Published online Mar 18, 2025. doi: 10.5500/wjt.v15.i1.95899
Revised: October 4, 2024
Accepted: November 4, 2024
Published online: March 18, 2025
Processing time: 219 Days and 17.5 Hours
Liver transplantation aims to increase the survival of patients with end-stage liver diseases and improve their quality of life. The number of organs available for transplantation is lower than the demand. To provide fair organ distribution, predictive mortality scores have been developed.
To compare the Acute Physiology and Chronic Health Evaluation IV (APACHE IV), balance of risk (BAR), and model for end-stage liver disease (MELD) scores as predictors of mortality.
Retrospective cohort study, which included 283 adult patients in the post
The transplant recipients were mainly male, with a mean age of 58.1 years. Donors were mostly male, with a mean age of 41.6 years. The median cold ische
The APACHE IV assessment score was more accurate than BAR and MELD in predicting mortality in deceased donor liver transplant recipients.
Core Tip: The organ allocation policy has been improving in recent decades, with the utilization of models and scales that reduce mortality among patients on the waiting list and enable a better assessment of post-transplant prognosis with appropriate donor-recipient matching. This article aims to compare the Acute Physiology and Chronic Health Evaluation IV, balance of risk, and model for end-stage liver disease scores as predictors of mortality.
- Citation: Hohenreuther R, Silveira AT, Filho EMR, Garcez A, Lacerda BG, Fernandes SA, Marroni CA. Physiology and health assessment, risk balance, and model for end-stage liver disease scores: Postoperative outcome of liver transplantation. World J Transplant 2025; 15(1): 95899
- URL: https://www.wjgnet.com/2220-3230/full/v15/i1/95899.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i1.95899
Brazil is one of the leading countries in transplantation in the world, ranking 2nd in absolute numbers of liver transplants among 35 countries, behind the United States, in the year 2019[1]. Nevertheless, the number of patients awaiting liver transplantation remains high, with 2688 adults on the waiting list in 2020, of whom 646 (24%), a significant number, died[1]. The organ allocation policy has been improving in recent decades, with the utilization of models and scales that reduce mortality among patients on the waiting list and enable a better assessment of post-transplant prognosis with appropriate donor-recipient matching. To distribute the organs offered fairly, considering the severity of the patient, scores have been developed to significantly predict mortality.
In 2006, Brazil adopted the model for end-stage liver disease (MELD) score as the scoring system for allocating candidates on the liver transplant waiting list, associating it with ABO system compatibility and the size of the organ to the recipient. Adopting the MELD score changed the priority criteria for allocation, which used to be based on the date of entry on the list, to considering the severity of the patient with objective criteria. The scale was developed in the United States and has been employed worldwide. The Score ranges from 6 to 40[2] in a scoring system that assesses the severity of cirrhosis and predicts survival in 90 days[3]. The Score is obtained through laboratory tests using a logarithmic formula, including international normalized ratio, total bilirubin, and creatinine[4]. The higher the MELD score, the worse the prognosis and the higher the risk of death. The MELD score underwent some modifications over time, such as the adaptation to MELD-Sodium (MELD-Na), where serum sodium is valued differently due to hyponatremia[3], used in the United States since 2016[5] and in Brazil since 2019[6]. Renal dysfunction is common in liver transplant candidates and significantly increases the risk of mortality, making careful management of renal function essential for optimizing transplant outcomes[7]. The search for improvements motivated researchers to develop other methods to predict mortality, some specific to liver transplantation in intensive care unit (ICU), such as the Acute Physiology and Chronic Health Evaluation (APACHE). The APACHE was developed by Knaus et al[8] through a study published in 1981 to assess mortality prediction in the ICU using data from the first 24 hours of admission. It has been updated over time, with the current version being IV, which considers 129 variables: Age, sex, admission, discharge or death dates, systolic and diastolic blood pressure, body temperature, heart rate, respiratory rate, blood glucose, urea, serum creatinine, hematocrit, white blood cells, albumin, and bilirubin levels, urinary output in the first 24 hours of ICU admission, pH, fraction of inspired oxygen, partial pressure of carbon dioxide, partial pressure of oxygen, bicarbonate, Glasgow Coma Scale, mechanical ventilation, diagnosis, admission information, and chronic patient condition[9].
Each item represents a value, and through a calculation, it results in an estimated percentage of mortality for the patient. Some studies demonstrate adequate correlation with prognosis and good calibration for predicting death[2,8,10-14]. The balance of risk (BAR) score was developed to find scores with good prognostic correlation and better calibration than MELD. The calculation is based on recipient data (MELD, retransplantation, life support, age, cold ischemia) and donor age. Each item is assigned a value from 0 to 27, where the higher value indicates a higher probability of death[15], calculated for one, three, and five years. A study published in Campinas/São Paulo (2015) evaluated the BAR score in a cohort, where the authors found moderate discrimination and adequate calibration in predicting mortality[16].
In an analysis conducted on the population of liver transplant recipients in the United States, using the United Network for Organ Sharing database by the University of Zurich, the superiority of the BAR system was demonstrated when compared to other predictive systems such as donor age MELD, donor risk index (DRI), and survival outcomes following liver transplantation (SOFT)[17]. The patient on the liver transplant waiting list is in a unique situation due to the certainty of the only available therapy for their survival, which is the allocation of a new organ[18] either from a deceased or living donor. The deceased donor must have brain death, meet legal criteria, and have family consent for donation[19]. The living donor must meet legal criteria, be a relative or have a court order, and the procedure should not (cannot) cause any harm to their health[19]. The liver has specificities in its transplantation, such as the cold ischemia time, which varies for deceased or living donors. Prolonged cold ischemia times are associated with a higher chance of liver injury[3,19]. The Score that classifies cirrhotic patients on the waiting list for transplantation can impact their prognosis and survival, which is why comparing the three studied methods can help predict the outcome and facilitate the choice of the best one.
Few studies address this topic, and the comparisons made in this work were not found in other research, which justifies the motivation and importance of this study and its conclusions.
This is a retrospective cohort study involving 283 adult recipients (above 18 years old) who underwent deceased donor liver transplantation (DDLT) at a referral center located in southern Brazil from January 1, 2014 to June 30, 2018. Only the first admission to the ICU within the same hospitalization was considered. The exclusion criteria included patients transplanted for severe acute liver failure, liver-kidney combined transplantation, retransplantation, and those for whom the required data for analysis were not available.
After the surgical procedure, patients were allocated to the transplant-specialized ICU. The data obtained in the ICU routine were stored in a spreadsheet using Microsoft Excel® (2010), and the SPSS® software (Statistical Package for the Social Sciences) was used[20]. The APACHE IV data were analyzed using the Epimed Monitor program[21], the BAR was calculated online[15] using data from the electronic medical records of the institution where the transplants took place, and the MELD was calculated using its original formula[17], since the data were obtained from the time of selection and within the stipulated time frame. The MELD and APACHE IV scores were routinely calculated in the hospital, while the BAR score was calculated based on the patient's medical records.
Some indices were not analyzed, such as the DRI and SOFT, due to missing data, which would make it impossible to perform a proper analysis of the patients. This work is part of a more extensive ongoing study that evaluates administrative aspects of patients admitted to the ICU of the Irmandade Santa Casa de Misericórdia of Porto Alegre-Brazil. The Research Ethics Committee approved it under the Brazil Platform, CAAE number 19687113.8.2001.5335. The present study complies with ethical norms and guidelines, including Resolution of the National Health Council No. 466/12[22], which exempts the application of the Informed Consent Form, as there is no exposure of personal data.
For data description, measures of central tendency and variability, including mean, standard deviation, median, and interquartile range, were used according to the distribution of the variables. The non-parametric Mann-Whitney test was used to assess differences in distribution between medians. For data description, descriptive statistics were used to calculate the mean and standard deviation or median and interquartile range according to the distribution of the variables. For categorical variables, absolute and relative frequencies were used, and the t-student test was applied to compare means.
To evaluate calibration (95%CI) and the ability to classify survivors and non-survivors (discharge and death in the ICU/hospital), respectively, receiver operating characteristic (ROC) curves were plotted and the respective areas under the curve (AUC) were calculated for APACHE IV, BAR, and MELD scores. For data analysis and comparison purposes, calibration is considered excellent when the AUC result is 0.9-0.99, very good (0.8-0.89), good (0.7-0.79), moderate (0.6-0.69), and poor (< 0.6)[10].
The survival curve to assess the probabilities of death over the follow-up period was constructed using the Kaplan-Meier method, with the P value calculated by the log-rank test. The sensitivity, specificity, and optimal cutoff point values, according to the percentage of correct classification, will be calculated later. The Hosmer-Lemeshow test will assess the degree of calibration (agreement between predicted and observed probability of death).
Data of 283 consecutive adult patients who underwent DDLT were analyzed after establishing the inclusion and exclusion criteria in their immediate postoperative period in the ICU (Table 1). The mean age of the recipients was 58.1 ± 9.4 years, with the majority being male (66.8%). The donors had a mean age of 41.6 ± 16.7 years, with the majority being male (68.2%). The mean cold ischemia time was 3.1 hours (2.4-3.8), and the median length of stay in the ICU after liver transplantation was 5 days (range: 3-7).
| Variables | n = 283 |
| Recipient age (years) | 58.1 ± 9.4 |
| Recipient gender | |
| Female | 94 (33.2) |
| Male | 189 (66.8) |
| Donor age (years) | 41.6 ± 16.7 |
| Donor gender | |
| Female | 90 (31.8) |
| Male | 193 (68.2) |
| Cold ischemia time (hours) | 3.1 (2.4-3.8) |
| ICU time (days) | 5 (3-7) |
| Transplant day MELD | 24.2 ± 5.3 |
| APACHE IV | 59.6 ± 23.4 |
| APACHE IV probability of death | 2.57 (1.51-4.78) |
| BAR score | 10.7 ± 3.2 |
| Death in 28 days | 27 (9.5) |
| Death in 90 days | 9/256 (3.5) |
The mean MELD score was 24.2 ± 5.3 points; the mean BAR score was 10.7 ± 3.2; for APACHE IV, the mean was 59.6, with a range of 23.4 points, and the median APACHE IV probability of death was 2.57 (1.51-4.78). There were 27 deaths within the first 28 days out of 283 patients (9.5%) and an additional 9 deaths out of 256 patients within 90 days (3.5%).
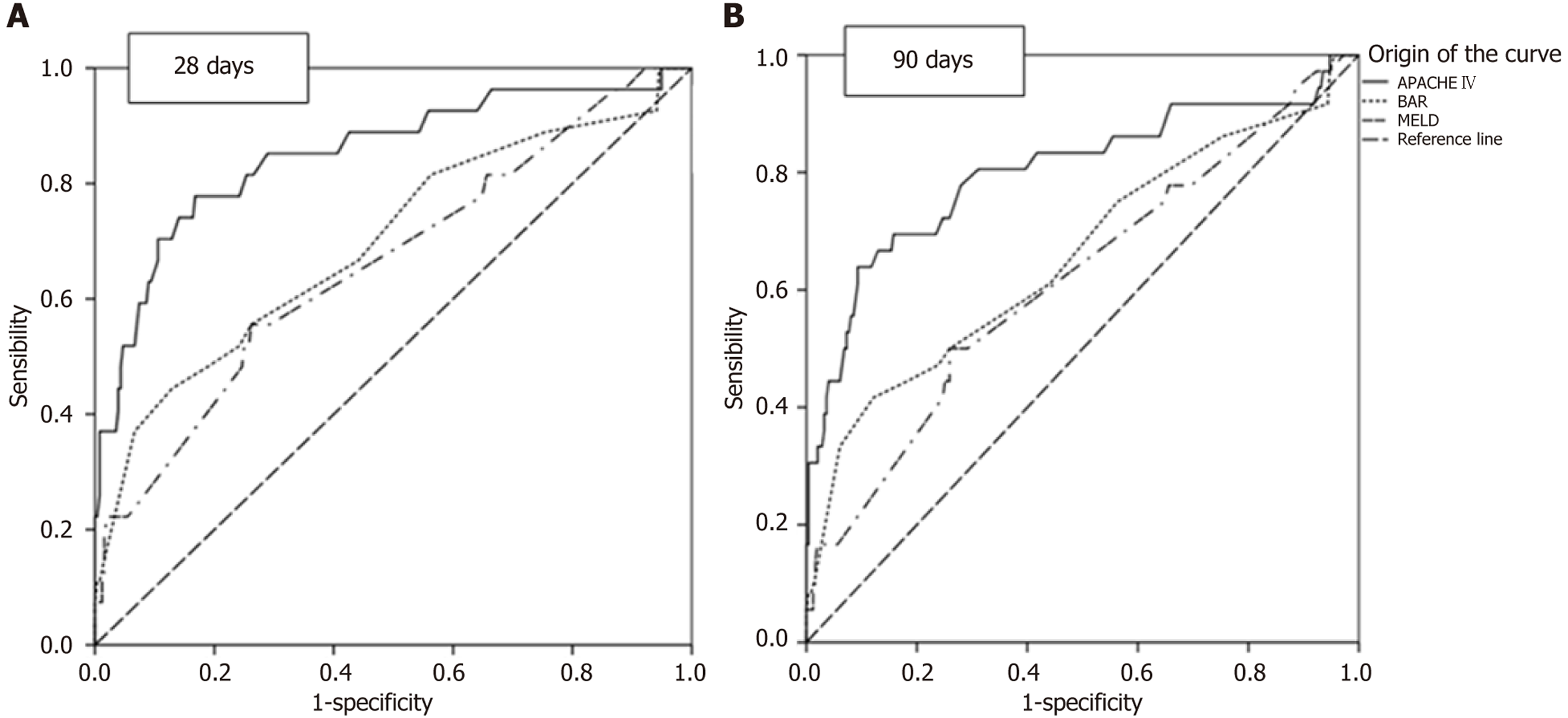
In Table 2, the survival rates up to the 5th year are demonstrated. The survival rates were 90.1% in the first month, 87.6% in the third month, and 82.5% in the first year. From there, the survival rate was stable up to the 5th year, with a value of 78.2%. Table 3 presents the AUC for 28 and 90 days after liver transplantation. Regarding 28 days, significant values for predicting death are demonstrated, with descending significance from APACHE IV (AUC: 0.85, P < 0.001, 95%CI: 0.76-0.94), to BAR (AUC: 0.70, P = 0.001, 95%CI: 0.58-0.81), and to MELD (AUC: 0.66, P = 0.006, 95%CI: 0.55-0.78).
| 1 month | 3 months | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | |
| Survival rate (%) | 90.1 | 87.6 | 85.9 | 82.5 | 79.6 | 78.2 | 78.2 | 78.2 |
| Variables | Death in 28 days | Death in 90 days | ||||||||||
| AUC | 95%CI | P value | Cutoff point | Sensitivity | Specificity | AUC | 95%CI | P value | Cutoff point | Sensitivity | Specificity | |
| APACHE IV | 0.85 | 0.76-0.94 | < 0.001 | 71.50 | 77.80% | 83.20% | 0.80 | 0.71-0.90 | < 0.001 | 64.50 | 77.80% | 72.10% |
| BAR score | 0.70 | 0.58-0.81 | 0.001 | 10.50 | 66.70% | 55.90% | 0.66 | 0.55-0.77 | 0.004 | 10.50 | 61.10% | 55.90% |
| MELD | 0.66 | 0.55-0.78 | 0.006 | 24 | 77.80% | 35.20% | 0.62 | 0.51-0.72 | 0.026 | 24 | 75.00% | 35.20% |
Also, a significant descending value is demonstrated for prediction at 90 days for the APACHE IV scale (AUC: 0.80, P < 0.001, 95%CI: 0.71-0.90), BAR (AUC: 0.66, P = 0.004, 95%CI: 0.55-0.77), and MELD (AUC: 0.62, P = 0.026, 95%CI: 0.51-0.72). ROC curves were used to compare the APACHE IV, BAR, and MELD scales to predict survival at 28 and 90 days, and the AUC were calculated (Figure 1). APACHE IV provided the most adequate prediction, followed by BAR and MELD.
The comparison of deaths and survivors at 28 days, 90 days, and overall deaths, as shown in Table 4, with variables including recipient age, ICU length of stay, cold ischemia time, APACHE IV scores (points and probability of death), BAR, and MELD, showed statistical significance for APACHE IV, BAR, and MELD at 28 days and overall mortality, but not for 90 days. The APACHE IV score demonstrated the highest discriminatory power.
| Variables | Death in 28 days | Death in 90 days | Death | ||||||
| Yes | No | P value | Yes | No | P value | Yes | No | P value | |
| Recipient's age | 59.2 ± 12.1 | 58.0 ± 9.1 | 0.604 | 62.4 ± 7.0 | 57.8 ± 9.2 | 0.135 | 59.3 ± 9.3 | 57.8 ± 9.5 | 0.275 |
| ICU stay time | 5 (3-8) | 5 (3-7) | 0.603 | 5 (4.5-18) | 5 (3-7) | 0.114 | 5 (3-10) | 5 (3-7) | 0.130 |
| Cold ischemia time | 3.3 (2.4-4.2) | 3.1 (2.4-3.8) | 0.634 | 2.9 (1.6-3.4) | 3.1 (2.4-3.8) | 0.208 | 3.3 (2.5-3.8) | 3.1 (2.4-3.8) | 0.571 |
| APACHE IV points | 92.0 ± 32.1 | 56.1 ± 19.4 | < 0.001 | 86.1 ± 32.5 | 55.7 ± 18.9 | < 0.001 | 76.5 ± 30.4 | 55.2 ± 19.0 | < 0.001 |
| APACHE IV prob death | 13.4 (4.6-26.9) | 2.4 (1.4-4.1) | < 0.001 | 11.6 (3.6-25.3) | 2.3 (1.4-3.9) | < 0.001 | 4.9 (2.2-18.7) | 2.3 (1.4-3.9) | < 0.001 |
| BAR | 13.2 ± 4.1 | 10.5 ± 2.9 | 0.002 | 12.7 ± 4.1 | 10.5 ± 2.9 | 0.003 | 11.8 ± 3.8 | 10.5 ± 2.9 | 0.016 |
| MELD | 28.3 ± 8.1 | 23.7 ± 4.7 | 0.008 | 27.1 ± 7.7 | 23.7 ± 4.7 | 0.016 | 26.1 ± 6.7 | 23.7 ± 4.8 | 0.013 |
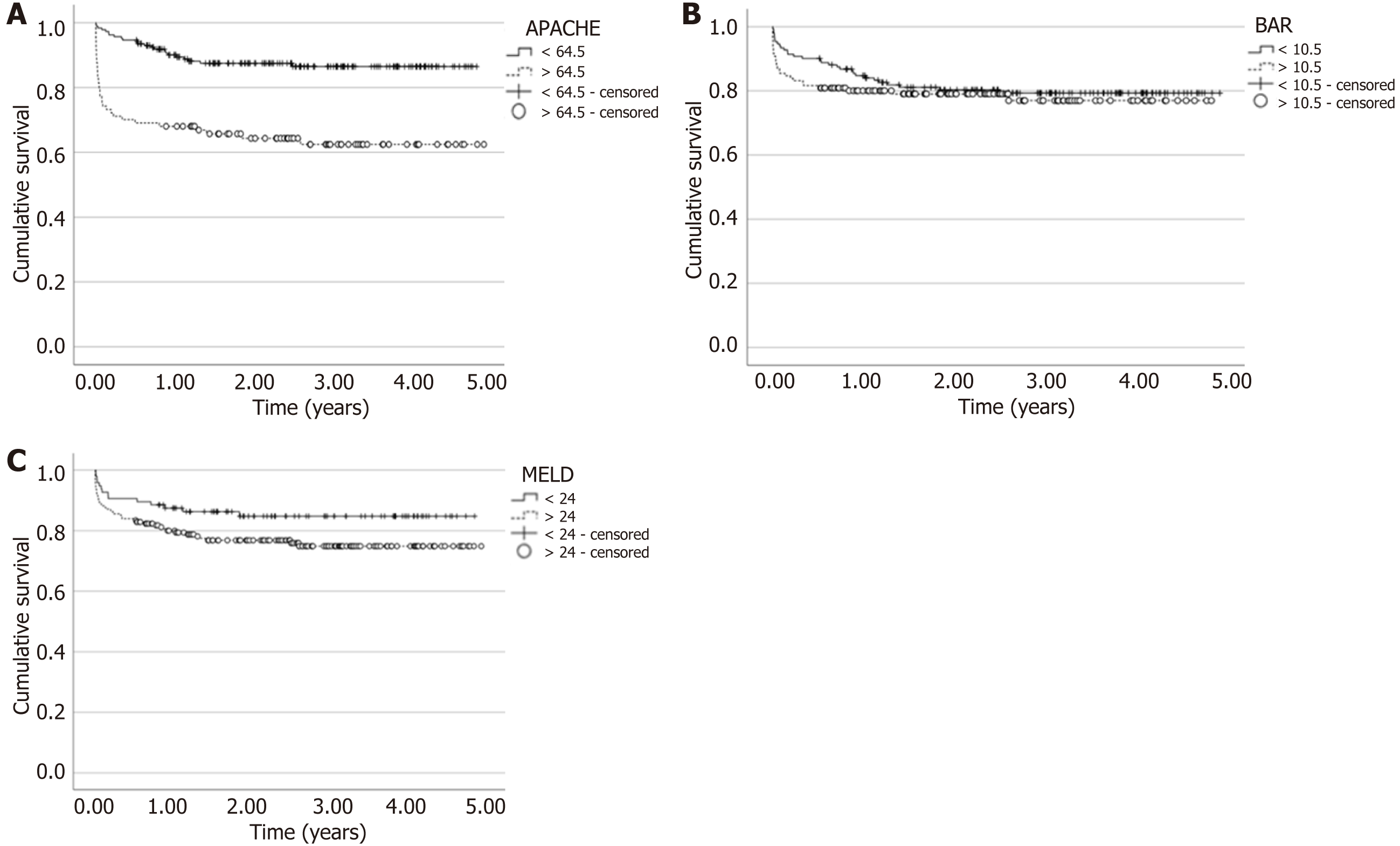
Analyzing which would be the best value for performing liver transplantation, as determined by the AUC for 90 days (Table 3), as it predicts death over a longer time frame, Figure 2A shows the difference in survival for each Score, considering APACHE 64.5 points. Through the log-rank test, P < 0.001 was found, indicating a significant difference in transplanting below 64.5, increasing the tendency towards survival. For BAR (Figure 2B), a score of 10.5 was used (P = 0.483), showing no significant difference in transplanting below this value. Finally, for MELD (Figure 2C), a value of 24 was used (P = 0.073), indicating a trend towards better survival when transplanting below 24, although not statistically significant.
The analysis of 283 Liver transplant patients regarding their demographic and clinical characteristics is shown in Table 1, with the mean age among them being 58.1 ± 9.4 years and the majority being male (66.8%), similar to the findings in other studies [2,16,23-26]. The mean age of the donors was 41.6 ± 16.7 years, with the majority being male (68.2%). This is similar to the findings reported by de Campos Junior et al[16] and Martínez et al[23], who also observed a mean age of 41.6 ± 13.2 years.
The mean cold ischemia time was 3.1 hours (2.4-3.8), which is shorter than the 6.9 hours reported by Victor Senna[24], 7.13 hours by Hu et al[2], and 7 hours by Martínez et al[23]. Possible explanations for the differences could be related to shorter distances traveled for organ procurement or the efficiency of the procurement team belonging to the same transplant center, which would streamline the procurement/transplant process[25]. The median length of stay in the ICU was 5 days (3-7), which agrees with the 5.6 days reported in the study by Lee et al[26] but is higher than the 3.71 days reported by Hu et al[2].
Regarding APACHE IV, the probability of death was 2.57%, with a mean score of 59.6 (± 23.4) for patients undergoing liver transplantation. As presented in Table 3, these values showed very good calibration for death at 28 and 90 days (AUC: 0.85, P < 0.001, 95%CI: 0.76-0.94) and (AUC: 0.80, P < 0.001, 95%CI: 0.71-0.90), respectively. The results of this study are consistent with other studies that also reported excellent calibrations for the outcome of death in patients undergoing liver transplantation[2,10,26]. According to the validation of using the APACHE IV score in patients undergoing liver transplantation by Rodrigues Filho EM, acceptable calibration was found to predict mortality in these patients[10,27].
The average score found for the BAR in our study was 10.7 ± 3.2 (Table 1), higher than the mean of 8 (4-14) reported by Martínez et al[23], considering the table available on the BAR scale website[15]. The score analysis establishes the perspective of one-year survival to be 90% for values between 5-8; for the 10.7 value found, it represents 86%, resulting in a difference of 4% between the two studies regarding the BAR score.
The mean MELD score was 24.2 ± 5.3 points (Table 1), similar to the study by Victor Senna[24], where the mean was 24.38. Other studies found lower mean MELD values, such as 16 for Volk et al[25], 18.09 for Hu et al[2], 20.16 for de Campos Junior et al[16], and 21 for Martínez et al[23]. When analyzing the MELD score concerning deaths at 28 days (Table 4), we found significant values of 28.3 ± 8.1 points for deaths and 23.7 ± 4.7 points for survivors (P = 0.008). At 90 days, there is also a statistical difference (P = 0.016).
When separately analyzing the MELD score for overall mortality (Table 4) between survivors and non-survivors, we found statistically significant differences, with 26.1 ± 6.7 for the deceased and 23.7 ± 4.8 for the survivors (P = 0.013). These results were similar to those of Lee et al[26] and Hu et al[2], who found a mean of 27 and 25.7 for non-survivors. As for survivors, they found a mean of 16 and 16.87, respectively. The differences among survivors may indicate that those in our study were more severe than those in the other studies.
Significant differences were also observed in all analyses between deceased and survivors for death at 28, 90 days, and overall mortality for the BAR and APACHE IV scores, as shown in Table 4. The survival rates of our patients at 1, 3, and 5 years were 82.5%, 78.2%, and 78.2% (Table 2). Deaths in the first 28 days were 27 (9.5%), and from there until 90 days, an additional 9 (3.5%) occurred. The data found by Volk et al[25] were similar in years 1 and 3, and worse in year 5, respectively, 83%, 75%, and 68%. It is observed that the highest mortality rate occurs in the immediate and early post-transplant periods, stabilizing after the third year. The numerical differences in the fifth year can be speculated and related to the etiology, presence or absence of hepatocellular carcinoma, greater long-term follow-up facilities, recurrences of the underlying disease, and treatment adherence.
To understand which of the three scores better predicts mortality, differences were observed in the prediction for 28 days, 90 days, and overall mortality. Other studies were similar[2,26,27], but there are no standardized criteria regarding the time frame for data evaluation, as in the study by Zakareya et al[28], which assessed the outcome at 3 months, 1 year, and 5 years. Others have relied solely on overall mortality[2,26].
A study conducted in Seoul by Lee et al[26] compared various mortality prediction scores (APACHE IV, Simplified Acute Physiology Score, APACHE II, MELD-Na, MELD, and Child-Turcotte-Pugh) post-liver transplantation and found 0.91 (95%CI: 0.86-0.96) for APACHE IV, which was the best Score for predicting mortality during in-hospital stay, which averaged 40 days for non-survivors. Another study conducted in China by Hu et al[2] found better calibration for APACHE IV 0.937 (95%CI: 0.892-0.981) compared to MELD 0.694 (95%CI: 0.51-0.817) during hospital stay. This study showed an APACHE IV of 0.85 (P < 0.001, 95%CI: 0.76-0.94) for death at 28 days, with very good calibration, similar to the results of the two studies, consistent with the outcome presented in Table 3, and a better mortality predictor when compared to BAR and MELD.
Regarding the 90-day period, Lee et al[26] found that APACHE IV had an AUC of 0.87 (95%CI: 0.79-0.95), indicating very good calibration, similar to what we found (Table 3), with an AUC of 0.80 (P < 0.001, 95%CI: 0.71-0.90). These findings for APACHE IV in both studies are better than those for the other scores.
The study conducted by Martínez et al[23], regarding BAR, found a mortality prediction of 0.755 (95%CI: 0.689-0.812), indicating good calibration in predicting mortality at 90 days. This study demonstrated an AUC of 0.66 (P = 0.004, 95%CI: 0.55-0.77) for the same time period, indicating moderate calibration despite showing statistical significance in distinguishing between those who died and those who did not.
According to Brandão et al[29], MELD showed an AUC of 0.60 (95%CI: 0.51-0.69) with moderate calibration at three months, similar to our study, where MELD had an AUC of 0.62 (P = 0.026, 95%CI: 0.51-0.72), also demonstrating moderate calibration but with statistical significance. In an integrative review conducted in 2015, it was concluded that the MELD system reduced mortality while waiting for an organ, but on its own, it does not appear to be a good predictor of mortality post-liver transplantation[30]. As for the APACHE IV, good calibration for predicting mortality was found. The comparison of APACHE IV, MELD, and BAR scores for predicting deaths at 28 days, 90 days, and overall mortality showed statistical significance concerning APACHE IV, BAR, and MELD at 28 days, 90 days, and overall mortality. The APACHE IV score demonstrated the highest discriminatory power.
Regarding the survival assessment, the calibration found (APACHE IV 64.5) was used for transplantation, with this being the only Score to show statistical significance (P < 0.001) by the log-rank test. In the study by de Campos Junior et al[16] for BAR, a calibration of 11 (P = 0.01) was found, unlike our study where, for a calibration of 10.5, the P value was equal to 0.483. Another study[31] found a calibration of 14 for BAR, obtaining a log-rank test with P = 0.42; whereas for Martínez et al[23], a value of 15 was verified with P < 0.01 for survival at three months, but their study includes both living and deceased donors. For APACHE IV and MELD, no studies with log-rank probability tests were found for adult transplanted patients. No current studies comparing APACHE IV, MELD, and BAR were found; therefore, there are no means to establish comparative analyses with results from other research[15].
In the present study, the APACHE IV score proved to be a better predictor of mortality than the BAR and MELD scores, accurately predicting mortality at 28 and 90 days and overall mortality. The performance of the APACHE IV score was very good at 28 days and good at 90 days. The performance of the BAR score was good at 28 days and poor at 90 days. And the performance of the MELD score was moderate at 28 days and poor at 90 days. However, for the data to be applied more broadly, further studies would be necessary, including analyses conducted at different transplant centers and with longer follow-up periods. This highlights a limitation of the study, as it is a retrospective research where some information was not filled out, and the surgical technique used, along with changes in renal function prior to tran
| 1. | Anual ABTO. Available from: https://site.abto.org.br/publicacao/xxvi-no-4-anual/. |
| 2. | Hu Y, Zhang X, Liu Y, Yan J, Li T, Hu A. APACHE IV is superior to MELD scoring system in predicting prognosis in patients after orthotopic liver transplantation. Clin Dev Immunol. 2013;2013:809847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Bittencourt PL, Zollinger CC, Lopes EP de A. Manual de cuidados intensivos em hepatologia. Barueri - SP. Available from: https://www.manole.com.br/. |
| 4. | Ministério da Saúde. BRASIL. Available from: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2009/prt2600_21_10_2009.html. |
| 5. | Mudanças na política de sódio sérico do MELD - OPTN. Available from: https://optn.transplant.hrsa.gov/news/meld-serum-sodium-policy-changes/. |
| 6. | Brasil. Portaria 2049. Available from: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2019/prt2049_12_08_2019.html. |
| 7. | O’leary J, Levitsky J, Wong F, Nadim M, Charlton M, Kim W. Protecting the Kidney in Liver Transplant Candidates: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant. 2016;16:2516-2531. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1445] [Article Influence: 32.1] [Reference Citation Analysis (33)] |
| 9. | Apache IV Score. Available from: https://intensivecarenetwork.com/Calculators/Files/Apache4.html. |
| 10. | Rodrigues Filho EM, Garcez A, Nedel WL. [Validation of APACHE IV score in postoperative liver transplantation in southern Brazil: a cohort study]. Braz J Anesthesiol. 2019;69:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Dahhan T, Jamil M, Al-tarifi A, Abouchala N, Kherallah M. Validation of the APACHE IV scoring system in patients with severe sepsis and comparison with the APACHE II system. Crit Care. 2009;13:P511. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Zimmerman JE, Kramer AA, Mcnair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297-1310. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1192] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 13. | Choi JW, Park YS, Lee YS, Park YH, Chung C, Park DI, Kwon IS, Lee JS, Min NE, Park JE, Yoo SH, Chon GR, Sul YH, Moon JY. The Ability of the Acute Physiology and Chronic Health Evaluation (APACHE) IV Score to Predict Mortality in a Single Tertiary Hospital. Korean J Crit Care Med. 2017;32:275-283. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Rodrigues-Filho EM, Garcez A. APACHE IV score in postoperative kidney transplantation. Rev Bras Ter Intensiva. 2018;30:181-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Online BAR Score Calculation. Available from: https://www.assessurgery.com/bar-score/bar-score-calculator/. |
| 16. | de Campos Junior ID, Stucchi RS, Udo EY, Boin Ide F. Application of the BAR score as a predictor of short- and long-term survival in liver transplantation patients. Hepatol Int. 2015;9:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, Geier A, Clavien PA. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745-53; discussion 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 18. | Ahmed A, Keeffe EB. Current indications and contraindications for liver transplantation. Clin Liver Dis. 2007;11:227-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Organ Donation: Transplants, Waiting Lists, and How to Become a Donor. Available from: https://antigo.saude.gov.br/saude-de-a-z/doacao-de-orgaos. |
| 20. | SPSS. Available from: https://bvsms.saude.gov.br/bvs/areas_tematicas/faq_transplantes.php. |
| 21. | Zampieri FG, Soares M, Borges LP, Salluh JIF, Ranzani OT. The Epimed Monitor ICU Database®: a cloud-based national registry for adult intensive care unit patients in Brazil. Rev Bras Ter Intensiva. 2017;29:418-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Conselho Nacional de Saúde. Available from: https://conselho.saude.gov.br/resolucoes/2012/Reso466.pdf. |
| 23. | Martínez JA, Pacheco S, Bachler JP, Jarufe N, Briceño E, Guerra JF, Benítez C, Wolff R, Barrera F, Arrese M. Accuracy of the BAR score in the prediction of survival after liver transplantation. Ann Hepatol. 2019;18:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Victor Senna D. Transplante de fígado no estado do Rio de Janeiro: análise retrospectiva do período 2013-2017. Αγαη. 2019;8. |
| 25. | Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Lee H, Yoon S, Oh SY, Shin J, Kim J, Jung CW, Ryu HG. Comparison of APACHE IV with APACHE II, SAPS 3, MELD, MELD-Na, and CTP scores in predicting mortality after liver transplantation. Sci Rep. 2017;7:10884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Ko M, Shim M, Lee SM, Kim Y, Yoon S. Performance of APACHE IV in Medical Intensive Care Unit Patients: Comparisons with APACHE II, SAPS 3, and MPM(0) III. Acute Crit Care. 2018;33:216-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Zakareya T, Taha M, Elzohry H, Darwiesh E, Aglan R, Elhelbawy M, Zakaria H, Deif M, Abbasy M. BAR Score Performance in Predicting Survival after Living Donor Liver Transplantation: A Single-Center Retrospective Study. Can J Gastroenterol Hepatol. 2022;2022:2877859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Brandão A, Fuchs SC, Gleisner AL, Marroni C, Zanotelli ML, Cantisani G; Liver Transplantation Group. MELD and other predictors of survival after liver transplantation. Clin Transplant. 2009;23:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Moraes ACO, Oliveira PC, Fonseca-Neto OCLD. The impact of the Meld score on allocation and outcome of liver transplantation: an review. Arq Bras Cir Dig. 2017;30:65-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Boecker J, Czigany Z, Bednarsch J, Amygdalos I, Meister F, Santana DAM, Liu WJ, Strnad P, Neumann UP, Lurje G. Potential value and limitations of different clinical scoring systems in the assessment of short- and long-term outcome following orthotopic liver transplantation. PLoS One. 2019;14:e0214221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/