INTRODUCTION
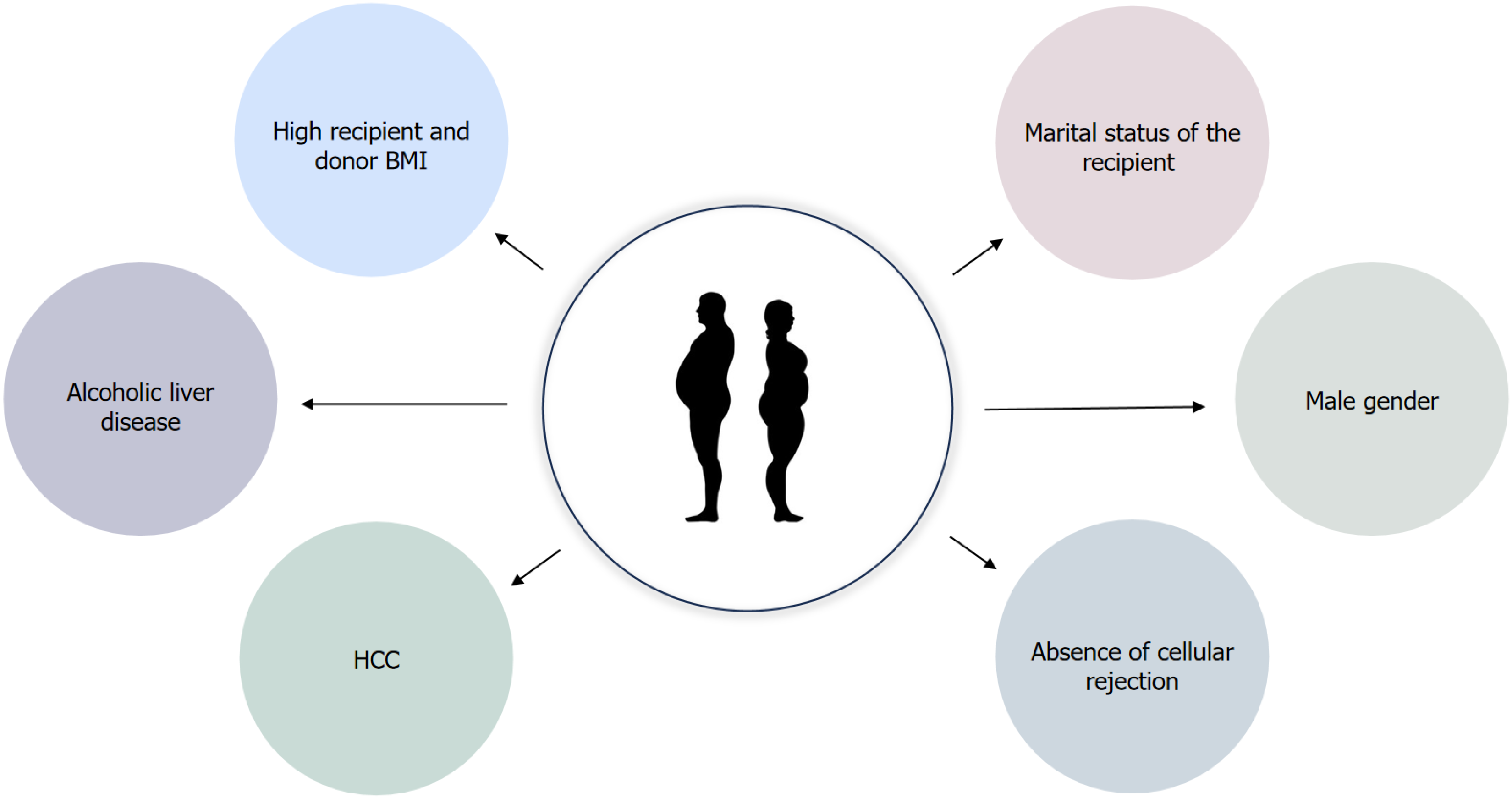
Liver transplantation (LT) is a life-saving intervention for patients afflicted with end-stage liver disease. Over the years, advances in surgical techniques and immunosuppressive therapies have notably enhanced the short-term survival rates of transplant recipients. However, long-term survival remains compromised, with roughly 50% of patients experiencing graft functionality two decades post-transplantation[1]. Already recognized risk factors affecting graft and patient survival are etiology of the primary liver disease, older donor and recipient age[2] and long-term use of immunosuppression which predisposes to the development of de novo malignant diseases[3], renal dysfunction[4], arterial hypertension, new-onset diabetes and hyperlipidemia[5]. Obesity is a risk factor for the development of all components of metabolic syndrome, therefore its influence on graft and patient survival is an important subject of clinical research. Moreover, obesity is associated with recurrent hepatocellular carcinoma (HCC) in liver allograft and it can be a contributing factor for other non-hepatic cancers[6]. In the era of metabolic associated steatotic liver disease (MASLD), as one of the primary indications for LT, post-transplant obesity emerged as a significant risk factor for allograft steatosis[7]. The factors contributing to post-transplant obesity development include the reversal of a catabolic state induced by liver cirrhosis, heightened appetite due to the disappearance of chronic illness and the metabolic effects of immunosuppressive therapy. Key risk factors for the development of obesity after transplantation are high recipient and donor body mass index (BMI), alcoholic liver disease, HCC, male gender, absence of cellular rejection and marital status of the recipient[8,9] (Figure 1). Weight accumulation is common after LT. The greatest weight gain occurs after the first 6 months post-LT in patients aged > 50 years and in those transplanted for chronic liver disease. By first and third year, 24% and 31% of patients became obese, defined as a BMI > 30 kg/m2 with the median weight gain of 5.1 and 9.5 kg above dry weight pre-transplant, respectively. In addition, a pre-transplant BMI > 30 is a strong indicator that the patient would still have a BMI > 30 in 3 years[10]. Patients with pre-transplant obesity usually remain overweight after the LT, and 15%-30% of patients with initially normal body weight are diagnosed with obesity during the first year after a LT. The incidence of concomitant obesity thereafter increases even further in 3-year observation[10,11]. Similar results were observed by Martinez-Camacho et al[12], who described that thirty-six percent (n = 1071) of LT recipients met body mass index (BMI) criteria for becoming either newly overweight (BMI ≥ 25 and < 30) or obese (BMI ≥ 30) in the second post-LT year. Beckmann et al[13] investigated weight changes after solid organ transplantation (SOT) in a prospective cohort study and found that 3 years post transplantation (Tx), compared to other organ groups, liver Tx recipients showed the greatest weight gain (mean 4.8 ± 10.4 kg) and that they had the highest incidence of obesity (38.1%). Information on the impact of new-onset obesity after liver transplantation on patient and graft survival is limited and conflicting. An increase in BMI after transplantation was associated with a significantly higher 5-year patient and graft survival (90% and 89%, respectively), compared to recipients who had a decrease in BMI (77% and 74%, respectively) or who remained of the same BMI (83%, and 82%, respectively)[12]. Yet, long-term effects of obesity on survival warrant further study, as these consequences may manifest over an extended period. Furthermore, weight gain does not imply that this group of patients were actually obese or overweight. On the other hand, obesity present one year after transplantation carries a 2-fold increased risk of total mortality[14]. Therefore, weight management seems to be an important topic for LT recipients. In this narrative review we will discuss different challenges and surgical and pharmacotherapy opportunities currently available for LT patients.
Figure 1 Key risk factors contributing to post-transplant obesity development.
BMI: Body mass index; HCC: Hepatocellular carcinoma.
ROLE OF PHARMACOTHERAPY
Although advancements in immunosuppressive therapies have led to a marked increase in the survival of transplant patients, these treatments come with significant side effects that impact the long-term health of transplant patients. They increase the risk of new malignant diseases, and have a significant influence on the development of metabolic syndrome. They are also nephrotoxic and neurotoxic. Given that metabolic syndrome is the primary risk factor for cardiovascular diseases in transplant patients, which causes 19%-42% of all deaths unrelated to the graft[15], selecting the optimal immunosuppression regimen is pivotal. The influence of pharmacotherapy on the body weight of transplant patients, relates to two categories: The influence of immunosuppressive therapy and the role of pharmacological treatment of obesity and metabolic syndrome.
The influence of immunosuppressive therapy on weight gain after solid organ transplantation
Corticosteroids remain the cornerstone of many induction regimens and one of the main immunosuppressive agents in the early post-transplant period. Their basic metabolic effects are an increase in appetite, a decrease in fatty acid oxidation, increased proteolysis and increased insulin resistance[15]. Despite the fact that corticosteroids are associated with an elevated risk of developing metabolic syndrome after LT[16], studies have not consistently found an association between corticosteroid use and obesity development[17,18], even in those who received steroids for > 3 months[10]. The study which evaluated 203 kidney recipients also showed that the use of steroid therapy had no impact on the percentage of weight gain post-transplantation[19]. Furthermore, a prospective study of 502 consecutive adult organ transplant recipients found no association between BMI levels and steroid doses (P = 0.98) for the overall sample, 12 months after transplant. No significant associations were seen for kidney (P = 0.48), liver (P = 0.11) and heart (P = 0.26) patients when this analysis was reassessed by the type of organ received[20]. On the other hand, prospective cohorts showed that higher cumulative doses of prednisone in the second year post-transplant may increase the risk of obesity[9]. An analysis of four prospective trials of glucocorticoid use in patients with rheumatoid arthritis found a 4% to 8% increase in mean body weight with the use of 5-10 mg/day of prednisone or equivalent for > 2 years[21]. However, most LT patients do not require corticosteroid use for such a long period. Results from a CORPOS prospective study in kidney transplant patients showed that lower corticosteroid doses were significantly associated with better body cell mass recovery after kidney transplantation[22]. Minimizing the duration of corticosteroid therapy is currently recommended to reduce the risk of metabolic syndrome, especially new-onset diabetes, which is the main cause of increased mortality in LT patients[17]. The use of calcineurin inhibitors (CNIs), cyclosporine and tacrolimus is associated with the development of metabolic syndrome, especially arterial hypertension, diabetes and dyslipidemia. However, no significant association was found with their use and the development of obesity. A retrospective analysis of 455 solid transplant patients showed no significant association between their use and the development of obesity one year after transplantation[23]. Meta-analysis of Beckmann et al[24] examined a shared parameter for body weight and found that neither tacrolimus [odds ratio (OR), 0.75; 95%CI: 0.47-1.21; P = 0.24] nor cyclosporine (OR, 1.40; 95%CI: 0.89-2.18; P = 0.14) were related significantly with post-LTx obesity. Furthermore, a retrospective study on kidney transplant recipients showed that CNI type was not a risk factor for the development of obesity in the 48th month[25]. Similar results were observed in a study by Lattanzi et al[26], where they failed to find a correlation with metabolic disorder and the different immunosuppressive regimens adopted. Regarding mTOR inhibitors and their impact on obesity, research is scarce. A randomized controlled study by Charlton et al[27], showed that everolimus may be linked to attenuated weight gain in LT patients compared with those receiving standard-dose tacrolimus. Similar results were described by a retrospective cohort study by Gilad et al[28],which showed that patients experienced no significant weight gain while on mTOR inhibitors, with no difference between the various treatment protocols, further supporting the favorable metabolic profile of mTOR inhibitors. Diekmann et al[29] analyzed body weight data in different patient populations at several time points: (1) Sirolimus (SRL) vs cyclosporine A (CsA) treatment de novo; (2) CsA + SRL vs CsA (SRL-free) treatment de novo; (3) SRL + tacrolimus elimination at 3 months vs SRL + mycophenolate mofetil vs tacrolimus + mycophenolate mofetil de novo; and (4) Conversion from CNI to SRL vs CNI in maintenance patients. The findings revealed that patients on SRL exhibited significantly less weight gain in de novo treatments compared to those on CsA. Additionally, in the conversion study, patients in the SRL group experienced weight loss, whereas those in the CNI group gained weight. Recent data from heart transplant recipients also showed that patients treated with SRL had significantly decreased new onset obesity and weight gain at 1 and 3 years post-heart transplantation, compared to ones treated with tacrolimus[30].
Pharmacological treatment of obesity and metabolic syndrome after solid organ transplantation
Glucagon-like peptide 1 receptor agonists (GLP-1RA) are a newer class of agents used for the treatment of type 2 diabetes mellitus (T2DM) (liraglutide, semaglutide, exenatide, dulaglutide and lixisenatide) and obesity (liraglutide and semaglutide). Due to their beneficial effects on insulin resistance and chronic inflammation, they were also studied in MASLD patients and showed significant improvements in hepatic fat content, liver biochemistry, body composition, metabolic parameters (such as glucose, lipids, and insulin sensitivity), as well as inflammatory markers, while there was also a tendency for improvement of fibrosis that did not reach statistical relevance[31,32]. Data available from rather small studies and primarily involving post-kidney transplant patients with previous or newly developed T2DM suggest their efficacy in glycemic control and weight management like that of non-transplant population while safety data support their use in transplant recipients while their metabolism does not affect CYP- or transporter-mediated drug-drug interactions[33]. One of the mechanisms of GLP-1Ras’ action is a delay of gastric emptying which could potentially alter the absorption of immunosuppressants, but data from small case series where liraglutide, one of the most widely prescribed GLP-1RA, was concomitantly used with tacrolimus did not show any clinically significant alterations in tacrolimus levels[34]. In a study by Thangavelu et al[35] and Singh et al[36], following SOT patients (among whom 7 and 12 post LT, respectively), different GLP-1RAs used were both efficacious and safe in terms of not affecting the level of immunosuppressive agents. Similarly, semaglutide in doses up to 2 mg sc in 23 post-LT patients led to significant weight loss and no additional safety concerns were observed[37]. Results coming from studies (retrospective case reports or small case series) involving GLP-1RA in obesity treatment in SOT, also mainly coming from RTx suggest beneficial effects similar to ones seen in non-renal transplant populations with oral semaglutide either alone or combined with sodium-glucose co-transporter type-2 inhibitors (SGLT-2i)[38]. Indeed, SGLT-2i are yet another anti-hyperglycemic agents with additional extraglycemic benefits transcribed into cardiovascular and renal protection in patients with and without diabetes, gaining approval for use in non-diabetic patients with heart failure and chronic kidney diseases[39]. SGLT-2i also aid weight reduction, and due to antioxidant and anti-inflammatory effects have a promising role in MASLD treatment, as shown from results of clinical trials mainly including T2DM patients reporting beneficial effects on liver fat accumulation and improvement of biological markers[40]. Again, the knowledge of their effectiveness in SOT patients is limited to reports (mainly retrospective of cases or small case series) primarily from kidney and to a lesser extent heart transplant patients, and suggests benefits in terms of weight loss, and HbA1c improvement[41]. It also remains to be seen if co-administration of SGLT-2i and GLP-1RAs would add additional benefits to SOT patients. Newer agents like tirzepatide, a dual GIP/GLP-1RA, showed superior weight loss effects to GLP-1RAs which were almost comparable to bariatric surgery and also steatosis improvement in randomized controlled trials[42], but there is no information on their use in post-LT patients. Currently, ongoing are trials investigating the weight loss potential of retatrutide, a triple-receptor-hormone-agonist (agonist of the glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and glucagon receptors), but until large scale results become available one can only guess their use in post-LT patients[43].
LIFESTYLE INTERVENTIONS: PHYSICAL ACTIVITY AND DIET
Lifestyle changes (diet and physical activity) should be advised for post LT patients[44]. Epidemiological data supporting the importance of physical activity come from the evidence linking 7.2% of all-cause deaths (exceeding four million deaths per year worldwide) with physical inactivity[45]. Mechanisms by which physical activity improves liver health potentially include improvement of hepatic (and extrahepatic) insulin sensitivity, reduction of inflammation, neutralization of reactive oxygen species and therefore promotion of antioxidant activity, reduction of intrahepatic triglyceride levels and de novo lipogenesis, leading to reduction of lipotoxicity[46-48]. In post-LT patients specifically, an aerobic exercise program lasting for 12 weeks was associated with improvement in functional capacity assessed through a 6-minute walk test[49], while a larger randomized control trial showed LT patients benefit from an individualized exercise program while it increases their cardiorespiratory fitness[50]. In addition, evidence supports improvement in quality of life measures in post-LT patients engaged in physical activity[51]. The observation of an inverse relationship between intensity of physical activity and metabolic syndrome in LT recipients coming from a cross-sectional analysis of data from 204 patients suggests its important role in reducing post-LT complications[52]. LT resolves MASLD-related cirrhosis but does not address other risk factors for metabolic syndrome. The long-term follow-up of LT patients still shows high mortality and morbidity rates related to cardiorenal and metabolic complications as well as non-hepatic malignancy burden[3]. Therefore, concomitant participation in physical activity might help in reduction of excessive weight, improve glycemia in case of diabetes mellitus, improve parameters of metabolic syndrome, cardiorespiratory fitness and cardiovascular risk factors[53]. Moreover, there are studies linking sarcopenia, which is closely related to sedentary life-style and low levels of physical activity, with poor post-LT outcomes in terms of higher mortality and graft failure risk, independent of other confounders[54]. It is important to consider/diagnose sarcopenia in overweight/obese post-LT patients. In a meta-analysis including 1515 patients, pre-transplant sarcopenic obesity was associated with higher post-LT mortality in 1, 3 and 5-years follow-up[55]. Therefore, physical activity intervention might also be an important means of targeting sarcopenia and indirectly affecting patients' survival and quality of life, and although programs are still lacking standardization, it seems that 150 minutes of mild/moderate intensity aerobic activity during 3-5 days weekly together with 2-3 weekly sessions of resistance training might be beneficial even for cirrhotic patients[56]. Dietary recommendations for patients with MASLD who are overweight or obese include calory restriction (at least 500 kcal/day), avoidance of fructose and targeting 5%-10% loss of initial BMI. On the other hand, there is no consensus on the upper limit of recommended calory restriction, with ranges between 1000 up to 1600 kcal per day, while, excessive and/or rapid weight loss (e.g., very low carbohydrate, high fat diets) might cause or exacerbate the insulin resistance and MASLD[44]. It seems that macronutrient composition of diet, independent of energy intake, can induce MASLD/MASH. With that respect, saturated fatty acids, trans-fats, simple sugars (sucrose, fructose) and animal proteins induce liver damage by modulating the accumulation of triglycerides and antioxidant activity in the liver, which affects insulin sensitivity and postprandial triglyceride metabolism, while consumption of monounsaturated fatty acids, PUFA ω3 fats, plant-based proteins and dietary fibers appear to be beneficial to the liver[57]. Moreover, micronutrients such as zinc, copper, vitamins A, C, D, E and carotenoids have antioxidant, antifibrotic, immunomodulatory and lipoprotective effects on hepatic cells, while excess iron and selenium might exacerbate liver inflammation[58]. In terms of different dietary patterns, and their role in promoting liver benefits in MASLD patients, a sample of data exists on the mediterranean diet (MD), which is high in fiber content, low in saturated fat and red meat. MD lowers oxidative stress, proinflammatory cytokines and insulin resistance, which decreases intrahepatic fat content in MASLD patients, as shown in one of the largest randomized clinical trial studies by Gepner et al[59]. Other popular dietary patterns, including different patterns of intermittent fasting and ketogenic diets, might result in deficit of vitamins, minerals, and bioactive compounds such as polyphenols leading to Wernicke’s encephalopathy, cardiac beriberi, and optic neuropathy in patients with low carbohydrate intake, or in case of a fasting to severe hypoglycemia, which might be of special concern in patients developing cirrhosis in transplanted liver. Therefore, these special dietary regimens, until enough evidence is gathered from high-scale and long-term prospective studies, still need caution and supervision[60]. In addition, nutritional supplements, together with physical exercise, may play an important role in preventing sarcopenia. For example, a recent Italian randomized pilot study reported that a 12-week supplementation after LT with β-hydroxy-β-methyl-butyrate, an active metabolite of leucine with anabolic effect that inhibits muscle proteolysis, seems to significantly improve muscle mass values in sarcopenic LT patients[26]. However, these supplementations are usually expensive and further studies with a larger cohort of patients are needed to confirm their positive clinical effects.
BARIATRIC SURGERY
The main goal of bariatric surgery after LT is to improve graft survival by reducing obesity-related comorbidities and the incidence of graft steatosis. Drug treatment alone for patients with obesity after LT has limited effectiveness due to drug-related adverse interactions, so surgical treatment remains the gold standard for these patients[61]. Ideal candidates for surgical intervention are patients with a BMI equal to or greater than 35 kg/m2 regardless of presence, absence or severity of comorbidities[62]. The main benefits of bariatric surgery include improvements in obesity-related comorbidities: T2DM (83%), dyslipidemia (63%), polycystic ovarian syndrome (78%), venous stasis disease (95%), obstructive sleep apnea (74%-98%), arterial hypertension (52%-92%) and MASLD (20%-37%)[63]. An interdisciplinary approach beside bariatric surgeons must also include hepatologists, psychiatrists and clinical nutritionists. The current recommendation is to wait at least one year after LT to minimize the risk of acute cellular rejection associated with bariatric surgery[64]. Four main types of bariatric surgical procedures can be performed for severely obese patients: Gastric band placement, sleeve gastrectomy, Roux-en-Y gastric bypass or biliopancreatic diversion with duodenal switch. The most preferred bariatric procedure after LT is a sleeve gastrectomy, which permanently removes most of the body and fundus of the stomach (60% to 80%)[63,65]. Besides reducing the gastric volume, its primary mechanism may be through alteration of neurohormonal pathways (reduction in the levels of ghrelin secreted by the stomach and increase in the levels of peptide-YY and GLP-1 which induce satiety)[64]. Sleeve gastrectomy has fewer metabolic consequences of malabsorption of nutrients and immunosuppressive medications as a restrictive bariatric surgical option compared to Roux-en-Y gastric bypass[65,66]. Patients after LT have an increased risk of wound complications (prolonged healing and infection) and dehiscence due to the use of steroids and other immunosuppressive medications with up to 16% compared to 1% in the general bariatric population[67,68]. Besides medication-related complications, significant adhesions can make bariatric surgery technically difficult in post-LT patients[69]. In the retrospective matched case-control series comparing bariatric surgery for post-LT patients vs non-transplant patients, no difference in BMI loss, remission of comorbidities, surgical time, complications and mortality was observed, except for an extended hospital stay in the post-LT group[70]. In the study by Morris et al[65] with patients who underwent laparoscopic sleeve gastrectomy (LSG) after LT compared to non-LT patients with LSG, there was no mortality and liver allograft rejection. After LSG, BMI decreased from 42.7 to 35.9 kg/m2 (P < 0.01). Following LSG in patients with diabetes, 60% discontinued insulin. Post-LT patients had a similar decrease in BMI and reduction in comorbidities in one year compared with a matched non-LT patient cohort. Bariatric surgery seems to represent the best treatment option for obesity and its related comorbidities in LT patients[71].
DISCUSSION
In accordance with current findings and extant literature, the management of obesity and its therapeutic strategies emerge as pivotal components in the comprehensive care regimen post liver transplantation, significantly influencing the long-term prognosis of this patient cohort. Moreover, given the escalating global prevalence of obesity and MASLD, an increase in the obesity prevalence among transplant recipients is foreseeable[72]. As delineated in our comprehensive review, pre-transplant obesity stands out as a paramount risk determinant for post-transplant adiposity[10,11]. Obesity has both direct impact on graft survival and an indirect influence on the development of metabolic syndrome, profoundly impacting patients' overall survival[14,73]. In light of these findings, heightened awareness regarding obesity in transplant populations is imperative, emphasizing timely recognition and intervention to safeguard optimal quality of life post-transplantation. Among the most important measures is the meticulous management of immunosuppressive regimens, entailing the right selection and duration of immunosuppression protocols. As described by Saunders et al[74], certain subgroups of patients exhibiting low rejection risk, as inferred from histological assessments and levels of donor-specific antibodies, may substantially benefit from a tailored immunosuppressive approach, characterized by significant early immunosuppressive dose reduction. In recent years we have witnessed significant improvements in pharmacotherapeutic interventions targeting obesity, particularly in individuals with concurrent diabetes mellitus. Contrary to initial concerns regarding potential interactions with immunosuppressive agents, recent investigations have debunked such concerns[33,35], announcing the prospective utility of these agents in treating obesity among transplant recipients. Concurrently, the promotion of physical activity concomitant with dietary modifications emerges as one of the cornerstone interventions, known to have positive effects on both quality of life and amelioration of metabolic derangements[52,53]. Bariatric surgery represents another efficacious tool against post-transplant obesity. Contrary to initial fear regarding its safety in individuals with portal hypertension, contemporary evidence suggests its viability and favorable impact on overall survival in this cohort[75]. However, the optimal timing of this intervention remains a subject of ongoing debate.
CONCLUSION
The management of post-liver transplantation obesity stands as a pivotal concern necessitating recognition and proactive intervention to optimize the quality of life and long-term survival outcomes in affected patients.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country of origin: Croatia
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Rodrigues AT S-Editor: Li L L-Editor: A P-Editor: Xu ZH