Published online Dec 18, 2024. doi: 10.5500/wjt.v14.i4.98155
Revised: July 18, 2024
Accepted: July 24, 2024
Published online: December 18, 2024
Processing time: 93 Days and 3.5 Hours
Focal segmental glomerulosclerosis (FSGS) often recurs after transplantation, leading to graft dysfunction and graft loss. Patients who have lost prior grafts due to recurrence are at particularly high risk of re-recurrence in subsequent grafts. Rituximab and plasma exchange have been used pre-emptively to prevent post-transplant recurrence. However, the efficacy of such preventative measures remains unclear.
To investigate the outcomes of preventative rituximab and plasma exchange for recurrent FSGS in transplant recipients after prior graft loss.
We conducted a systematic review of 11 studies with 32 patients who had experienced prior graft loss due to post-transplant FSGS recurrence and were treated with either pre-emptive plasma exchange alone, rituximab alone, or a combination of both.
Overall, 47% of the 32 patients experienced recurrence despite prophylactic treatment. Re-recurrence was seen in 25% (1/4) with pre-emptive rituximab alone, and 45% recurrence (9/20) with plasma exchange alone. Re-recurrence was noted in 63% with the use of combined plasma exchange and rituximab.
There is a paucity of available evidence in the literature to draw clear conclusions on the benefits of pre-emptive measures to prevent FSGS re-recurrence. The small sample sizes and variations in protocols call for larger and controlled studies to serve this patient population at high risk of recurrence and graft loss.
Core Tip: Patients with prior graft losses due to post-transplant recurrence of focal segmental glomerulosclerosis are at particularly high risk of re-recurrence in subsequent transplants. With scarcity of transplants and limited options, prevention becomes vital. However, there has been a recommendation against the use of pre-emptive measures to prevent recurrence. Currently, there is too little evidence described in the literature to draw clear conclusions on the efficacy of preventative treatment. This lack of evidence for benefit does not necessarily equate to evidence of lack of benefit. Larger controlled studies are needed to investigate the effectiveness of pre-emptive measures.
- Citation: Gharaei S, Abbas H, Kanigicherla DA. Review of plasma exchange and rituximab for prevention of recurrent focal segmental glomerulosclerosis after a prior graft loss. World J Transplant 2024; 14(4): 98155
- URL: https://www.wjgnet.com/2220-3230/full/v14/i4/98155.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i4.98155
Primary focal segmental glomerulosclerosis (FSGS) is a common cause of nephrotic syndrome in both adults and children and can lead to end-stage kidney failure in a significant proportion. Recurrence of FSGS (rFSGS) causes graft dysfunction and significantly increases risk of graft loss. Rates of rFSGS vary widely in reported studies and can be seen in about 30% of first transplants. Several risk factors were identified, although inconsistently in prior studies. The incidence can be seen in more than 80% in patients receiving subsequent grafts after graft loss from prior rFSGS[1]. Although the absolute number of recipients with this devastating scenario is very small, such patients with failed grafts from a prior rFSGS have a difficult choice to make regarding re-transplantation. With such high risk of developing re-recurrence in further grafts, there is a need for effective preventative measures in this patient cohort.
It is believed that circulating factors possibly of immune origin affect podocytes and glomerular permeability and contribute to the pathogenesis of primary FSGS and rFSGS[2,3]. This underpins the use of plasma exchange (PE) as treatment since its first report[4]. In addition, rituximab (RTX) was found surreptitiously to improve recurrence of post-transplant nephrotic syndrome[5], and since has been used alone or in combination with PE to treat rFSGS. Treatment with these, although not standardized, can improve remission. Based on a similar rationale, it is hypothesized that the combination of PE and rituximab can prevent rFSGS. Studies describing such prophylactic use included patients with a variety of risk factors, many being retrospective and having disparate methodologies. A recent review[6] found no significant difference in recurrence between the group that received rituximab (with or without PE) vs the standard treatment group or PE alone vs no PE. A prospective observational study of perioperative rituximab and PE also did not show difference in rates of rFSGS[7]. The recent consensus statement from a working group concluded that pre-transplant intervention to prevent rFSGS is not recommended[8]. However, it is uncertain if these conclusions could be applied to specific patient populations such as those with a prior graft loss from rFSGS who are deemed to be at very high risk of re-recurrence.
We, therefore, evaluated the reported efficacy of prophylactic treatment with rituximab and plasma exchange in second and third renal graft recipients following a prior graft failure with rFSGS. A systematic review of published literature was undertaken to assess this.
A literature search was conducted to identify studies that included adult and pediatric patients with the following pre-specified inclusion criteria: Diagnosis of primary FSGS in native kidneys, lost one or two prior transplants due to rFSGS, and received prophylactic treatment with PE, RTX or combination of both.
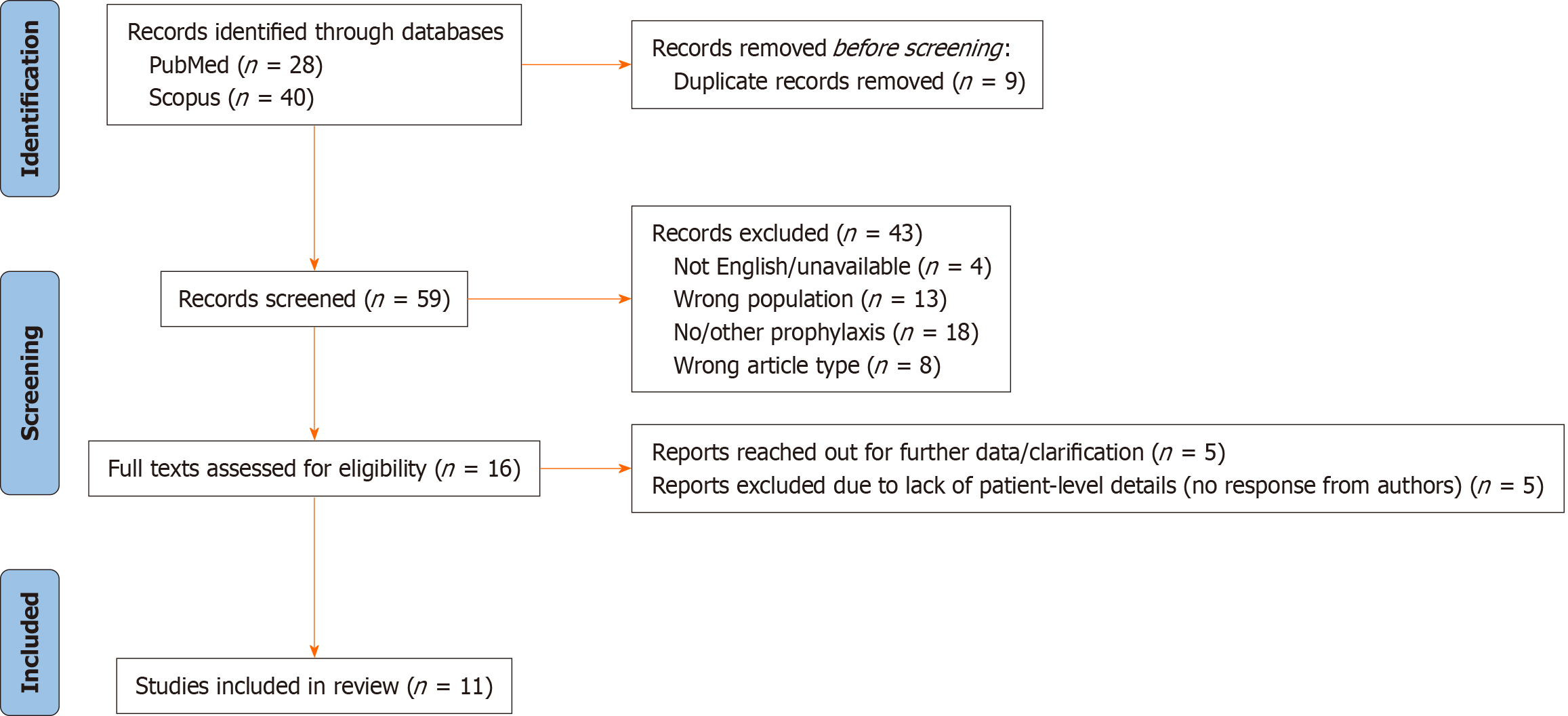
Articles were searched using PubMed and Scopus and standard methodology was applied to undertake systematic review (Figure 1).
Search terms included: “FSGS”, “focal segmental glomerulosclerosis”, “nephrotic syndrome”, “podocytopathy”, “recurrence”, “re-recurrence”, “relapse”, “transplant”, “allograft”, “rituximab”, “monoclonal antibody”, “plasma exchange” and “plasmapheresis”. After applying the filters full text, English language, and articles, 28 and 40 results were found, respectively. After removing duplicates, the total number of papers was 59, which underwent initial screening by two reviewers.
Two reviewers conducted the eligibility screening independently, excluding studies of patients with first grafts alone, those wherein recipients had no rFSGS in prior transplants, those who had received no or alternative prophylaxis, and studies that were not primary research. The two reviewers then discussed any conflicts and mutually agreed on the inclusion of the patients that consisted of adult and pediatric patients with the pre-specified criteria.
PRISMA reporting guidelines were used[9].
59 studies were screened for titles and abstracts, of which 16 papers met the pre-specified criteria as above and were included for full-text review. We reached out to authors of 5 papers where patient-level data was not available in the publications. However, no response was received and they were excluded from this review. 11 studies were included for full analysis. All included articles were published between 2005 and 2022. Data was extracted from all patients, where available. There was a total of 32 patients across these 11 studies; details are in Table 1.
| No. | Ref. | Patient number | Number of prior KTs | Gender | Age at index KT | pPE | pRTX | Recurrence | Follow-up time | Outcome at last follow-up |
| 1 | Gohh et al[10] | 3/6 | 8-41 months | |||||||
| 1 | 1 | M | 33 | + | + | Graft dysfunction | ||||
| 2 | 1 | F | 46 | + | + | GF | ||||
| 3 | 1 | F | 9 | + | + | GF | ||||
| 4 | 1 | F | 45 | + | - | |||||
| 5 | 2 | M | 41 | + | - | |||||
| 6 | 1 | M | 45 | + | - | |||||
| 2 | Audard et al[11] | 0/4 | 15–54 months | |||||||
| 7 | 1 | F | 33 | + | + | - | ||||
| 8 | 1 | M | 43 | + | - | |||||
| 9 | 1 | F | 28 | + | - | |||||
| 10 | 1 | M | 40 | + | + | - | ||||
| 3 | Couloures et al[12] | 0/1 | 12 months | |||||||
| 11 | 1 | M | 18 | + | - | |||||
| 4 | Meyer et al[13] | 1/1 | ||||||||
| 12 | 1 | F | 28 | + (IA) | + | 24 months | Stable, creatinine clearance: 97 mL/min | |||
| 5 | Chikamoto et al[14] | 0/1 | 36 months | |||||||
| 13 | 1 | F | 8 | + | + | - | ||||
| 6 | Valdivia et al[15] | 1/3 | 10 months | All have functioning grafts, 2 had complete response | ||||||
| 14 | 1 | + | + | |||||||
| 15 | 2 | + | - | |||||||
| 16 | 2 | + | - | |||||||
| 7 | Vallianou et al[16] | 3/3 | 12 months | |||||||
| 17 | 1 | + | + | |||||||
| 18 | 1 | + | + | Remission | ||||||
| 19 | 2 | + | + | GF | ||||||
| 8 | Naciri Bennani et al[17] | 5/5 | 4-10 years | 1 GF, 2 dependent on IA, 2 in remission | ||||||
| 20 | 2 | + | + | + | ||||||
| 21 | 2 | + | + | + | ||||||
| 22 | 2 | + | + | + | ||||||
| 23 | 2 | + | + | + | ||||||
| 24 | 1 | + | + | + | ||||||
| 9 | Gonzalez et al[18] | 1/3 | 3 years | 2 GF | ||||||
| 25 | 2 | + | + | |||||||
| 26 | 2 | + | - | |||||||
| 27 | 2 | + | - | |||||||
| 10 | Mahesh et al[19] | 0/3 | ||||||||
| 28 | 1 | + | - | Death | ||||||
| 29 | 1 | + | - | |||||||
| 30 | 1 | + | - | GF (rejection) | ||||||
| 11 | Auñón et al[20] | 1/2 | 71 months | |||||||
| 31 | 1 | + | + | |||||||
| 32 | 1 | + | - |
Biopsy-proven recurrence of FSGS was confirmed in 5 studies, whilst clinical recurrence was used for diagnosis (nephrotic range proteinuria, in the absence of acute graft rejection or other disease) in 6 studies. Definitions of remission varied across studies. In most studies, complete remission was defined as a reduction of proteinuria < 0.5 g/day or < 0.3 g/day and clinical improvement of renal function. Partial remission was mostly defined as a reduction of proteinuria of 50% of the initial value or < 3.5 g/day.
Plasma exchange regimes varied across studies, from a minimum of 3, to a maximum of 17 sessions in the pre-and the immediate post-transplant period. Rituximab doses mostly consisted of 375 mg/m2 as a single dose pre-transplant, or on the day of transplantation, with an additional dose at day 7 post-transplant.
Table 2 summarizes the recurrence rates after different prophylactic treatments. 17 of the 32 patients (53%) remained free of FSGS in the index transplant, with a minimum follow-up of 8 months. The other 15 (47%) experienced recurrence, either immediately or within the first three months after transplantation. Two patients (#21 and #23, Table 2) experienced recurrence several years later. 9 of these patients received PE alone, one patient RTX only and 5 patients received a combination of both. 11 patients, in these studies together, had lost more than one prior graft due to FSGS recurrence. Of these, 6 (56%) experienced recurrence in their third graft despite prophylactic treatment.
| Prophylaxis (n) | Recurrence (%) | No recurrence (%) |
| PE only (20) | 9 (45) | 11 (55) |
| RTX only (4) | 1 (25) | 3 (75) |
| PE + RTX (8) | 5 (63) | 3 (37) |
| Total (32) | 15 (47) | 17 (53) |
11 of the 15 patients that experienced re-recurrence despite pre-emptive treatment received therapeutic PE, 8 patients received therapeutic rituximab in addition. 3 patients showed no remission on treatment, 2 had complete remission and 6 had partial remission of the disease (Table 3).
| Patient number | tPE | tPE regime | tRTX | tRTX regime | Treatment outcome |
| 1 | + | PE with replacement fluid of one plasma volume of 3.33% albumin solution (0.9% saline and 5% albumin n a 1:2 ratio) | - | - | PR |
| 2 | + | - | - | PR | |
| 3 | + | - | - | NR | |
| 12 | + | Immunoadsorption 4 times, 10-g of immunoglobulins substituted once | + | 375 mg/m2, 3 doses | PR |
| 18 | + | 1.5 times plasma volume exchange, replaced by a human albumin solution 5%. 3 daily sessions, followed by three-times-a-week for 3 weeks | + | 2 doses of 1 g each, 2 weeks apart | CR for 1 year |
| 19 | + | + | NR | ||
| 20 | + | Alternate day for 2 weeks, then adapted to proteinuria level. Steroids at 1 mg/kg/day for 3 weeks and then slowly tapered | + | 375 mg/m2 of body surface area at the 4th and 8th apheresis sessions | PR |
| 21 | + | + | GF | ||
| 22 | + | + | PR | ||
| 23 | + | + | CR | ||
| 24 | + | + | PR |
To the best of our knowledge, this is the only review of studies wherein prophylactic plasma exchange and rituximab were employed for the prevention of FSGS in all patients who had previously experienced a failed allograft due to rFSGS. While pre-emptive treatment has been proposed as a potential strategy to reduce rFSGS, this study underscores the complexities associated with its application in specific patient groups who have experienced rFSGS. This systematic review identified only 32 patients across 11 studies that met the three pre-specified criteria. Our findings indicate that prophylactic treatments varied, with 62.5% receiving only plasma exchange, 25% receiving combined treatments, and 12.5% treated solely with rituximab. The effectiveness appeared similar across all three interventions, but due to the small sample size, it was challenging to draw definitive conclusions from individual regimes.
This review highlights the essential consideration that lack of evidence for the benefit of such interventions is not the same as conclusive evidence of ineffectiveness. Conclusions from previous reviews and recommendations are based on the absence of supportive data, owing to inadequately powered studies. Through this review, we advocate for a more nuanced perspective that may pave the way for future research, particularly through well-designed prospective studies on pre-emptive treatments in patients with similar risk factors. Encouraging studies that aim to fill this evidence gap can potentially lead to breakthroughs in preventing recurrent disease, ultimately enhancing patient outcomes in these conditions.
Few limitations of this study include: (1) Some studies did not report gender, age at diagnosis and age at last transplant for all patients; (2) Exclusion of genetic causes was not explicit in the reports; (3) Studies defined recurrence and remission slightly differently; and (4) Owing to small numbers, formal statistical analysis could be undertaken.
In conclusion, there is a dearth of literature supporting or refuting the efficacy of pre-emptive interventions using plasma exchange and/or rituximab in preventing rFSGS in this specific recipient population. We suggest that prospective controlled studies are urgently required to investigate the role of pre-emptive strategies in the prevention of rFSGS in such very high-risk individuals.
| 1. | Uffing A, Pérez-Sáez MJ, Mazzali M, Manfro RC, Bauer AC, de Sottomaior Drumond F, O'Shaughnessy MM, Cheng XS, Chin KK, Ventura CG, Agena F, David-Neto E, Mansur JB, Kirsztajn GM, Tedesco-Silva H Jr, Neto GMV, Arias-Cabrales C, Buxeda A, Bugnazet M, Jouve T, Malvezzi P, Akalin E, Alani O, Agrawal N, La Manna G, Comai G, Bini C, Muhsin SA, Riella MC, Hokazono SR, Farouk SS, Haverly M, Mothi SS, Berger SP, Cravedi P, Riella LV. Recurrence of FSGS after Kidney Transplantation in Adults. Clin J Am Soc Nephrol. 2020;15:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Sharma M, Sharma R, McCarthy ET, Savin VJ. The focal segmental glomerulosclerosis permeability factor: biochemical characteristics and biological effects. Exp Biol Med (Maywood). 2004;229:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Maas RJ, Deegens JK, Wetzels JF. Permeability factors in idiopathic nephrotic syndrome: historical perspectives and lessons for the future. Nephrol Dial Transplant. 2014;29:2207-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Zimmerman SW. Plasmapheresis and dipyridamole for recurrent focal glomerular sclerosis. Nephron. 1985;40:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Nozu K, Iijima K, Fujisawa M, Nakagawa A, Yoshikawa N, Matsuo M. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol. 2005;20:1660-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Boonpheng B, Hansrivijit P, Thongprayoon C, Mao SA, Vaitla PK, Bathini T, Choudhury A, Kaewput W, Mao MA, Cheungpasitporn W. Rituximab or plasmapheresis for prevention of recurrent focal segmental glomerulosclerosis after kidney transplantation: A systematic review and meta-analysis. World J Transplant. 2021;11:303-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Alasfar S, Matar D, Montgomery RA, Desai N, Lonze B, Vujjini V, Estrella MM, Manllo Dieck J, Khneizer G, Sever S, Reiser J, Alachkar N. Rituximab and Therapeutic Plasma Exchange in Recurrent Focal Segmental Glomerulosclerosis Postkidney Transplantation. Transplantation. 2018;102:e115-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Raina R, Jothi S, Haffner D, Somers M, Filler G, Vasistha P, Chakraborty R, Shapiro R, Randhawa PS, Parekh R, Licht C, Bunchman T, Sethi S, Mangat G, Zaritsky J, Schaefer F, Warady B, Bartosh S, McCulloch M, Alhasan K, Swiatecka-Urban A, Smoyer WE, Chandraker A, Yap HK, Jha V, Bagga A, Radhakrishnan J. Post-transplant recurrence of focal segmental glomerular sclerosis: consensus statements. Kidney Int. 2024;105:450-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 9. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51705] [Article Influence: 10341.0] [Reference Citation Analysis (2)] |
| 10. | Gohh RY, Yango AF, Morrissey PE, Monaco AP, Gautam A, Sharma M, McCarthy ET, Savin VJ. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant. 2005;5:2907-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Audard V, Kamar N, Sahali D, Cardeau-Desangles I, Homs S, Remy P, Aouizerate J, Matignon M, Rostaing L, Lang P, Grimbert P. Rituximab therapy prevents focal and segmental glomerulosclerosis recurrence after a second renal transplantation. Transpl Int. 2012;25:e62-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Couloures K, Pepkowitz SH, Goldfinger D, Kamil ES, Puliyanda DP. Preventing recurrence of focal segmental glomerulosclerosis following renal transplantation: a case report. Pediatr Transplant. 2006;10:962-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Meyer TN, Thaiss F, Stahl RA. Immunoadsorbtion and rituximab therapy in a second living-related kidney transplant patient with recurrent focal segmental glomerulosclerosis. Transpl Int. 2007;20:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Chikamoto H, Hattori M, Kuroda N, Kajiho Y, Matsumura H, Fujii H, Ishizuka K, Hisano M, Akioka Y, Nozu K, Kaito H, Shimizu M. Pretransplantation combined therapy with plasmapheresis and rituximab in a second living-related kidney transplant pediatric recipient with a very high risk for focal segmental glomerulosclerosis recurrence. Pediatr Transplant. 2012;16:E286-E290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Valdivia P, Gonzalez Roncero F, Gentil MA, Jiménez F, Algarra G, Pereira P, Rivera M, Suñer M, Cabello V, Toro J, Mateos J. Plasmapheresis for the prophylaxis and treatment of recurrent focal segmental glomerulosclerosis following renal transplant. Transplant Proc. 2005;37:1473-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Vallianou K, Marinaki S, Skalioti C, Lionaki S, Darema M, Melexopoulou C, Boletis I. Therapeutic Options for Recurrence of Primary Focal Segmental Glomerulonephritis (FSGS) in the Renal Allograft: Single-Center Experience. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Naciri Bennani H, Elimby L, Terrec F, Malvezzi P, Noble J, Jouve T, Rostaing L. Kidney Transplantation for Focal Segmental Glomerulosclerosis: Can We Prevent Its Recurrence? Personal Experience and Literature Review. J Clin Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Gonzalez E, Ettenger R, Rianthavorn P, Tsai E, Malekzadeh M. Preemptive plasmapheresis and recurrence of focal segmental glomerulosclerosis in pediatric renal transplantation. Pediatr Transplant. 2011;15:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Mahesh S, Del Rio M, Feuerstein D, Greenstein S, Schechner R, Tellis V, Kaskel F. Demographics and response to therapeutic plasma exchange in pediatric renal transplantation for focal glomerulosclerosis: a single center experience. Pediatr Transplant. 2008;12:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Auñón P, Polanco N, Pérez-Sáez MJ, Rodrigo E, Sancho A, Pascual J, Andrés A, Praga M. Pre-emptive rituximab in focal and segmental glomerulosclerosis patients at risk of recurrence after kidney transplantation. Clin Kidney J. 2021;14:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/