Published online Dec 18, 2024. doi: 10.5500/wjt.v14.i4.98718
Revised: August 19, 2024
Accepted: August 23, 2024
Published online: December 18, 2024
Processing time: 78 Days and 17.2 Hours
Liver transplantation (LT) for metabolic dysfunction-associated steatotic liver disease (MASLD) is increasing globally due to rising rates of obesity and metabolic syndrome, posing significant challenges. MASLD patients typically present with advanced age, higher body mass index (BMI), and metabolic com
Core Tip: Managing liver transplantation (LT) in patients with metabolic dysfunction-associated steatotic liver disease (MASLD) presents unique challenges due to the high prevalence of obesity, diabetes, and metabolic syndrome. Pre-transplant evaluation should assess these factors to optimize patient selection and surgical outcomes. Intraoperative challenges, such as prolonged surgical times in obese MASLD patients, require careful management. Post-transplant monitoring for metabolic syndrome and cardiovascular complications is critical, as MASLD patients are at increased risk. Addressing steatosis recurrence through targeted metabolic management is crucial for long-term graft health and patient survival. A comprehensive, multidisciplinary approach is key to improving outcomes for MASLD recipients undergoing LT.
- Citation: Sato-Espinoza K, Chotiprasidhi P, Liza E, Placido-Damian Z, Diaz-Ferrer J. Evolution of liver transplantation in the metabolic dysfunction-associated steatotic liver disease era: Tracking impact through time. World J Transplant 2024; 14(4): 98718
- URL: https://www.wjgnet.com/2220-3230/full/v14/i4/98718.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i4.98718
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease, is currently the most common type of chronic liver disease, affecting around 30% of the global population[1]. MASLD’s development involves a combination of environmental, genetic, and metabolic factors. Due to its diverse causes, MASLD often remains undiagnosed or untreated, which can progress to more severe conditions, such as metabolic dysfunction-associated steatohepatitis (MASH), cirrhosis, and potentially, hepatocellular carcinoma (HCC), necessitating liver transplantation (LT).
According to the latest annual data report from the United States Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) in 2022[2], MASLD was the second most common indication for LT in non-HCC patients and the most common indication in HCC patients, with prevalence steadily increasing. Similar trends have been observed in Europe[3-5] and Latin America[6-9]. However, indications for LT due to viral hepatitis have declined over time, largely due to the widespread use of direct-acting antiviral agents (DAAs)[10], while indications related to cholestasis or autoimmune diseases have remained unchanged.
The rising prevalence of MASLD as the primary indication for LT has resulted in changes in the clinical characteristics of both donors and recipients, including a higher prevalence of metabolic disease, obesity, and associated comorbidities. This emphasizes the importance of accurately assessing MASLD and associating comorbidities to prevent further complications before, during, and after LT.
Controversial data still exist regarding short-term and long-term outcomes in patients who received LT due to MASLD. Therefore, this review aims to analyze the current literature on LT in the context of MASLD and discuss important considerations before, during, and after LT.
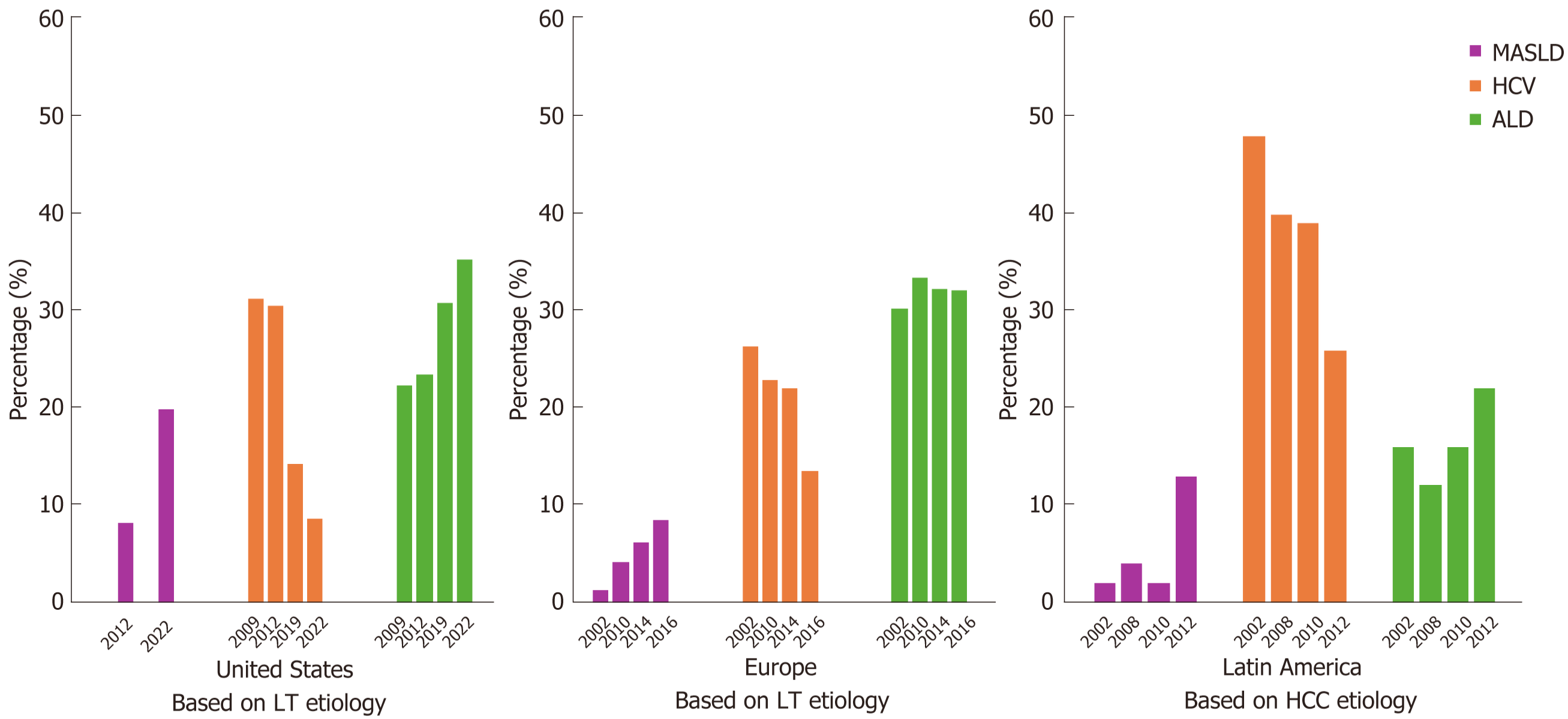
The etiologies for LT have evolved over the decades. Since 1980[11], hepatitis C virus (HCV) was the leading cause worldwide, but its prevalence began to steadily decline in the mid-2010s due to the widespread implementation of DAA therapies[10,12,13]. In the United States, the OPTN/SRTR reported a decrease in HCV-related LTs from 31.2% in 2009 to 8.6% in 2022[2]. A similar trend was observed in Europe, with HCV-related LTs decreasing from 26.4% in 2002 to 13.5% in 2016[3].
Conversely, MASLD has seen exponential growth in the 21st century, driven by the increasing prevalence of obesity and metabolic syndrome[14]. Currently, MASLD is the leading cause of LT in patients with HCC and the second most common cause in non-HCC patients. The OPTN/SRTR began attributing most cases previously classified as "other or unknown disease" to MASH in 2009, with this etiology steadily rising as an indication for LT[15]. The 2022 OPTN/SRTR report noted that MASLD began being reported as an individual etiology in 2012, accounting for 8.1% of LT etiologies, and had increased to 19.9% by 2022[2]. Similarly, the European Liver Transplant Registry database, which encompasses 33 countries, reported that LTs due to MASH increased from 1.2% in 2002 to 8.4% in 2016[3]. An Italian study also showed an increase in LTs due to MASH from 2.5% in 2006 to 23% in 2020[16]. In Canada, the prevalence of LT due to MASH increased from 0% in 2008 to 13.2% in 2018, with an annual increase of 1.2%[17].
In Latin America, reports regarding LT prevalence due to MASLD or MASH are limited, with some countries having no data. However, some studies indicate that the prevalence of MASLD in Brazil is 35.2%, Chile 23%, Mexico 17%, and Colombia 26.6%[6,18,19]. The high prevalence of MASLD in these populations contributes to a similar trend observed in western countries, where MASLD has become the leading indication for LT over the past decade.
The OPTN/SRTR also reported an increase in HCC as an indication for LT, rising from 3.6% in 2009 to 5.8% in 2012, and reaching 11.2% in 2022[2]. An Italian study found that HCC arising from MASH increased from 4% in 2006 to 30% in 2020[16].
Alcohol liver disease (ALD) remains an important etiology for LT. The OPTN/SRTR documented a steady increase in ALD-related LTs from 22.3% in 2009 to 35.3% in 2022[2]. In Europe, a similar trend was observed, with ALD-related LTs increasing from 30.2% in 2002 to 32.3% in 2016[3]. A single-center study in Italy found that ALD accounted for 30% of all LT registrations, maintaining a steady course and becoming the primary indication for transplantation over the past 5 years[16].
Acute liver failure, autoimmune diseases, and cholestasis have remained stable over the years in both the United States[2,15] and Europe[3].
The landscape of LT etiologies has shifted significantly over recent decades (Figure 1). While hepatitis C was once the leading cause, the advent of DAAs has reduced its prevalence. Conversely, the rise in obesity and metabolic syndrome rates has driven the increase in MASLD, which is now a leading indication for LT, especially in HCC patients. ALD continues to be a significant cause of LT. Understanding these trends is crucial for anticipating future healthcare needs and improving LT outcomes.
Patients waiting for LT due to MASLD exhibit similar metabolic disorder characteristics compared to those with other etiologies. These shared characteristic include older age[5,16,17,20-34], diabetes mellitus (DM)[5,20-22,28,30,32,34-36], hypertension (HTN)[5,20-22,26,28,30,31,36], dyslipidemia[5,20,28,30-32], coronary artery disease[20,21,28], chronic kidney disease (CKD)[20,28], obesity, and a higher body mass index (BMI)[5,16,17,20-22,24,27,30,31,33-36] (Table 1).
| Ref. | Population | Outcome | MASLD patient characteristics |
| Kennedy et al[20] | 904 patients: NASH 129, Non-NASH 775 | No difference in overall survival rate at 1, 3 and 5 years, but at 4 months was twice high in NASH | Older, white, higher BMI, higher MELD score, higher rate of HTN-DM, dyslipidemia, CKD, CAD, CVD |
| Danford et al[21] | 955 patients: NASH 74, Non-NASH 881 | No difference in overall survival rate at 5 years | Older, female, higher rate of HTN, DM, CVD, BMI, less rate of HCC |
| Piazza et al[22] | 143 patients: NASH 78, ALD 65 | No difference in cardiovascular events in 1 and 3 years | Older, female higher rate of HTN, DM, BMI |
| Kern et al[23] | 513 patients: NASH 65, ALD 183, HCV 116, Others 149 | No difference overall survival between groups at 1, 3 and 5 years. Higher postoperative complications in NASH | Older, high MELD and BMI, higher incidence of advance HCC |
| O’Neill et al[24] | 279 patients: NAFLD 84, ALD 195 | No difference in patient survival rate at 1, 3, 5 and 10 years and in graft survival | Older, higher BMI |
| Van Herck et al[5] | 232 patients: NAFLD 112, HCV 120 | No difference in overall survival rate at 1, 3, 5 and 10 years. Higher total cardiovascular morbidity in NAFLD patients | Older, higher MELD score, higher rate DM, dyslipidemia, HTN, metabolic syndrome, and BMI |
| Sourianarayanane et al[25] | 185 patients: NASH 77, ALD 108 | No difference in overall survival rate at 1 year | Older, female, less liver rejection episodes |
| Malik et al[26] | 686 patients: NASH 98, ALD 196, HCV 196, PBC/PSC 196 | Higher early mortality (30 days and 1 year). No difference in overall survival at 5 years | Older, female, higher BMI, DM, HTN |
| Reddy et al[27] | 214 patients: NASH 52, HCV/ALD 162 | No difference in overall survival rate at 90 days | Older, female, higher BMI and metabolic syndrome, lower MELD score |
| Kwong et al[28] | 1023 patients: NASH 207, viral hepatitis 395, ALD 198. Autoimmune or cholestatic disease 110. Unspecified/other 113 | No difference in overall survival rate at 1 and 3 years | Older, female, higher MELD scores, higher rate of DM, HTN, dyslipidemia, CAD, and CKD, less rate of HCC |
| Holzner et al[29] | 635 patients: NASH-HCC 51 Non-NASH HCC 584 | No difference in overall survival rate at 1, 3 and 5 years | Older, higher MELD score |
| Bhagat et al[30] | 154 patients: NASH 71, ALD 83 | No difference in overall survival rate at 1, 3, and 5 years | Higher rate of HTN, DM, dyslipidemia, BMI |
| Vanwagner et al[31] | 242 patients: NASH 115, ALD 127 | No difference in overall survival rate at 1, 3, and 5 years | Older, females, higher rate of HTN, dyslipidemia, BMI |
| Castelló et al[35] | 54 patients: NASH 18, ALD 36 | No difference in overall survival rate at 1, 3, and 5 years | Higher rate of DM, BMI, higher rate of HCC, less MELD score |
| Houlihan et al[36] | 96 patients: NASH 48, Non-NASH 48 | No difference in overall survival rate at 1 and 5 years. NASH group progress in higher rates to relevant kidney disease within 2 years | High rate of HTN, DM, and BMI |
| Unger et al[33] | 27 patients: NASH 15, Cryptogenic 12 | No difference in overall survival rate at 5 years | Older, higher BMI |
| Rajendran et al[17] | 20672 patients: NASH-HCC 2071, Non-NASH HCC 18601 | No difference in overall survival rate at 1, 3 and 5 years | Older, higher BMI and MELD score |
| Verna et al[34] | 4981 patients: NAFLD 538, Non-NAFLD 4443 | No difference in overall survival rate at 1, 3 and 5 years | Older, females higher rate of DM, higher BMI, and MELD score |
| Ferrarese et al[16] | 1491 patients: NASH 179, Non-NASH 1312 | No difference in overall survival rate at 1, 3 and 5 years | Older, higher BMI |
At the time of enlistment, MASLD patients present with more comorbidities, predisposing them to worse morbidity compared to non-MASLD patients. A Belgian study found that MASLD patients with metabolic disorders had a two-fold increased risk of cardiovascular events before LT compared to patients with hepatitis C[5]. Several studies have also reported that MASLD patients often undergo additional cardiac testing beyond echocardiography during pre-transplant evaluations[21,31]. A multicenter study indicated that MASLD patients underwent stress testing and left heart catheterization more frequently than non-MASLD patients[28]. Additionally, another study found that MASLD patients were more likely to have high vascular resistance[22]. A comparative study noted that although MASLD patients were less likely to have left ventricular hypertrophy, they were more likely to undergo cardiac catheterization[31]. MASLD patients were more likely to be on aspirin, statins, antidiabetic, and antihypertensive medications at the time of enlistment[31,37].
Regarding kidney health, some authors reported no differences in pre-LT estimated glomerular filtration rate (eGFR) between MASLD and non-MASLD patients[36,38]. However, another study found that obese patients had a lower mean eGFR at the time of enlistment[37].
MASLD patients were more likely to experience complications from cirrhosis, such as ascites, hepatic encephalopathy, variceal bleeding, and spontaneous bacterial peritonitis[28]. However, an Italian study found no differences in incidence of portal vein thrombosis and refractory ascites before LT[16].
Controversy exists regarding the baseline model for end-stage liver disease (MELD) score in MASLD patients. Several studies report that MASLD patients had higher MELD scores than non-MASLD patients[17,29,34]. However, a study in Spain found that MASLD patients had lower MELD scores[35], while other studies reported no difference in MELD scores between MASLD and non-MASLD patients[16,38].
A retrospective study showed that MASLD patients have a higher likelihood of being declined for LT list due to medical comorbidities[21].
MASLD patients awaiting LT present with significant comorbidities, including higher rates of cardiovascular disease and metabolic disorders, leading to more intensive pre-transplant evaluations and potential challenges in managing these patients both before and after LT. Their complex health profiles underscore the need for comprehensive pretransplant assessments to optimize outcomes.
According to the American Association for the Study of liver diseases guideline, a BMI > 40 is a relative contraindication for LT[39]. The European Association for the Study of the Liver (EASL) guidelines recommend that patients with BMI > 35 should be discussed within the multidisciplinary team—including dietician, psychologist, hepatologist, anesthesiologist and surgeon—before being listed for transplantation. Additionally, EASL guidelines categorize patients with a BMI > 30 as marginal donors, describing them as being at high risk for unfavorable outcomes[40]. Furthermore, in different transplant centers, a BMI > 30 or > 40 is considered an absolute contraindication for receiving a LT. This exclusion of high-risk patients with higher BMIs may contribute to the observed lack of difference in overall survival and outcomes between recipients. Consequently, a significant number of patients who potentially need a LT due to MASLD may not be considered due to the higher prevalence of comorbidities associated with obesity. This may lead to an underestimation of risk within this population, resulting from a selection bias in LT candidates.
Data regarding the prevalence of HCC in MASLD vs non-MASLD patients are controversial. A multicenter study found that MASLD patients were less likely to have HCC at the time of transplant than non-MASLD patients[28]. However, other studies reported that HCC was more common in MASLD patients[35,36].
Compared to HCV and ALD recipients, MASLD recipients presented with better baseline synthetic liver function and fewer tumors[27]. However, there were no differences in the size of the largest tumor, frequency of satellite lesions, incidence of T3/T4 disease, tumor differentiation, rates of macro/microvascular invasion, and presence of pathologic nodal or metastatic disease[27]. Similar findings were reported in another study, which found no differences in radiographic tumor characteristics, such as the number of tumors at diagnosis and the size of the largest tumor[29].
Liver histology in MASLD patients showed higher steatosis, lobular inflammation, and hepatocyte ballooning but a lower rate of end-stage fibrosis[27]. MASLD patients also had lower alpha-fetoprotein (AFP) levels, with a maximum value of 20 ng/mL[17,29,34].
The prevalence and characteristics of HCC in MASLD patients compared to non-MASLD patients show contradictory results. While some studies suggest a lower prevalence of HCC in MASLD patients, others report the opposite. Despite better baseline liver function and fewer tumors, MASLD patients exhibit similar tumor characteristics and histology compared to HCV and ALD recipients. Lower AFP levels are also noted in MASLD patients, indicating a distinct tumor marker profile.
The increasing number of MASLD patients listed for LT has introduced additional challenges for surgeons, hepatologists, and the multidisciplinary team in managing these patients during surgery. A Japanese study found that patients with a BMI > 30 kg/m2 who underwent liver surgery had prolonged operative times extended by approximately 50 minutes compared to patients with a normal BMI[41]. Additionally, a comparative study showed the negative impact of obesity on resection time and blood loss during surgery, with increased morbidity due to pulmonary complications[42]. Furthermore, another study showed that patients with a BMI > 35 kg/m2 had an increased risk of receiving unplanned intubation due to complications or deterioration of their condition[43].
A study comparing ALD and MASLD patients who received LT found no difference in cold ischemia time, operating room time, and length of stay in the hospital and intensive care unit[23,31]. Similarly, another study reported no difference in warm ischemia between ALD vs MASLD patients[25]. Consistent with these findings, another study reported no differences in operative characteristics, including warm and cold ischemia times, and days to discharge between MASLD and non-MASLD recipients[32]. Additionally, a study compared populations based on BMI, found no differences in cold and warm ischemia times among normal, overweight, and obese populations[37].
Managing MASLD patients during LT presents unique challenges due to their higher BMI and associated comor
During explanation, MASLD patients were found to have more viable tumors but with a similar maximum viable tumor diameter compared to non-MASLD patients[29]. There were no significant differences in tumor features, such as vascular invasion and tumor differentiation. Maximum viable tumor size greater than 5 cm, vascular invasion, and pre-LT AFP > 20 ng/mL were found to be independent predictors of poor survival. Another study found no differences in the number or size of tumors, rate of viable tumor cells, tumor grade, macro or microvascular invasion, and tumor staging after liver explant[17].
Interestingly, MASLD patients were more likely to have undiagnosed HCC discovered during explanation compared to non-MASLD patients[34]. These undiagnosed HCC cases in MASLD patients were associated with younger age, higher BMI, higher MELD score, higher neutrophil-to-lymphocyte ratio, and lower AFP levels. Additionally, these patients had fewer total tumors, smaller maximum tumor diameters, earlier tumor stages, and well-differentiated tumors without vascular invasion compared to patients with previously identified HCC. However, when comparing undiagnosed HCC in MASLD vs non-MASLD patients, there were no differences in explant characteristics, including maximum tumor diameter, tumor number, vascular invasion, and tumor differentiation.
Current guidelines recommend screening for HCC using ultrasound (US), with or without AFP, every 6 months, primarily for patients with cirrhosis[44]. However, MASLD patients often present with a lower rate of end-stage fibrosis, meaning they are not typically subjected to strict periodic surveillance for HCC. This lack of surveillance increases the risk of discovering tumors after liver explanation. Additionally, this population faces significant challenges due to body habitus, with many being obese and fat around the liver, complicating the visualization and interpretation of US examinations. While multiphase computed tomography or magnetic resonance imaging is not the primary recommendation, careful evaluation and selection of MASLD patients could reveal benefits from these modalities due to their higher sensitivity for HCC detection compared to the US[45].
In conclusion, MASLD patients undergoing liver explanation often have more viable tumors and are more likely to have undiagnosed HCC at the time of transplant. Despite these findings, there are no significant differences in key tumor characteristics between MASLD and non-MASLD patients, emphasizing the need for vigilant monitoring and thorough pre-transplant evaluations.
The outcomes following LT encompass a broad spectrum, including the evaluation of morbidity, complications, short-term and long-term survival, and overall quality of life. Given the high prevalence of MASLD as an indication for LT and its associated comorbidities, the post-transplant period requires careful monitoring and management to optimize patient outcomes.
As discussed previously, patients undergoing LT for MASLD often present with high-risk metabolic features at the time of enlistment. Several studies reported that these high-risk metabolic characteristics persist after LT[5,36,38,46]. Additionally, the prevalence of dyslipidemia increases post-LT in patients with and without previous diagnosis[47]. In the Indian population, 61% of MASLD LT recipients developed post-transplant metabolic syndrome[38]. Factors asso
A study in Scotland reported that BMI in MASLD recipients decreased at 3 and 6 months post-LT but returned to pre-LT levels by the end of the first year[24]. However, BMI in ALD patients remained stable for 2 years. Another study at Karolinska University reported that BMI of MASLD recipients remained stable after 1 year post-LT but decreased in ALD recipients[47]. Although BMI can predict the risk of metabolic diseases and post-LT complications, it remains an imperfect index and cannot accurately assess the nutritional status of these patients[48]. Regardless of the measurement technique used, the results have limitations. However, it is crucial to thoroughly evaluate the nutritional status of these patients, considering factors such as ascites, edema levels, and skeletal muscle mass[49]. Interestingly, some studies suggest that adjusting BMI for ascites to estimate a patient’s dry weight offers a more accurate assessment of nutritional status[50].
A comparative study found that MASLD patients were more likely to experience adverse cardiovascular events during the first year after LT[31]. Seventy percent of these cardiovascular events occurred in the immediate perioperative period (0-30 days), with MASLD recipients having a higher risk of sudden cardiac arrest compared to ALD recipients. Additionally, a study found that patients with a previous history of peripheral artery disease had a higher prevalence of cardiovascular events after LT[22]. MASLD recipients had a higher prevalence of acute kidney failure during the first month after LT, with 31.3% of patients progressing to significant CKD (stage > III) within 2 years of follow-up[36]. Steatosis was identified as an independent predictor of CKD stage > III after LT[51]. A multicenter study found that the eGFR at 1-, 3-, 6-, and 12-months after LT was consistently lower in MASLD patients compared to non-MASLD patients[28]. Furthermore, a history of CKD before LT is a critical risk factor for post-LT mortality in MASLD patients (hazard ratio = 1.16, P < 0.001)[51]. A study based on the UNOS database indicated an increasing trend in simultaneous liver-kidney transplant indications in the United States for cirrhotic patients. The liver graft survival rate was compared between MASLD and other non-viral etiologies (78% vs 74%, P = 0.14)[52]. However, kidney graft loss in MASLD patients was 1.5 times more likely. It is essential to evaluate the baseline conditions in patients before LT to address specific strategies, such as the use of immunosuppressive drugs and the prevention or treatment of metabolic conditions in patients with pre-existing CKD or those at risk of developing renal injury[52]. The diagnostic criteria and prevention strategies for acute kidney failure in post-LT MASLD patients should be the same as for other etiologies, with consideration of the higher risk in this population.
In a combined analysis, MASLD LT recipients exhibit higher rates of infection, sepsis, wound healing, biliary complications, secondary surgery, and bleeding compared to recipients with other etiologies. However, no statistical significance was observed for these events when individually assessed[23]. Additionally, another study analyzed recipients based on BMI and found that overweight and obese patients had higher morbidity rates from infection complications and more extended hospital stays[53].
Quality of life post-LT is an important aspect of long-term outcomes. Due to their comorbidities, MASLD patients may experience a different quality of life trajectory than non-MASLD recipients.
Persistent metabolic issues and higher rates of complications such as cardiovascular events and kidney failure complicate post-LT outcomes for MASLD patients. While quality of life generally improves post-transplant, MASLD patients often continue to face significant health challenges, necessitating thorough post-operative care and monitoring.
A retrospective study from Karolinska University showed a 41% recurrence rate of steatosis in MASLD patients post-LT, with 32% developing moderate to severe steatosis and 50% developing perisinusoidal fibrosis[47]. Pre-LT insulin-dependent DM was associated with recurrent steatosis in MASLD patients. A study in Scotland reported a 20% recurrence rate[24], while a Spain study reported a 30% recurrence rate of steatosis at 3 years post-LT in MASLD patients[35]. Similar findings were observed in another study, which showed a 33% recurrence rate at 6 months post-LT with moderate to severe steatosis characteristics. In contrast, none of the ALD recipients exhibited de novo steatosis[30]. A study in Turkey observed a 12.3% recurrence rate and a 22.1% rate of de novo steatosis[54]. DM was found to be an independent predictor of steatosis post-LT. An Indian study reported a 39% recurrence rate and a 31% rate of de novo steatosis[38].
In Belgium, a study periodically evaluated the rate of steatosis recurrence in MASLD patients, reporting rates of 12.8%, 23.7%, and 43.5% at 1-, 3-, and 5-years post-LT, respectively[5]. Risk factors associated with recurrent steatosis included older age at the time of transplantation, obesity, and higher BMI post-LT. Patients with recurrent steatosis had a higher incidence of myocardial infarction after LT. However, a study involving centers in Switzerland and France found higher recurrence rates with steatosis grade > 1 occurring in 68% at 1 year and 85% at 5 years[4]. MASH recurrence was lower, with 14.9% and 60.3% at 1 and 5 years, respectively, post-LT. BMI > 31 kg/m2 was associated with an increased risk of steatosis recurrence, while age < 65 years at the time of LT, low post-LT high-density lipoprotein (< 45 mg/dL), and grade 1 or 2 steatosis after 1 year LT was associated with an increased risk for MASH.
Additionally, a study comparing MASLD and ALD patients post-LT reported no difference between the groups in the progression rate of steatosis or NAS score at 1 year post-LT[25]. However, the rate of fibrosis was higher in ALD patients. MASLD as an etiology and the NAS progression rate was associated with a reduced risk for fibrosis progression, whereas the steatosis progression rate was linked to an increased risk of fibrosis.
Recurrence of MASLD after LT is a significant concern due to the underlying metabolic risk factors that persist post-transplant (Table 2). Studies indicate that recurrent MASLD can occur in a substantial proportion of patients, potentially affecting long-term graft function and patient health. Managing metabolic risk factors through lifestyle modifications and medical interventions is crucial for preventing recurrence and ensuring better post-transplant outcomes.
| Ref. | Population | Recurrence/de novo steatosis |
| Tokodai et al[47] | 95 patients: NASH 27, ALD 68 | Recurrence 41% in NASH patients |
| O’Neill et al[24] | 279 patients: NAFLD 84, ALD 195 | Recurrence 20% in NAFLD patients |
| Van Herck et al[5] | 232 patients: NAFLD 112, HCV 120 | Recurrence 429% in NAFLD patients (mean at 3 years): -12.8% at 1 year, -23.7% at 3 years, -43.5% at 5 years |
| Sourianarayanane et al[25] | 185 patients: NASH 77, ALD 108 | Recurrence 546% at 1 year in NASH patients |
| Bhagat et al[30] | 154 patients: NASH 71, ALD 83 | Recurrence in 33% after 6 months in NASH patients |
| Castelló et al[35] | 54 patients: NASH 18, ALD 36 | Recurrence in 20% in NASH patients at 1 year and 30% at 3 years |
| Unger et al[33] | 27 patients: NASH 15, Cryptogenic 12 | Recurrence in 20% after 6 months in NASH patients |
Multiple studies have shown no difference in overall survival rate between MASLD patients and non-MASLD patients at 1-, 3-, and 5-years post-LT (Table 1)[5,16,7,20-31,33-36,47]. Despite similar overall survival rate, MASLD patients exhibit a higher incidence of comorbidities.
A Belgian study reported higher overall cardiovascular morbidity in MASLD patients compared to HCV patients, especially in later follow-ups[5]. In MASLD patients, 25% of deaths were related to cardiovascular events, and 8.3% were due to infections. A comparative study reported that experiencing at least one cardiovascular event was associated with 50% of overall mortality in MASLD patients, with 27% of deaths due to the cardiovascular event itself and 20% due to sepsis[31]. Similar results were found in a study involving populations from the United States and Canada, where MASLD recipients had a higher rate of cardiovascular events (22%), but fewer deaths related to liver causes (6.3%) compared to non-MASLD recipients[17]. However, the rate of death from infections was similar in both groups.
An analysis based on DM status reported that patients with DM had higher incidence rates of all-cause mortality and graft failure, particularly from cardiovascular and renal events, though they had a low rate of death due to graft rejection[55]. Another study found no difference in the cause of death between normal, overweight, and obese BMI categories, but obese patients had a significantly higher risk of all-cause mortality compared to normal-weight patient[37]. The percentage of deaths was 44% in obese patients, compared to 25% and 29% in normal and overweight patients, respectively. A BMI > 40 was identified as an independent predictor of worse survival rates[56].
Some studies reclassified patients into higher-risk categories, noting that patients older than 60 years, with a BMI > 30 kg/m², and comorbidities such as DM and HTN had worse prognoses for both short- and long-term survival and higher morbidity risks[20,26,57].
While overall survival rates between MASLD and non-MASLD LT recipients appear comparable, MASLD patients exhibit distinctive post-transplant challenges. Cardiovascular morbidity and mortality are notably higher in MASLD recipients, highlighting the need for vigilant cardiovascular monitoring and management. Additionally, comorbid conditions like DM, obesity, and HTN significantly impact survival and graft outcomes. Addressing these comorbidities through targeted interventions could improve long-term survival and quality of life for MASLD patient post-transplant.
Bariatric surgery (BS) has been proven to be the most effective treatment for morbidly obese patients[58,59]. This surgery not only aids in weight reduction but also reduces the risk of metabolic disorders and their complications. MASLD patients, who typically have a higher BMI than other LT candidates, could benefit significantly from BS. However, the optimal timing for performing BS—whether before, during, or after LT—remains undefined.
Some studies suggest that sleeve gastrectomy is preferable to Roux-en-Y gastric bypass for LT patients due to a lower risk profile and fewer nutrient absorption issues[60,61]. Despite fewer side effects associated with sleeve gastrectomy, the potential risk of malnutrition and sarcopenia must be considered. It is important to note that BS is not suitable for all patients, as decompensated cirrhosis is a contraindication[62,63].
A multicenter study reported that three patients received BS before LT and two patients after LT, with a median weight loss of 20 kg post-LT and no significant differences in complications among these patients[4]. One patient with recurrent steatosis one year after LT underwent sleeve gastrectomy three years post-LT, resulting in improved hepatic histology, complete regression of steatosis, resolution of NASH, and reduced fibrosis stage on biopsy five years post-LT. Another study evaluated 13 patients who underwent simultaneous LT and BS vs 36 patients who received LT and standard weight loss interventions[64]. During follow-up, the BS group maintained more consistent weight loss and had a lower prevalence of HTN, insulin resistance, and hepatic steatosis. The survival rate did not differ between the groups.
Despite these promising outcomes, determining the optimal timing for BS remains challenging due to the limited number of patients in each study. Further research involving more extensive and diverse populations is needed to understand this issue better.
LT for MASLD is increasing due to rising global rates of obesity and metabolic syndrome. This shift in transplant indications presents unique challenges. MASLD patients typically present at an older age with higher BMI and multiple metabolic comorbidities, such as DM and HTN. These comorbidities influence both pre-transplant evaluations and surgical outcomes. Obesity in MASLD patients can lead to prolonged operative times and impact surgical outcomes. Immediate post-transplant complications are comparable between MASLD and other etiologies; however, long-term outcomes reveal higher rates of metabolic syndrome, cardiovascular events, and renal complications in MASLD recipients. While overall survival rates are similar, MASLD patients experience higher cardiovascular morbidity and hepatic steatosis recurrence, necessitating vigilant post-transplant care to manage these risks and prevent graft complications. Despite comparable survival rates, MASLD patients with higher BMI and comorbidities such as DM face increased mortality rates from cardiovascular events. Comprehensive cardiovascular management is essential post-transplantation. Optimizing outcomes for MASLD patients undergoing LT requires a multidisciplinary approach focusing on pre-transplant risk assessment, intraoperative care, and robust post-transplant monitoring and management. Addressing metabolic and cardiovascular health post-transplant is crucial for improving long-term outcomes and quality of life.
| 1. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 2006] [Article Influence: 668.7] [Reference Citation Analysis (3)] |
| 2. | Kwong AJ, Kim WR, Lake JR, Schladt DP, Schnellinger EM, Gauntt K, McDermott M, Weiss S, Handarova DK, Snyder JJ, Israni AK. OPTN/SRTR 2022 Annual Data Report: Liver. Available from: https://srtr.transplant.hrsa.gov/annual_reports/2022/Liver.aspx. |
| 3. | Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, Fritz J, Feurstein B, Popp W, Karam V, Muiesan P, O'Grady J, Jamieson N, Wigmore SJ, Pirenne J, Malek-Hosseini SA, Hidalgo E, Tokat Y, Paul A, Pratschke J, Bartels M, Trunecka P, Settmacher U, Pinzani M, Duvoux C, Newsome PN, Schneeberger S; European Liver and Intestine Transplant Association (ELITA). Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019;71:313-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 4. | Villeret F, Dharancy S, Erard D, Abergel A, Barbier L, Besch C, Boillot O, Boudjema K, Coilly A, Conti F, Corpechot C, Duvoux C, Faitot F, Faure S, Francoz C, Giostra E, Gugenheim J, Hardwigsen J, Hilleret MN, Hiriart JB, Houssel-Debry P, Kamar N, Lassailly G, Latournerie M, Pageaux GP, Samuel D, Vanlemmens C, Saliba F, Dumortier J. Inevitability of disease recurrence after liver transplantation for NAFLD cirrhosis. JHEP Rep. 2023;5:100668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 5. | Van Herck J, Verbeek J, van Malenstein H, Laleman W, Cassiman D, Verslype C, van der Merwe S, Jochmans I, Sainz-Barriga M, Monbaliu D, Pirenne J, Nevens F. Liver-Related and Cardiovascular Outcome of Patients Transplanted for Nonalcoholic Fatty Liver Disease: A European Single-Center Study. Transplant Proc. 2021;53:1674-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Arab JP, Dirchwolf M, Álvares-da-Silva MR, Barrera F, Benítez C, Castellanos-Fernandez M, Castro-Narro G, Chavez-Tapia N, Chiodi D, Cotrim H, Cusi K, de Oliveira CPMS, Díaz J, Fassio E, Gerona S, Girala M, Hernandez N, Marciano S, Masson W, Méndez-Sánchez N, Leite N, Lozano A, Padilla M, Panduro A, Paraná R, Parise E, Perez M, Poniachik J, Restrepo JC, Ruf A, Silva M, Tagle M, Tapias M, Torres K, Vilar-Gomez E, Costa Gil JE, Gadano A, Arrese M. Latin American Association for the study of the liver (ALEH) practice guidance for the diagnosis and treatment of non-alcoholic fatty liver disease. Ann Hepatol. 2020;19:674-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 7. | Karnikowski M, Córdova C, Oliveira RJ, Karnikowski MG, Nóbrega Ode T. Non-alcoholic fatty liver disease and metabolic syndrome in Brazilian middle-aged and older adults. Sao Paulo Med J. 2007;125:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | López-velázquez JA, Silva-vidal KV, Ponciano-rodríguez G, Chávez-tapia NC, Arrese M, Uribe M, Méndez-sánchez N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann Hepatol. 2014;13:166-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Lizardi-Cervera J, Laparra IB, Chávez-Tapia NC, Ostos MER, Esquivel MU. Prevalencia de hígado graso no alcohólico y síndrome metabólico en población asintomática. Rev Gastroenterol Méx. 2006;71:453-459. |
| 10. | Khan AS, Adams N, Vachharajani N, Dageforde L, Wellen J, Shenoy S, Crippin JS, Doyle MB, Chapman WC. Liver transplantation for hepatitis C patients in the era of direct-acting antiviral treatment: A retrospective cohort study. Int J Surg. 2020;75:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Lake JR. Changing indications for liver transplantation. Gastroenterol Clin N. 1993;22:213-229. [DOI] [Full Text] |
| 12. | Belli LS, Perricone G, Adam R, Cortesi PA, Strazzabosco M, Facchetti R, Karam V, Salizzoni M, Andujar RL, Fondevila C, De Simone P, Morelli C, Fabregat-Prous J, Samuel D, Agarwaal K, Moreno Gonzales E, Charco R, Zieniewicz K, De Carlis L, Duvoux C; all the contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 13. | Gane EJ, Agarwal K. Directly acting antivirals (DAAs) for the treatment of chronic hepatitis C virus infection in liver transplant patients: "a flood of opportunity". Am J Transplant. 2014;14:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 14. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1634] [Article Influence: 233.4] [Reference Citation Analysis (0)] |
| 15. | Liver. OPTN/SRTR 2019 Annual Data Report: Liver. Available from: https://srtr.transplant.hrsa.gov/annual_reports/2019/Liver.aspx#LI_tx_counts_diag_b64. |
| 16. | Ferrarese A, Battistella S, Germani G, Russo FP, Senzolo M, Gambato M, Vitale A, Cillo U, Burra P. Nash Up, Virus Down: How the Waiting List Is Changing for Liver Transplantation: A Single Center Experience from Italy. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Rajendran L, Murillo Perez CF, Ivanics T, Claasen MPAW, Hansen BE, Wallace D, Yoon PD, Sapisochin G. Outcomes of liver transplantation in non-alcoholic steatohepatitis (NASH) versus non-NASH associated hepatocellular carcinoma. HPB (Oxford). 2023;25:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 18. | Díaz LA, Ayares G, Arnold J, Idalsoaga F, Corsi O, Arrese M, Arab JP. Liver Diseases in Latin America: Current Status, Unmet Needs, and Opportunities for Improvement. Curr Treat Options Gastroenterol. 2022;20:261-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Gallardo-Rincón H, Cantoral A, Arrieta A, Espinal C, Magnus MH, Palacios C, Tapia-Conyer R. Review: Type 2 diabetes in Latin America and the Caribbean: Regional and country comparison on prevalence, trends, costs and expanded prevention. Prim Care Diabetes. 2021;15:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Kennedy C, Redden D, Gray S, Eckhoff D, Massoud O, McGuire B, Alkurdi B, Bloomer J, DuBay DA. Equivalent survival following liver transplantation in patients with non-alcoholic steatohepatitis compared with patients with other liver diseases. HPB (Oxford). 2012;14:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Danford CJ, Iriana S, Shen C, Curry MP, Lai M. Evidence of bias during liver transplant evaluation of non-alcoholic steatohepatitis cirrhosis patients. Liver Int. 2019;39:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Piazza NA, Singal AK. Frequency of Cardiovascular Events and Effect on Survival in Liver Transplant Recipients for Cirrhosis Due to Alcoholic or Nonalcoholic Steatohepatitis. Exp Clin Transplant. 2015;14:79-85. |
| 23. | Kern B, Feurstein B, Fritz J, Fabritius C, Sucher R, Graziadei I, Bale R, Tilg H, Zoller H, Newsome P, Eschertzhuber S, Margreiter R, Öfner D, Schneeberger S. High incidence of hepatocellular carcinoma and postoperative complications in patients with nonalcoholic steatohepatitis as a primary indication for deceased liver transplantation. Eur J Gastroenterol Hepatol. 2019;31:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | O'Neill S, Napetti S, Cornateanu S, Sutherland AI, Wigmore S, Oniscu GC, Adair A. Impact of body mass index in liver transplantation for nonalcoholic fatty liver disease and alcoholic liver disease. HPB (Oxford). 2017;19:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Sourianarayanane A, Arikapudi S, McCullough AJ, Humar A. Nonalcoholic steatohepatitis recurrence and rate of fibrosis progression following liver transplantation. Eur J Gastroenterol Hepatol. 2017;29:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Malik SM, deVera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Kwong AJ, Devuni D, Wang C, Boike J, Jo J, VanWagner L, Serper M, Jones L, Sharma R, Verna EC, Shor J, German MN, Hristov A, Lee A, Spengler E, Koteish AA, Sehmbey G, Seetharam A, John N, Patel Y, Kappus MR, Couri T, Paul S, Salgia RJ, Nhu Q, Frenette CT, Lai JC, Goel A; Re-Evaluating Age Limits in Transplantation (REALT) Consortium. Outcomes of Liver Transplantation Among Older Recipients With Nonalcoholic Steatohepatitis in a Large Multicenter US Cohort: the Re-Evaluating Age Limits in Transplantation Consortium. Liver Transpl. 2020;26:1492-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Holzner ML, Florman S, Schwartz ME, Tabrizian P. Outcomes of liver transplantation for nonalcoholic steatohepatitis-associated hepatocellular carcinoma. HPB (Oxford). 2022;24:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Barritt AS 4th, Dellon ES, Kozlowski T, Gerber DA, Hayashi PH. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol. 2011;45:372-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Unger LW, Herac M, Staufer K, Salat A, Silberhumer G, Hofmann M, Trauner M, Rasoul-Rockenschaub S, Soliman T, Reiberger T, Berlakovich GA. The post-transplant course of patients undergoing liver transplantation for nonalcoholic steatohepatitis versus cryptogenic cirrhosis: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2017;29:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Verna EC, Phipps MM, Halazun KJ, Markovic D, Florman SS, Haydel BM, Ruiz R, Klintmalm G, Lee DD, Taner B, Hoteit MA, Tevar AD, Humar A, Chapman WC, Vachharajani N, Aucejo FN, Melcher ML, Nguyen MH, Nydam TL, Markmann JF, Mobley C, Ghobrial RM, Langnas AN, Carney C, Berumen J, Schnickel GT, Sudan D, Hong JC, Rana A, Jones CM, Fishbein TM, Busuttil RW, Agopian V; US Multicenter HCC Transplant Consortium. Outcomes in liver transplant recipients with nonalcoholic fatty liver disease-related HCC: results from the US multicenter HCC transplant consortium. Liver Transpl. 2023;29:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Castelló B, Aguilera V, Blázquez MT, Rubín Á, García M, Vinaixa C, Benlloch S, SanJuan F, Montalva E, López R, Berenguer M. Post-transplantation outcome in non-alcoholic steatohepatitis cirrhosis: Comparison with alcoholic cirrhosis. Ann Hepatol. 2019;18:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Houlihan DD, Armstrong MJ, Davidov Y, Hodson J, Nightingale P, Rowe IA, Paris S, Gunson BK, Bramhall SB, Mutimer DJ, Neuberger JM, Newsome PN. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens? Liver Transpl. 2011;17:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | van Son J, Stam SP, Gomes-Neto AW, Osté MCJ, Blokzijl H, van den Berg AP, Porte RJ, Bakker SJL, de Meijer VE. Post-transplant obesity impacts long-term survival after liver transplantation. Metabolism. 2020;106:154204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Choudhary NS, Dhampalwar S, Saraf N, Rastogi A, Bhangui P, Soin AS. Post-transplant Non-alcoholic Fatty Liver Disease and Metabolic Syndrome After Living Donor Liver Transplantation in Indians. J Clin Exp Hepatol. 2024;14:101281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 730] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 40. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 746] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 41. | Ri M, Miyata H, Aikou S, Seto Y, Akazawa K, Takeuchi M, Matsui Y, Konno H, Gotoh M, Mori M, Motomura N, Takamoto S, Sawa Y, Kuwano H, Kokudo N. Effects of body mass index (BMI) on surgical outcomes: a nationwide survey using a Japanese web-based database. Surg Today. 2015;45:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Langella S, Russolillo N, Forchino F, Lo Tesoriere R, D'Eletto M, Ferrero A. Impact of obesity on postoperative outcome of hepatic resection for colorectal metastases. Surgery. 2015;158:1521-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Yokoo H, Miyata H, Konno H, Taketomi A, Kakisaka T, Hirahara N, Wakabayashi G, Gotoh M, Mori M. Models predicting the risks of six life-threatening morbidities and bile leakage in 14,970 hepatectomy patients registered in the National Clinical Database of Japan. Medicine (Baltimore). 2016;95:e5466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3439] [Article Influence: 429.9] [Reference Citation Analysis (3)] |
| 45. | Pocha C, Choudhry S. MRI for screening and surveillance for hepatocellular cancer in NAFLD and NASH. Hepatoma Res. 2023;9:22. [DOI] [Full Text] |
| 46. | Younossi ZM. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation. Liver Transpl. 2018;24:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Tokodai K, Karadagi A, Kjaernet F, Romano A, Ericzon BG, Nowak G. Characteristics and risk factors for recurrence of nonalcoholic steatohepatitis following liver transplantation. Scand J Gastroenterol. 2019;54:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Sato-Espinoza K, Chotiprasidhi P, Huaman MR, Díaz-Ferrer J. Update in lean metabolic dysfunction-associated steatotic liver disease. World J Hepatol. 2024;16:452-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 49. | Campillo B, Richardet JP, Bories PN. Validation of body mass index for the diagnosis of malnutrition in patients with liver cirrhosis. Gastroenterol Clin Biol. 2006;30:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Lamarti E, Hickson M. The contribution of ascitic fluid to body weight in patients with liver cirrhosis, and its estimation using girth: a cross-sectional observational study. J Hum Nutr Diet. 2020;33:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020;2:100192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 52. | Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation. 2016;100:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Hakeem AR, Cockbain AJ, Raza SS, Pollard SG, Toogood GJ, Attia MA, Ahmad N, Hidalgo EL, Prasad KR, Menon KV. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl. 2013;19:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 54. | Adali G, Bilgic NM, Kalaman AE, Ozturk O, Ozdil K. Prevalence and predictors of metabolic-associated fatty liver disease in liver transplant recipients: A cross-sectional prospective study. Hepatol Forum. 2023;4:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 55. | Lee DU, Ponder R, Lee KJ, Chou H, Lee K, Jung D, Fan GH, Urrunaga NH. The prognostic relationship between donor age and infectious risk in liver transplant patients with nonalcoholic steatohepatitis: Analysis of UNOS database. Dig Liver Dis. 2023;55:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Conzen KD, Vachharajani N, Collins KM, Anderson CD, Lin Y, Wellen JR, Shenoy S, Lowell JA, Doyle MB, Chapman WC. Morbid obesity in liver transplant recipients adversely affects longterm graft and patient survival in a single-institution analysis. HPB (Oxford). 2015;17:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Dare AJ, Plank LD, Phillips AR, Gane EJ, Harrison B, Orr D, Jiang Y, Bartlett AS. Additive effect of pretransplant obesity, diabetes, and cardiovascular risk factors on outcomes after liver transplantation. Liver Transpl. 2014;20:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 297] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 59. | Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres A, Weiner R, Yashkov Y, Frühbeck G; International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); European Association for the Study of Obesity Obesity Management Task Force (EASO OMTF). Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24:42-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 434] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 60. | Ahmed Z, Khan MA, Vazquez-Montesino LM, Ahmed A. Bariatric surgery, obesity and liver transplantation. Transl Gastroenterol Hepatol. 2022;7:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Alqahtan SA, Brown RS. Management and Risks Before, During, and After Liver Transplant in Individuals With Obesity. Gastroenterol Hepatol (N Y). 2023;19:20-29. [PubMed] |
| 62. | Spengler EK, O'Leary JG, Te HS, Rogal S, Pillai AA, Al-Osaimi A, Desai A, Fleming JN, Ganger D, Seetharam A, Tsoulfas G, Montenovo M, Lai JC. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation. 2017;101:2288-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 63. | Terrault NA, Francoz C, Berenguer M, Charlton M, Heimbach J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin Gastroenterol Hepatol. 2023;21:2150-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 64. | Zamora-Valdes D, Watt KD, Kellogg TA, Poterucha JJ, Di Cecco SR, Francisco-Ziller NM, Taner T, Rosen CB, Heimbach JK. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology. 2018;68:485-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/