open. 2024;7:e240427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Rangaswami J, Mathew RO, Parasuraman R, Tantisattamo E, Lubetzky M, Rao S, Yaqub MS, Birdwell KA, Bennett W, Dalal P, Kapoor R, Lerma EV, Lerman M, McCormick N, Bangalore S, McCullough PA, Dadhania DM. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant. 2019;34:760-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 24. | Birdwell KA, Park M. Post-Transplant Cardiovascular Disease. Clin J Am Soc Nephrol. 2021;16:1878-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Lim K, Ting SMS, Hamborg T, McGregor G, Oxborough D, Tomkins C, Xu D, Thadhani R, Lewis G, Bland R, Banerjee P, Fletcher S, Krishnan NS, Higgins R, Zehnder D, Hiemstra TF. Cardiovascular Functional Reserve Before and After Kidney Transplant. JAMA Cardiol. 2020;5:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, Navaneethan SD, Ramanathan V. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996-2014). Am J Nephrol. 2018;48:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Andersson C, Hansen D, Sørensen SS, McGrath M, McCausland FR, Torp-Pedersen C, Schou M, Køber L, Pfeffer MA. Long-term cardiovascular events, graft failure, and mortality in kidney transplant recipients. Eur J Intern Med. 2024;121:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 29. | Lenihan CR, Montez-Rath ME, Scandling JD, Turakhia MP, Winkelmayer WC. Outcomes after kidney transplantation of patients previously diagnosed with atrial fibrillation. Am J Transplant. 2013;13:1566-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Lentine KL, Schnitzler MA, Abbott KC, Li L, Burroughs TE, Irish W, Brennan DC. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis. 2005;46:720-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Cardinal H, Dieudé M, Hébert MJ. Endothelial Dysfunction in Kidney Transplantation. Front Immunol. 2018;9:1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16:496-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Stewart G, Jardine AG, Briggs JD. Ischaemic heart disease following renal transplantation. Nephrol Dial Transplant. 2000;15:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Mathur AK, Chang YH, Steidley DE, Heilman R, Khurmi N, Wasif N, Etzioni D, Moss AA. Patterns of Care and Outcomes in Cardiovascular Disease After Kidney Transplantation in the United States. Transplant Direct. 2017;3:e126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Zhang J, Wang Z, Wang C, Ma D. Endothelial-cell-mediated mechanism of coronary microvascular dysfunction leading to heart failure with preserved ejection fraction. Heart Fail Rev. 2023;28:169-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S, Fink JC, Fisher ML, Bartlett ST, Weir MR. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Dennis MR, Pires PW, Banek CT. Vascular Dysfunction in Polycystic Kidney Disease: A Mini-Review. J Vasc Res. 2023;60:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Chedid M, Kaidbay HD, Wigerinck S, Mkhaimer Y, Smith B, Zubidat D, Sekhon I, Prajwal R, Duriseti P, Issa N, Zoghby ZM, Hanna C, Senum SR, Harris PC, Hickson LJ, Torres VE, Nkomo VT, Chebib FT. Cardiovascular Outcomes in Kidney Transplant Recipients With ADPKD. Kidney Int Rep. 2022;7:1991-2005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Yoo DJ, Agodoa L, Yuan CM, Abbott KC, Nee R. Risk of intracranial hemorrhage associated with autosomal dominant polycystic kidney disease in patients with end stage renal disease. BMC Nephrol. 2014;15:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Snyder JJ, Kasiske BL, Maclean R. Peripheral arterial disease and renal transplantation. J Am Soc Nephrol. 2006;17:2056-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Patel SI, Chakkera HA, Wennberg PW, Liedl DA, Alrabadi F, Cha SS, Hooley DD, Amer H, Wadei HM, Shamoun FE. Peripheral arterial disease preoperatively may predict graft failure and mortality in kidney transplant recipients. Vasc Med. 2017;22:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Galié N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004;61:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 43. | Lentine KL, Villines TC, Axelrod D, Kaviratne S, Weir MR, Costa SP. Evaluation and Management of Pulmonary Hypertension in Kidney Transplant Candidates and Recipients: Concepts and Controversies. Transplantation. 2017;101:166-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Kwaifa IK, Bahari H, Yong YK, Noor SM. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 45. | Berkman ER, Richardson KL, Clark JD, Dick AAS, Lewis-Newby M, Diekema DS, Wightman AG. An ethical analysis of obesity as a contraindication of pediatric kidney transplant candidacy. Pediatr Nephrol. 2023;38:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 46. | Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J; FREEDOM Study Group. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Chang S, Jiang J. Association of Body Mass Index and the Risk of New-Onset Diabetes After Kidney Transplantation: A Meta-analysis. Transplant Proc. 2018;50:1316-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Freeman CM, Woodle ES, Shi J, Alexander JW, Leggett PL, Shah SA, Paterno F, Cuffy MC, Govil A, Mogilishetty G, Alloway RR, Hanseman D, Cardi M, Diwan TS. Addressing morbid obesity as a barrier to renal transplantation with laparoscopic sleeve gastrectomy. Am J Transplant. 2015;15:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Scheuermann U, Babel J, Pietsch UC, Weimann A, Lyros O, Semmling K, Hau HM, Seehofer D, Rademacher S, Sucher R. Recipient obesity as a risk factor in kidney transplantation. BMC Nephrol. 2022;23:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 50. | Mallamaci F, Leonardis D, Tripepi R, Parlongo G, Catalano C, Tripepi G, Castronovo V, Ferini-Strambi L, Zoccali C. Sleep disordered breathing in renal transplant patients. Am J Transplant. 2009;9:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Kholdani C, Fares WH, Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ. 2015;5:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 834] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 53. | Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 366] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 54. | Weinrauch LA, Claggett B, Liu J, Finn PV, Weir MR, Weiner DE, D'Elia JA. Smoking and outcomes in kidney transplant recipients: a post hoc survival analysis of the FAVORIT trial. Int J Nephrol Renovasc Dis. 2018;11:155-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010;55:1988-1995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Mohammadi L, Han DD, Xu F, Huang A, Derakhshandeh R, Rao P, Whitlatch A, Cheng J, Keith RJ, Hamburg NM, Ganz P, Hellman J, Schick SF, Springer ML. Chronic E-Cigarette Use Impairs Endothelial Function on the Physiological and Cellular Levels. Arterioscler Thromb Vasc Biol. 2022;42:1333-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 57. | Skulratanasak P, Larpparisuth N. Lipid management to mitigate poorer postkidney transplant outcomes. Curr Opin Nephrol Hypertens. 2023;32:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Riella LV, Gabardi S, Chandraker A. Dyslipidemia and its therapeutic challenges in renal transplantation. Am J Transplant. 2012;12:1975-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Kumar J, Reccia I, Virdis F, Podda M, Sharma AK, Halawa A. Belatacept in renal transplantation in comparison to tacrolimus and molecular understanding of resistance pattern: Meta-analysis and systematic review. World J Transplant. 2021;11:70-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 60. | Chatauret N, Favreau F, Giraud S, Thierry A, Rossard L, Le Pape S, Lerman LO, Hauet T. Diet-induced increase in plasma oxidized LDL promotes early fibrosis in a renal porcine auto-transplantation model. J Transl Med. 2014;12:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Bae S, Ahn JB, Joseph C, Whisler R, Schnitzler MA, Lentine KL, Kadosh BS, Segev DL, McAdams-DeMarco MA. Statins in Kidney Transplant Recipients: Usage, All-Cause Mortality, and Interactions with Maintenance Immunosuppressive Agents. J Am Soc Nephrol. 2023;34:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Wissing KM, Unger P, Ghisdal L, Broeders N, Berkenboom G, Carpentier Y, Abramowicz D. Effect of atorvastatin therapy and conversion to tacrolimus on hypercholesterolemia and endothelial dysfunction after renal transplantation. Transplantation. 2006;82:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | USRDS. Annual Data Report. [cited 23 October, 2024]. Available from: https://usrds-adr.niddk.nih.gov/2024. |
| 64. | Ueberdiek L, Jehn U, Pavenstädt H, Gebauer K, Reuter S. Novel Therapeutic Strategies for Dyslipidemia: First Report of Inclisiran Therapy in a Kidney Transplanted Patient. Transpl Int. 2023;36:11104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Weir MR, Burgess ED, Cooper JE, Fenves AZ, Goldsmith D, McKay D, Mehrotra A, Mitsnefes MM, Sica DA, Taler SJ. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015;26:1248-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 66. | Tantisattamo E, Molnar MZ, Ho BT, Reddy UG, Dafoe DC, Ichii H, Ferrey AJ, Hanna RM, Kalantar-Zadeh K, Amin A. Approach and Management of Hypertension After Kidney Transplantation. Front Med (Lausanne). 2020;7:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 67. | Carpenter MA, John A, Weir MR, Smith SR, Hunsicker L, Kasiske BL, Kusek JW, Bostom A, Ivanova A, Levey AS, Solomon S, Pesavento T, Weiner DE. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol. 2014;25:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Kim CS, Oh TR, Suh SH, Choi HS, Bae EH, Ma SK, Jung JH, Kim B, Han KD, Kim SW. Uncontrolled hypertension is associated with increased risk of graft failure in kidney transplant recipients: a nationwide population-based study. Front Cardiovasc Med. 2023;10:1185001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 69. | Guzik TJ, Nosalski R, Maffia P, Drummond GR. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol. 2024;21:396-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 156] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 70. | Seeman T, Myette RL, Feber J. Hypertension in pediatric kidney transplantation. Pediatr Transplant. 2023;27:e14522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 71. | Jeelani H, Sharma A, Halawa AM. Posterior Reversible Encephalopathy Syndrome in Organ Transplantation. Exp Clin Transplant. 2022;20:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 72. | Wang CC, Lee AS, Liu SH, Chang KC, Shen MY, Chang CT. Spironolactone ameliorates endothelial dysfunction through inhibition of the AGE/RAGE axis in a chronic renal failure rat model. BMC Nephrol. 2019;20:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Mortensen LA, Bistrup C, Stubbe J, Carlström M, Checa A, Wheelock CE, Palarasah Y, Bladbjerg EM, Thiesson HC, Jensen BL. Effect of spironolactone for 1 yr on endothelial function and vascular inflammation biomarkers in renal transplant recipients. Am J Physiol Renal Physiol. 2019;317:F529-F539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Hirano H, Fujiwara Y, Maenosono R, Minami K, Uehara H, Okabe T, Nakamori K, Nomi H, Komura K, Inamoto T, Azuma H. Usefulness of Dietary Salt Restriction in Kidney Transplant Recipients: Analysis of Blood Pressure Levels Depending on the Differences in the Levels of Salt Intake. Transplant Proc. 2023;55:841-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 75. | Dickinson KM, Clifton PM, Keogh JB. Endothelial function is impaired after a high-salt meal in healthy subjects. Am J Clin Nutr. 2011;93:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Pisano A, Mallamaci F, D'Arrigo G, Bolignano D, Wuerzner G, Ortiz A, Burnier M, Kanaan N, Sarafidis P, Persu A, Ferro CJ, Loutradis C, Boletis IN, London G, Halimi JM, Sautenet B, Rossignol P, Vogt L, Zoccali C. Blood pressure monitoring in kidney transplantation: a systematic review on hypertension and target organ damage. Nephrol Dial Transplant. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Ozkayar N, Altun B, Yildirim T, Yilmaz R, Dede F, Arik G, Turkmen E, Hayran M, Aki FT, Arici M, Erdem Y. Blood pressure measurements, blood pressure variability and endothelial function in renal transplant recipients. Clin Exp Hypertens. 2014;36:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Magavern EF, Kapil V, Saxena M, Gupta A, Caulfield MJ. Use of Genomics to Develop Novel Therapeutics and Personalize Hypertension Therapy. Arterioscler Thromb Vasc Biol. 2024;44:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Jenssen T, Hartmann A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol. 2019;15:172-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 80. | Khairoun M, de Koning EJ, van den Berg BM, Lievers E, de Boer HC, Schaapherder AF, Mallat MJ, Rotmans JI, van der Boog PJ, van Zonneveld AJ, de Fijter JW, Rabelink TJ, Reinders ME. Microvascular damage in type 1 diabetic patients is reversed in the first year after simultaneous pancreas-kidney transplantation. Am J Transplant. 2013;13:1272-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Topitz D, Schwaiger E, Frommlet F, Werzowa J, Hecking M. Cardiovascular events associate with diabetes status rather than with early basal insulin treatment for the prevention of post-transplantation diabetes mellitus. Nephrol Dial Transplant. 2020;35:544-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Wauters RP, Cosio FG, Suarez Fernandez ML, Kudva Y, Shah P, Torres VE. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation. 2012;94:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 83. | Gora IM, Ciechanowska A, Ladyzynski P. NLRP3 Inflammasome at the Interface of Inflammation, Endothelial Dysfunction, and Type 2 Diabetes. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 84. | Maruhashi T, Higashi Y. Pathophysiological Association between Diabetes Mellitus and Endothelial Dysfunction. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 85. | Strøm Halden TA, Åsberg A, Vik K, Hartmann A, Jenssen T. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant. 2014;29:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Nafisa A, Gray SG, Cao Y, Wang T, Xu S, Wattoo FH, Barras M, Cohen N, Kamato D, Little PJ. Endothelial function and dysfunction: Impact of metformin. Pharmacol Ther. 2018;192:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 87. | Shamshad F, Tungsanga S, Senior P, Shojai S, Ghimire A, Ye F, Kung JY, Hariramani VK, Abdulrahman A, Penney M, Sultana N, Muneer S, Okpechi I, Bello AK. Effect of metformin use on graft and patient survival in kidney transplant recipients with type 2 diabetes: a systematic review protocol. BMJ Open. 2024;14:e078393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull Exp Biol Med. 2021;171:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 89. | Schwaiger E, Krenn S, Kurnikowski A, Bergfeld L, Pérez-Sáez MJ, Frey A, Topitz D, Bergmann M, Hödlmoser S, Bachmann F, Halleck F, Kron S, Hafner-Giessauf H, Eller K, Rosenkranz AR, Crespo M, Faura A, Tura A, Song PXK, Port FK, Pascual J, Budde K, Ristl R, Werzowa J, Hecking M. Early Postoperative Basal Insulin Therapy versus Standard of Care for the Prevention of Diabetes Mellitus after Kidney Transplantation: A Multicenter Randomized Trial. J Am Soc Nephrol. 2021;32:2083-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, Kountouri A, Balampanis K, Parissis J, Andreadou I, Katogiannis K, Dimitriadis G, Bamias A, Iliodromitis E, Lambadiari V. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients With Type 2 Diabetes Mellitus After 12-Month Treatment. J Am Heart Assoc. 2020;9:e015716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 91. | Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, Santulli G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights From Frail Hypertensive and Diabetic Patients. Hypertension. 2022;79:1633-1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 92. | Alshnbari AS, Millar SA, O'Sullivan SE, Idris I. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Endothelial Function: A Systematic Review of Preclinical Studies. Diabetes Ther. 2020;11:1947-1963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 93. | Jankowski V, Saritas T, Kjolby M, Hermann J, Speer T, Himmelsbach A, Mahr K, Heuschkel MA, Schunk SJ, Thirup S, Winther S, Bottcher M, Nyegard M, Nykjaer A, Kramann R, Kaesler N, Jankowski J, Floege J, Marx N, Goettsch C. Carbamylated sortilin associates with cardiovascular calcification in patients with chronic kidney disease. Kidney Int. 2022;101:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 94. | Gajjala PR, Fliser D, Speer T, Jankowski V, Jankowski J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol Dial Transplant. 2015;30:1814-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 95. | Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18:666-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 96. | Cormican S, Negi N, Naicker SD, Islam MN, Fazekas B, Power R, Griffin TP, Dennedy MC, MacNeill B, Malone AF, Griffin MD. Chronic Kidney Disease Is Characterized by Expansion of a Distinct Proinflammatory Intermediate Monocyte Subtype and by Increased Monocyte Adhesion to Endothelial Cells. J Am Soc Nephrol. 2023;34:793-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Baskal S, Post A, Kremer D, Bollenbach A, Bakker SJL, Tsikas D. Urinary excretion of amino acids and their advanced glycation end-products (AGEs) in adult kidney transplant recipients with emphasis on lysine: furosine excretion is associated with cardiovascular and all-cause mortality. Amino Acids. 2021;53:1679-1693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 98. | Post A, Bollenbach A, Bakker SJL, Tsikas D. Whole-body arginine dimethylation is associated with all-cause mortality in adult renal transplant recipients. Amino Acids. 2021;53:541-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Ponticelli C, Campise MR. The inflammatory state is a risk factor for cardiovascular disease and graft fibrosis in kidney transplantation. Kidney Int. 2021;100:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Soleimanian S, Yaghobi R, Karimi MH, Geramizadeh B, Roozbeh J. Altered Signatures of Plasma Inflammatory Proteins and Phonotypic Markers of NK Cells in Kidney Transplant Patients upon CMV Reactivation. Curr Microbiol. 2022;80:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Couzi L, Pitard V, Moreau JF, Merville P, Déchanet-Merville J. Direct and Indirect Effects of Cytomegalovirus-Induced γδ T Cells after Kidney Transplantation. Front Immunol. 2015;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 102. | Molema G, Zijlstra JG, van Meurs M, Kamps JAAM. Renal microvascular endothelial cell responses in sepsis-induced acute kidney injury. Nat Rev Nephrol. 2022;18:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 103. | Campbell PM, Humphreys GJ, Summers AM, Konkel JE, Knight CG, Augustine T, McBain AJ. Does the Microbiome Affect the Outcome of Renal Transplantation? Front Cell Infect Microbiol. 2020;10:558644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 104. | Efentakis P, Choustoulaki A, Kwiatkowski G, Varela A, Kostopoulos IV, Tsekenis G, Ntanasis-Stathopoulos I, Georgoulis A, Vorgias CE, Gakiopoulou H, Briasoulis A, Davos CH, Kostomitsopoulos N, Tsitsilonis O, Dimopoulos MA, Terpos E, Chłopicki S, Gavriatopoulou M, Andreadou I. Early microvascular coronary endothelial dysfunction precedes pembrolizumab-induced cardiotoxicity. Preventive role of high dose of atorvastatin. Basic Res Cardiol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 105. | Maruhashi T, Hisatome I, Kihara Y, Higashi Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis. 2018;278:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 106. | Farouk SS, Rein JL. The Many Faces of Calcineurin Inhibitor Toxicity-What the FK? Adv Chronic Kidney Dis. 2020;27:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 107. | Akalin E, Ganeshan SV, Winston J, Muntner P. Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation. 2008;86:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 108. | Dahle DO, Jenssen T, Holdaas H, Asberg A, Soveri I, Holme I, Mjøen G, Eide IA, Pihlstrøm H, Dörje C, Halden TA, Hartmann A. Uric acid and clinical correlates of endothelial function in kidney transplant recipients. Clin Transplant. 2014;28:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Armstrong KA, Johnson DW, Campbell SB, Isbel NM, Hawley CM. Does uric acid have a pathogenetic role in graft dysfunction and hypertension in renal transplant recipients? Transplantation. 2005;80:1565-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang CC, Struthers AD. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 111. | Deftereos SG, Beerkens FJ, Shah B, Giannopoulos G, Vrachatis DA, Giotaki SG, Siasos G, Nicolas J, Arnott C, Patel S, Parsons M, Tardif JC, Kovacic JC, Dangas GD. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation. 2022;145:61-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 119] [Reference Citation Analysis (0)] |
| 112. | Borghi C, Agnoletti D, Cicero AFG, Lurbe E, Virdis A. Uric Acid and Hypertension: a Review of Evidence and Future Perspectives for the Management of Cardiovascular Risk. Hypertension. 2022;79:1927-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 113. | Renner B, Klawitter J, Goldberg R, McCullough JW, Ferreira VP, Cooper JE, Christians U, Thurman JM. Cyclosporine induces endothelial cell release of complement-activating microparticles. J Am Soc Nephrol. 2013;24:1849-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 114. | Martin N, Tu X, Egan AJ, Stover C. Complement Activation on Endothelial Cell-Derived Microparticles-A Key Determinant for Cardiovascular Risk in Patients with Systemic Lupus Erythematosus? Medicina (Kaunas). 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Woda CB, Bruneau S, Mak AL, Haskova Z, Liu K, Ghosh CC, Briscoe DM. Calcineurin inhibitors augment endothelial-to-mesenchymal transition by enhancing proliferation in association with cytokine-mediated activation. Biochem Biophys Res Commun. 2019;519:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Akoglu H, Seringec N, Yildirim T, Yilmaz R, Okutucu S, Turkmen E, Evranos B, Kaya EB, Dikmenoglu N, Arici M, Erdem Y, Turgan C. Relationship between hemorheology and endothelial dysfunction in renal transplant patients receiving calcineurin inhibitors. J Nephrol. 2013;26:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Blaheta RA, Leckel K, Wittig B, Zenker D, Oppermann E, Harder S, Scholz M, Weber S, Schuldes H, Encke A, Markus BH. Inhibition of endothelial receptor expression and of T-cell ligand activity by mycophenolate mofetil. Transpl Immunol. 1998;6:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Ponticelli C. The pros and the cons of mTOR inhibitors in kidney transplantation. Expert Rev Clin Immunol. 2014;10:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Bredewold OW, van Oeveren-Rietdijk AM, Florijn B, Rotmans JI, de Fijter JW, van Kooten C, van Zonneveld AJ, de Boer HC. Conversion from calcineurin inhibitors to belatacept-based immunosuppressive therapy skews terminal proliferation of non-classical monocytes and lowers lymphocyte counts. Transpl Immunol. 2024;82:101976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Yakubu I, Moinuddin I, Gupta G. Use of belatacept in kidney transplantation: what's new? Curr Opin Organ Transplant. 2023;28:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Durrbach A, Pestana JM, Florman S, Del Carmen Rial M, Rostaing L, Kuypers D, Matas A, Wekerle T, Polinsky M, Meier-Kriesche HU, Munier S, Grinyó JM. Long-Term Outcomes in Belatacept- Versus Cyclosporine-Treated Recipients of Extended Criteria Donor Kidneys: Final Results From BENEFIT-EXT, a Phase III Randomized Study. Am J Transplant. 2016;16:3192-3201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 122. | Cicora F, Paz M, Mos F, Roberti J. Use of belatacept as alternative immunosuppression in three renal transplant patients with de novo drug-induced thrombotic microangiopathy. Case Rep Med. 2013;2013:260254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Malinoski D, Saunders C, Swain S, Groat T, Wood PR, Reese J, Nelson R, Prinz J, Kishish K, Van De Walker C, Geraghty PJ, Broglio K, Niemann CU. Hypothermia or Machine Perfusion in Kidney Donors. N Engl J Med. 2023;388:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 124. | Phillips BL, Ibrahim M, Greenhall GHB, Mumford L, Dorling A, Callaghan CJ. Effect of delayed graft function on longer-term outcomes after kidney transplantation from donation after circulatory death donors in the United Kingdom: A national cohort study. Am J Transplant. 2021;21:3346-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 125. | Ponticelli C, Reggiani F, Moroni G. Delayed Graft Function in Kidney Transplant: Risk Factors, Consequences and Prevention Strategies. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 126. | Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. 2019;3:CD011671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 127. | Langewisch E, Mannon RB. Chronic Allograft Injury. Clin J Am Soc Nephrol. 2021;16:1723-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 128. | Lefaucheur C, Viglietti D, Bouatou Y, Philippe A, Pievani D, Aubert O, Duong Van Huyen JP, Taupin JL, Glotz D, Legendre C, Loupy A, Halloran PF, Dragun D. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019;96:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 129. | Konvalinka A, Tinckam K. Utility of HLA Antibody Testing in Kidney Transplantation. J Am Soc Nephrol. 2015;26:1489-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 130. | Guidicelli G, Guerville F, Lepreux S, Wiebe C, Thaunat O, Dubois V, Visentin J, Bachelet T, Morelon E, Nickerson P, Merville P, Taupin JL, Couzi L. Non-Complement-Binding De Novo Donor-Specific Anti-HLA Antibodies and Kidney Allograft Survival. J Am Soc Nephrol. 2016;27:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 131. | Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 132. | Delville M, Lamarthée B, Pagie S, See SB, Rabant M, Burger C, Gatault P, Giral M, Thaunat O, Arzouk N, Hertig A, Hazzan M, Matignon M, Mariat C, Caillard S, Kamar N, Sayegh J, Westeel PF, Garrouste C, Ladrière M, Vuiblet V, Rivalan J, Merville P, Bertrand D, Le Moine A, Duong Van Huyen JP, Cesbron A, Cagnard N, Alibeu O, Satchell SC, Legendre C, Zorn E, Taupin JL, Charreau B, Anglicheau D. Early Acute Microvascular Kidney Transplant Rejection in the Absence of Anti-HLA Antibodies Is Associated with Preformed IgG Antibodies against Diverse Glomerular Endothelial Cell Antigens. J Am Soc Nephrol. 2019;30:692-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 133. | Loupy A, Vernerey D, Viglietti D, Aubert O, Duong Van Huyen JP, Empana JP, Bruneval P, Glotz D, Legendre C, Jouven X, Lefaucheur C. Determinants and Outcomes of Accelerated Arteriosclerosis: Major Impact of Circulating Antibodies. Circ Res. 2015;117:470-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 134. | Lahmer T, Hermans R, Schmaderer C, Chang J, Stock K, Lutz J, Heemann U, Baumann M. Mineralocorticoid receptor antagonism and aldosterone synthesis inhibition do not improve glomerulosclerosis and renal interstitial fibrosis in a model of chronic kidney allograft injury. Kidney Blood Press Res. 2012;35:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Garg N, Samaniego MD, Clark D, Djamali A. Defining the phenotype of antibody-mediated rejection in kidney transplantation: Advances in diagnosis of antibody injury. Transplant Rev (Orlando). 2017;31:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 136. | Selby NM, McIntyre CW. The vicious cycle of dialysis-induced cardiac injury--are dynamic changes in diastolic function involved? Am J Kidney Dis. 2013;62:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 137. | Skinner SC, Derebail VK, Poulton CJ, Bunch DC, Roy-Chaudhury P, Key NS. Hemodialysis-Related Complement and Contact Pathway Activation and Cardiovascular Risk: A Narrative Review. Kidney Med. 2021;3:607-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 138. | Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23:1900-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 139. | Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid Med Cell Longev. 2017;2017:3081856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 140. | Chung AW, Yang HH, Kim JM, Sigrist MK, Brin G, Chum E, Gourlay WA, Levin A. Arterial stiffness and functional properties in chronic kidney disease patients on different dialysis modalities: an exploratory study. Nephrol Dial Transplant. 2010;25:4031-4041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 141. | Lee MJ, Han SH, Lee JE, Choi HY, Yoon CY, Kim EJ, Han JH, Han JS, Oh HJ, Park JT, Kang SW, Yoo TH. Endothelial dysfunction is associated with major adverse cardiovascular events in peritoneal dialysis patients. Medicine (Baltimore). 2014;93:e73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 142. | McIntyre CW. Update on Hemodialysis-Induced Multiorgan Ischemia: Brains and Beyond. J Am Soc Nephrol. 2024;35:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 143. | Bellien J, Fréguin-Bouilland C, Joannidès R, Hanoy M, Rémy-Jouet I, Monteil C, Iacob M, Martin L, Renet S, Vendeville C, Godin M, Thuillez C, Le Roy F. High-efficiency on-line haemodiafiltration improves conduit artery endothelial function compared with high-flux haemodialysis in end-stage renal disease patients. Nephrol Dial Transplant. 2014;29:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 144. | Inamdar N, Tomer S, Kalmath S, Bansal A, Yadav AK, Sharma V, Bahuguna P, Gorsi U, Arora S, Lal A, Kumar V, Rathi M, Kohli HS, Gupta KL, Ramachandran R. Reversal of endothelial dysfunction post-immunosuppressive therapy in adult-onset podocytopathy and primary membranous nephropathy. Atherosclerosis. 2020;295:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 145. | Diamond JR, Karnovsky MJ. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988;33:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 229] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 146. | Shoji J, Mii A, Terasaki M, Shimizu A. Update on Recurrent Focal Segmental Glomerulosclerosis in Kidney Transplantation. Nephron. 2020;144 Suppl 1:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Corban MT, Prasad A, Nesbitt L, Loeffler D, Herrmann J, Lerman LO, Lerman A. Local Production of Soluble Urokinase Plasminogen Activator Receptor and Plasminogen Activator Inhibitor-1 in the Coronary Circulation Is Associated With Coronary Endothelial Dysfunction in Humans. J Am Heart Assoc. 2018;7:e009881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 148. | Roca N, Jatem E, Martín ML, Muñoz M, Molina M, Martínez C, Segarra A. Relationship between soluble urokinase-type plasminogen activator receptor and serum biomarkers of endothelial activation in patients with idiopathic nephrotic syndrome. Clin Kidney J. 2021;14:543-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 149. | Abuzeineh M, Aala A, Alasfar S, Alachkar N. Angiotensin II receptor 1 antibodies associate with post-transplant focal segmental glomerulosclerosis and proteinuria. BMC Nephrol. 2020;21:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | Pietrzak-Nowacka M, Safranow K, Płońska-Gościniak E, Nowacki A, Późniak P, Gutowski P, Ciechanowski K. Cardiovascular Involvement in Patients with Autosomal Dominant Polycystic Kidney Disease: A Review. Kidney Blood Press Res. 2024;49:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 151. | Sagar PS, Rangan GK. Cardiovascular Manifestations and Management in ADPKD. Kidney Int Rep. 2023;8:1924-1940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 152. | Blasco M, Guillén-Olmos E, Diaz-Ricart M, Palomo M. Complement Mediated Endothelial Damage in Thrombotic Microangiopathies. Front Med (Lausanne). 2022;9:811504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 153. | Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, Bedrosian CL, Faas SJ. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125:3253-3262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 154. | Norby GE, Leivestad T, Mjøen G, Hartmann A, Midtvedt K, Gran JT, Holdaas H. Premature cardiovascular disease in patients with systemic lupus erythematosus influences survival after renal transplantation. Arthritis Rheum. 2011;63:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 155. | Mak A, Chan JKY. Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nat Rev Rheumatol. 2022;18:286-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 156. | Zhai Y, Liu Y, Qi Y, Long X, Gao J, Yao X, Chen Y, Wang X, Lu S, Zhao Z. The soluble VEGF receptor sFlt-1 contributes to endothelial dysfunction in IgA nephropathy. PLoS One. 2020;15:e0234492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 157. | Halbwachs L, Lesavre P. Endothelium-neutrophil interactions in ANCA-associated diseases. J Am Soc Nephrol. 2012;23:1449-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 158. | Kaneyama K, Kobayashi H, Yamataka A, Lane GJ, Miyano T. Serum soluble vascular cell adhesion molecule-1 (VCAM-1) concentrations in children with reflux nephropathy. Pediatr Surg Int. 2005;21:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 159. | Dogra GK, Watts GF, Herrmann S, Thomas MA, Irish AB. Statin therapy improves brachial artery endothelial function in nephrotic syndrome. Kidney Int. 2002;62:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 160. | Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutiérrez OM, Bansal V, Rosas SE, Nigwekar S, Yee J, Kramer H. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2017;70:737-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 161. | Sprague SM, Martin KJ, Coyne DW. Phosphate Balance and CKD-Mineral Bone Disease. Kidney Int Rep. 2021;6:2049-2058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 162. | Haarhaus M, Evenepoel P; European Renal Osteodystrophy (EUROD) workgroup; Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD) working group of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA). Differentiating the causes of adynamic bone in advanced chronic kidney disease informs osteoporosis treatment. Kidney Int. 2021;100:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 163. | Baia LC, Heilberg IP, Navis G, de Borst MH; NIGRAM investigators. Phosphate and FGF-23 homeostasis after kidney transplantation. Nat Rev Nephrol. 2015;11:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 164. | Yadav AK, Tiwana S, Steel M, Ramachandran R, Kaski JC, Jha V, Banerjee D. Vitamin D deficiency, endothelial function and bone biomarkers in post-kidney transplantation patients from North India. Int Urol Nephrol. 2019;51:181-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 165. | Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018;19: 285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 166. | Thongprayoon C, Neyra JA, Hansrivijit P, Medaura J, Leeaphorn N, Davis PW, Kaewput W, Bathini T, Salim SA, Chewcharat A, Aeddula NR, Vallabhajosyula S, Mao MA, Cheungpasitporn W. Serum Klotho in Living Kidney Donors and Kidney Transplant Recipients: A Meta-Analysis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 167. | de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 168. | Theodoridis X, Chourdakis M, Papaemmanouil A, Chaloulakou S, Georgakou AV, Chatzis G, Triantafyllou A. The Effect of Diet on Vascular Aging: A Narrative Review of the Available Literature. Life (Basel). 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 169. | Gomes-Neto AW, Osté MCJ, Sotomayor CG, van den Berg E, Geleijnse JM, Berger SP, Gans ROB, Bakker SJL, Navis GJ. Mediterranean Style Diet and Kidney Function Loss in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2020;15:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 170. | Li J, Chong A, Carey S. Dietary interventions on the prevention and management of diabetes in post-kidney transplantation - A systematic review. Nephrology (Carlton). 2022;27:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 171. | de Vries LV, Dobrowolski LC, van den Bosch JJ, Riphagen IJ, Krediet CT, Bemelman FJ, Bakker SJ, Navis G. Effects of Dietary Sodium Restriction in Kidney Transplant Recipients Treated With Renin-Angiotensin-Aldosterone System Blockade: A Randomized Clinical Trial. Am J Kidney Dis. 2016;67:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 172. | Osté MCJ, Gomes-Neto AW, Corpeleijn E, Gans ROB, de Borst MH, van den Berg E, Soedamah-Muthu SS, Kromhout D, Navis GJ, Bakker SJL. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am J Transplant. 2018;18:2523-2533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 173. | Cupisti A, Ghiadoni L, D'Alessandro C, Kardasz I, Morelli E, Panichi V, Locati D, Morandi S, Saba A, Barsotti G, Taddei S, Arnoldi A, Salvetti A. Soy protein diet improves endothelial dysfunction in renal transplant patients. Nephrol Dial Transplant. 2007;22:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 174. | Pihlstrøm HK, Mjøen G, Mucha S, Franke A, Jardine A, Fellström B, Dahle DO, Holdaas H, Melum E. Genetic markers associated with long-term cardiovascular outcome in kidney transplant recipients. Am J Transplant. 2019;19:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 175. | Shaked A, Loza BL, Van Loon E, Olthoff KM, Guan W, Jacobson PA, Zhu A, Fishman CE, Gao H, Oetting WS, Israni AK, Testa G, Trotter J, Klintmalm G, Naesens M, Asrani SK, Keating BJ. Donor and recipient polygenic risk scores influence the risk of post-transplant diabetes. Nat Med. 2022;28:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 176. | Uyar M, Sezer S, Ozdemir FN, Kulah E, Arat Z, Atac FB, Haberal M. Endothelial nitric oxide synthase polymorphism influences renal allograft outcome. Clin Transplant. 2014;28:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 177. | Nobakht E, Jagadeesan M, Paul R, Bromberg J, Dadgar S. Precision Medicine in Kidney Transplantation: Just Hype or a Realistic Hope? Transplant Direct. 2021;7:e650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Leite AR, Borges-Canha M, Cardoso R, Neves JS, Castro-Ferreira R, Leite-Moreira A. Novel Biomarkers for Evaluation of Endothelial Dysfunction. Angiology. 2020;71:397-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 179. | Gorog DA, Storey RF, Gurbel PA, Tantry US, Berger JS, Chan MY, Duerschmied D, Smyth SS, Parker WAE, Ajjan RA, Vilahur G, Badimon L, Berg JMT, Cate HT, Peyvandi F, Wang TT, Becker RC. Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol. 2022;19:475-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 202] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 180. | Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, Neil D, Hoefer IE, Fragiadaki M, Waltenberger J, Weber C, Bochaton-Piallat ML, Bäck M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 181. | Gyöngyösi M, Alcaide P, Asselbergs FW, Brundel BJJM, Camici GG, Martins PDC, Ferdinandy P, Fontana M, Girao H, Gnecchi M, Gollmann-Tepeköylü C, Kleinbongard P, Krieg T, Madonna R, Paillard M, Pantazis A, Perrino C, Pesce M, Schiattarella GG, Sluijter JPG, Steffens S, Tschöpe C, Van Linthout S, Davidson SM. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc Res. 2023;119:336-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 182. | Xu SW, Ilyas I, Weng JP. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44:695-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 249] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 183. | Jaime Garcia D, Chagnot A, Wardlaw JM, Montagne A. A Scoping Review on Biomarkers of Endothelial Dysfunction in Small Vessel Disease: Molecular Insights from Human Studies. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 184. | Chia PY, Teo A, Yeo TW. Overview of the Assessment of Endothelial Function in Humans. Front Med (Lausanne). 2020;7:542567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 185. | Alexander Y, Osto E, Schmidt-Trucksäss A, Shechter M, Trifunovic D, Duncker DJ, Aboyans V, Bäck M, Badimon L, Cosentino F, De Carlo M, Dorobantu M, Harrison DG, Guzik TJ, Hoefer I, Morris PD, Norata GD, Suades R, Taddei S, Vilahur G, Waltenberger J, Weber C, Wilkinson F, Bochaton-Piallat ML, Evans PC. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc Res. 2021;117:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 186. | Tamargo IA, Baek KI, Kim Y, Park C, Jo H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat Rev Cardiol. 2023;20:738-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 166] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 187. | Zhou HL, Jiang XZ, Ventikos Y. Role of blood flow in endothelial functionality: a review. Front Cell Dev Biol. 2023;11:1259280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 188. | Samuels BA, Shah SM, Widmer RJ, Kobayashi Y, Miner SES, Taqueti VR, Jeremias A, Albadri A, Blair JA, Kearney KE, Wei J, Park K, Barseghian El-Farra A, Holoshitz N, Janaszek KB, Kesarwani M, Lerman A, Prasad M, Quesada O, Reynolds HR, Savage MP, Smilowitz NR, Sutton NR, Sweeny JM, Toleva O, Henry TD, Moses JW, Fearon WF, Tremmel JA; Microvascular Network (MVN). Comprehensive Management of ANOCA, Part 1-Definition, Patient Population, and Diagnosis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2023;82:1245-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 189. | Rocco E, Grimaldi MC, Maino A, Cappannoli L, Pedicino D, Liuzzo G, Biasucci LM. Advances and Challenges in Biomarkers Use for Coronary Microvascular Dysfunction: From Bench to Clinical Practice. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 190. | Mathew RC, Bourque JM, Salerno M, Kramer CM. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc Imaging. 2020;13:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 191. | Slomka P, Berman DS, Alexanderson E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging. 2014;2:343-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 192. | Hanssen H, Streese L, Vilser W. Retinal vessel diameters and function in cardiovascular risk and disease. Prog Retin Eye Res. 2022;91:101095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 193. | Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1463] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 194. | Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 760] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 195. | Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 555] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 196. | Wenceslau CF, McCarthy CG, Earley S, England SK, Filosa JA, Goulopoulou S, Gutterman DD, Isakson BE, Kanagy NL, Martinez-Lemus LA, Sonkusare SK, Thakore P, Trask AJ, Watts SW, Webb RC. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am J Physiol Heart Circ Physiol. 2021;321:H77-H111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 197. | Poredos P, Jezovnik MK. Testing endothelial function and its clinical relevance. J Atheroscler Thromb. 2013;20:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 198. | Maruhashi T, Kajikawa M, Kishimoto S, Hashimoto H, Takaeko Y, Yamaji T, Harada T, Han Y, Aibara Y, Mohamad Yusoff F, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Tomiyama H, Takase B, Kohro T, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A, Higashi Y. Diagnostic Criteria of Flow-Mediated Vasodilation for Normal Endothelial Function and Nitroglycerin-Induced Vasodilation for Normal Vascular Smooth Muscle Function of the Brachial Artery. J Am Heart Assoc. 2020;9:e013915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 199. | Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L. Reproducibility of different methods to measure the endothelial function. Vasc Med. 2012;17:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 200. | Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 431] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 201. | Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 635] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 202. | Faita F, Masi S, Loukogeorgakis S, Gemignani V, Okorie M, Bianchini E, Charakida M, Demi M, Ghiadoni L, Deanfield JE. Comparison of two automatic methods for the assessment of brachial artery flow-mediated dilation. J Hypertens. 2011;29:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Heiss C, Rodriguez-Mateos A, Bapir M, Skene SS, Sies H, Kelm M. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc Res. 2023;119:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 204. | Akamatsu D, Sato A, Goto H, Watanabe T, Hashimoto M, Shimizu T, Sugawara H, Sato H, Nakano Y, Miura T, Zukeran T, Serizawa F, Hamada Y, Tsuchida K, Tsuji I, Satomi S. Nitroglycerin-mediated vasodilatation of the brachial artery may predict long-term cardiovascular events irrespective of the presence of atherosclerotic disease. J Atheroscler Thromb. 2010;17:1266-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 205. | Kajikawa M, Maruhashi T, Hida E, Iwamoto Y, Matsumoto T, Iwamoto A, Oda N, Kishimoto S, Matsui S, Hidaka T, Kihara Y, Chayama K, Goto C, Aibara Y, Nakashima A, Noma K, Higashi Y. Combination of Flow-Mediated Vasodilation and Nitroglycerine-Induced Vasodilation Is More Effective for Prediction of Cardiovascular Events. Hypertension. 2016;67:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 206. | Theodorakopoulou MP, Schoina M, Sarafidis P. Assessment of Endothelial and Microvascular Function in CKD: Older and Newer Techniques, Associated Risk Factors, and Relations with Outcomes. Am J Nephrol. 2020;51:931-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 207. | Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985). 2006;101:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 208. | Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 209. | Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I, Kremastinos D, Landmesser U, Protogerou A, Stefanadis C, Tousoulis D, Vassalli G, Vink H, Werner N, Wilkinson I, Vlachopoulos C. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 210. | Schnall RP, Sheffy JK, Penzel T. Peripheral arterial tonometry-PAT technology. Sleep Med Rev. 2022;61:101566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 211. | Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A, de Jong PT. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension. 2006;47:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 250] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 212. | Heitmar R, Lip GYH, Ryder RE, Blann AD. Retinal vessel diameters and reactivity in diabetes mellitus and/or cardiovascular disease. Cardiovasc Diabetol. 2017;16:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 213. | Caglar K, Yilmaz MI, Saglam M, Cakir E, Kilic S, Eyileten T, Sonmez A, Oguz Y, Oner K, Ors F, Vural A, Yenicesu M. Endothelial dysfunction and fetuin A levels before and after kidney transplantation. Transplantation. 2007;83:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 214. | Yilmaz MI, Saglam M, Caglar K, Cakir E, Ozgurtas T, Sonmez A, Eyileten T, Yenicesu M, Acikel C, Oguz Y, Ozcan O, Bozlar U, Erbil K, Aslan I, Vural A. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation. 2005;80:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 215. | Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease--a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 216. | Junarta J, Hojs N, Ramphul R, Lowe-Jones R, Kaski JC, Banerjee D. Progression of endothelial dysfunction, atherosclerosis, and arterial stiffness in stable kidney transplant patients: a pilot study. BMC Cardiovasc Disord. 2020;20:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 217. | Langberg NE, Jenssen TG, Haugen AJ, Mjøen G, Birkeland KI, Åsberg A, Hartmann A, Dahle DO. Endothelial Dysfunction and 6-Year Risk of Mortality in Kidney Transplant Recipients. Transplant Direct. 2022;8:e1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 218. | Päivärinta J, Metsärinne K, Löyttyniemi E, Teuho J, Tolvanen T, Knuuti J, Koivuviita N. Myocardial perfusion reserve of kidney transplant patients is well preserved. EJNMMI Res. 2020;10:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 219. | Zbroch E, Małyszko J, Małyszko J, Koc-Żórawska E, Myśliwiec M. Renalase, kidney function, and markers of endothelial dysfunction in renal transplant recipients. Pol Arch Med Wewn. 2012;122:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 220. | Frenay AR, van den Berg E, de Borst MH, Beckmann B, Tsikas D, Feelisch M, Navis G, Bakker SJ, van Goor H. Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino Acids. 2015;47:1941-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/