Published online Sep 18, 2024. doi: 10.5500/wjt.v14.i3.95233
Revised: May 31, 2024
Accepted: June 26, 2024
Published online: September 18, 2024
Processing time: 117 Days and 2 Hours
Hypothermic machine perfusion (HMP) has demonstrated benefits in terms of early kidney transplant function compared to static cold storage. While longer preservation times have shown detrimental effects, a previous paired study indicated that longer pump times (the second kidney in a pair) might lead to improved outcomes.
To revisit the prior paired study's somewhat unexpected results by reviewing our program's experience.
A total of 61 pairs of transplant recipients who received kidneys from the same donor (2012-2021) were analyzed. Patients were divided into two groups depending on whether they were transplanted first (K1) or second (K2). Therefore, the patients in each pair had identical donor characteristics, except for time on the pump. Statistical analyses included Kaplan-Meyer analysis and paired tests, including McNemar's test, student's paired t-test, or Wilcoxon's test, as appropriate.
The two groups of recipients had similar demographics (age, body mass index, diabetes, time on dialysis, sensitization and retransplants). Cold ischemic times for K1 and K2 were 8.9 (95%CI: 7.9, 9.8) and 14.7 hours (13.7, 15.8)
Our results agree with a previous study that suggested possible advantages to longer pump times. Both studies should encourage further research into HMP's potential anti-inflammatory effect.
Core Tip: Our study assesses the impact of longer hypothermic machine perfusion (HMP) times on kidney transplant outcomes using a matched pair analysis of 61 kidney pairs. Our results agree with previous findings, showing that the second kidney, subjected to longer HMP times, has improved freedom from biopsy-proven acute rejection within the first year. These outcomes suggest that extended HMP durations, when applied judiciously, may confer anti-inflammatory benefits. This supports the potential for further basic science research and future optimization of perfusion protocols.
- Citation: Verdiales C, Baxter L, Lim HJ, Beck G, Moser MA. Matched pair analysis of the effect of longer hypothermic machine perfusion time on kidney transplant outcomes. World J Transplant 2024; 14(3): 95233
- URL: https://www.wjgnet.com/2220-3230/full/v14/i3/95233.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i3.95233
It has long been established that longer cold ischemic time (CIT) is associated with decreased kidney transplant function[1]. It has even been suggested that increased injury from increased CIT may increase the immunogenicity of kidney grafts, leading to a higher incidence of early acute rejection[2].
Hypothermic machine perfusion (HMP) has been shown to improve early kidney function and increase long-term graft survival compared to static cold storage[3]. The effect may even be more pronounced for donation after circulatory determination of death (DCDD) donor kidneys[4]. As such, HMP is our program's primary preservation method for all deceased (and living) donor kidneys retrieved for transplantation.
A study analyzing unpaired kidneys from three trials[5] found a somewhat unexpected result: The kidneys transplanted second, with a longer time on HMP, had better freedom from biopsy-proven acute rejection (BPAR). This finding was subsequently confirmed with a paired kidney study by the same group (n = 66)[6]. A recent study analyzed data from over 79000 patients in the UNOS registry and found, after careful statistical adjustment, that kidneys that underwent HMP were significantly less likely to experience acute rejection within the first year [(odds ratio (OR) 0.91, P = 0.002) compared to kidneys that underwent static cold storage[7]. We aimed to validate the previous findings of the paired study by evaluating data from a single center with standardized practices.
A database, including all consecutive deceased-donor kidney transplant pairs in our institution between 2012 and 2021, was constructed from medical records and transplant charts. The time period was chosen as our surgical program underwent a 'rebirth' in 2012 with the recruitment of three new transplant surgeons who had all completed their fellowships at the same institution. Our study included all pairs in which both donor kidneys were retrieved by our program, and both kidneys were also transplanted within our program (n = 61 pairs). This approach allows us to use the matched-pair design in deceased donor kidney transplant studies, which helps reduce variability and controls for donor factors. There was a mandatory 5-minute 'no touch' time after cardiac arrest for all DCDD donors. Aortic cannulation and flushing with cold University of Wisconsin solution occurred within 10 minutes of cardiac arrest in all DCDD donors. Whether DCDD or donation after neurological determination of death (DNDD), all kidneys were dissected 'in the cold' after cross-clamping and flushing; all retrieved kidneys (DCDD and DNDD) received approximately 500 cc of back table flush per kidney. Both kidneys retrieved from a donor were treated the same way, including placement on Lifeport (Organ Recovery Systems, Chicago) HMP device for a minimum of 4 hours, using KPS-1 solution (Organ Recovery Systems, Chicago). Both kidneys were maintained at a temperature of 4 °C throughout the perfusion period.
One or two of three surgeons, all of whom trained at the same institution, performed the transplant surgery. They employed a standard surgical technique that included anastomosis of the renal artery and vein to the external iliac artery and vein, respectively, followed by removal of the clamps and reperfusion. Once hemostasis was satisfactory, anastomosis of the spatulated ureter to the bladder using 5-0 PDS was performed over a double-J stent.
All DCDD and highly sensitized recipients received thymoglobulin induction (1.5 mg/kg/day for 7 days), followed by the gradual introduction of tacrolimus-based maintenance immunosuppression (target trough levels of 8-12 ng/mL for the first 3 months, then 5-8 ng/mL after that). Recipients of other kidney types received basiliximab induction (20 mg iv on day 0 and day 4) and maintenance immunosuppression with tacrolimus with trough levels as above (except two patients who received cyclosporine-based immunosuppression) in combination with mycophenolate mofetil (1 gram twice daily) and steroids with rapid taper.
Delayed graft function (DGF) was defined as the requirement for dialysis during the first seven days post-transplant. Transplant renal biopsies were not protocol-driven but instead obtained as part of a standard workup for a rise in serum creatinine by more than 10% in patients where calcineurin inhibitor toxicity, inadequate hydration, and vascular and ureteric problems had first been ruled out. BPAR was defined by pathology report, with all biopsies read by a fellowship-trained pathologist, with most cases reported as a consensus between two pathologists. Graft failure was defined as the return to chronic scheduled dialysis. No patients were lost to follow-up, and all recipients were followed for at least two years (average 3.4 years) or until graft loss or death.
Analyses were performed with Stata ver 18 (College Station, TX) using paired tests whenever possible because we studied pairs of kidneys from the same donor. The paired student's t-test was used for normally distributed variables, such as age, weight, creatinine clearance, and CIT; the distribution of each was first plotted to confirm their normality. Time on dialysis prior to transplant was inconsistently recorded (i.e., "this patient has been on dialysis for over 5 years" was recorded as 60 months). Therefore, non-parametric paired statistical testing was used (Wilcoxon's paired rank sum test), as it is less sensitive to outliers and does not require the assumption of normality. McNemar's paired test was used for nominal data, appropriate for dichotomous' yes/no' variables such as diabetes or DGF. Finally, Kaplan-Meier analysis was used for survival analysis, the standard test for time-to-event comparisons; although a matched survival analysis would have been optimal, none could be found at this time.
The demographics of the 61 pairs of recipients, with each pair receiving kidneys from the same donor, are shown in Table 1. First (K1) or second (K2) kidney recipients had similar characteristics, including age, sex, weight, height, body mass index, diabetes, sensitization, retransplant, and time on dialysis prior to transplant. All 122 patients were followed to the time of graft loss or death, with none lost to follow-up.
| Characteristics | Received kidney 1 (n = 61) | Received kidney 2 (n = 61) | P value |
| Age (years, 95%CI) | 52.2 (48.8, 55.4) | 53.3 (49.8, 56.7) | 0.64 |
| Sex (M:F) | 42:19 | 42:19 | 1.0 |
| Weight (kg, 95%CI) | 82.1 (77.4, 86.7) | 80.8 (76.2, 85.5) | 0.71 |
| Height (cm, 95%CI) | 173 (170, 175) | 171 (168, 173) | 0.28 |
| BMI (kg/m2, 95%CI) | 27.4 (26.1, 28.7) | 27.6 (26.3, 28.9) | 0.79 |
| Diabetes (%) | 20 (33) | 15 (25) | 0.32 |
| HSP (%) | 3 (5) | 3 (5) | 1.0 |
| Average HLA mismatches (95%CI) | 6.3 (5.9, 6.7) | 6.4 (6, 6.85) | 0.65 |
| Retransplant (%) | 5 (8) | 5 (8) | 1.0 |
| Time on dialysis prior to transplant (months, IQR) | 47 (27, 60) | 48 (33, 64) | 0.22 |
Factors relating to donation are shown in Table 2. The majority of the 61 paired kidneys were obtained from DCDD (42/61, 69%). Because the second kidney had to await the completion of the first transplant surgery before being transplanted, there are expected significant differences between the two groups in terms of pump time and total CIT, but no difference in terms of static cold storage time, nor warm ischemic time at the time of anastomosis.
| Factor | Received kidney 1 (n = 61) | Received kidney 2 (n = 61) | P value |
| DCDD (%) | 42 (69) | 42 (69) | 1.0 |
| ECD (%) | 19 (31) | 19 (31) | 1.0 |
| HMP time (HR, 95%CI) | 5.2 (4.5, 5.8) | 10.8 (9.9, 11.7) | < 0.0001b |
| Static cold storage time (HR, 95%CI) | 3.7 (3.1, 4.3) | 3.9 (3.1, 4.7) | 0.38 |
| Total CIT (HR, 95%CI) | 8.9 (7.9, 9.8) | 14.7 (13.7, 15.8) | < 0.0001b |
| WIT2 (min, 95%CI) | 52 (47, 57) | 53(48, 59) | 0.81 |
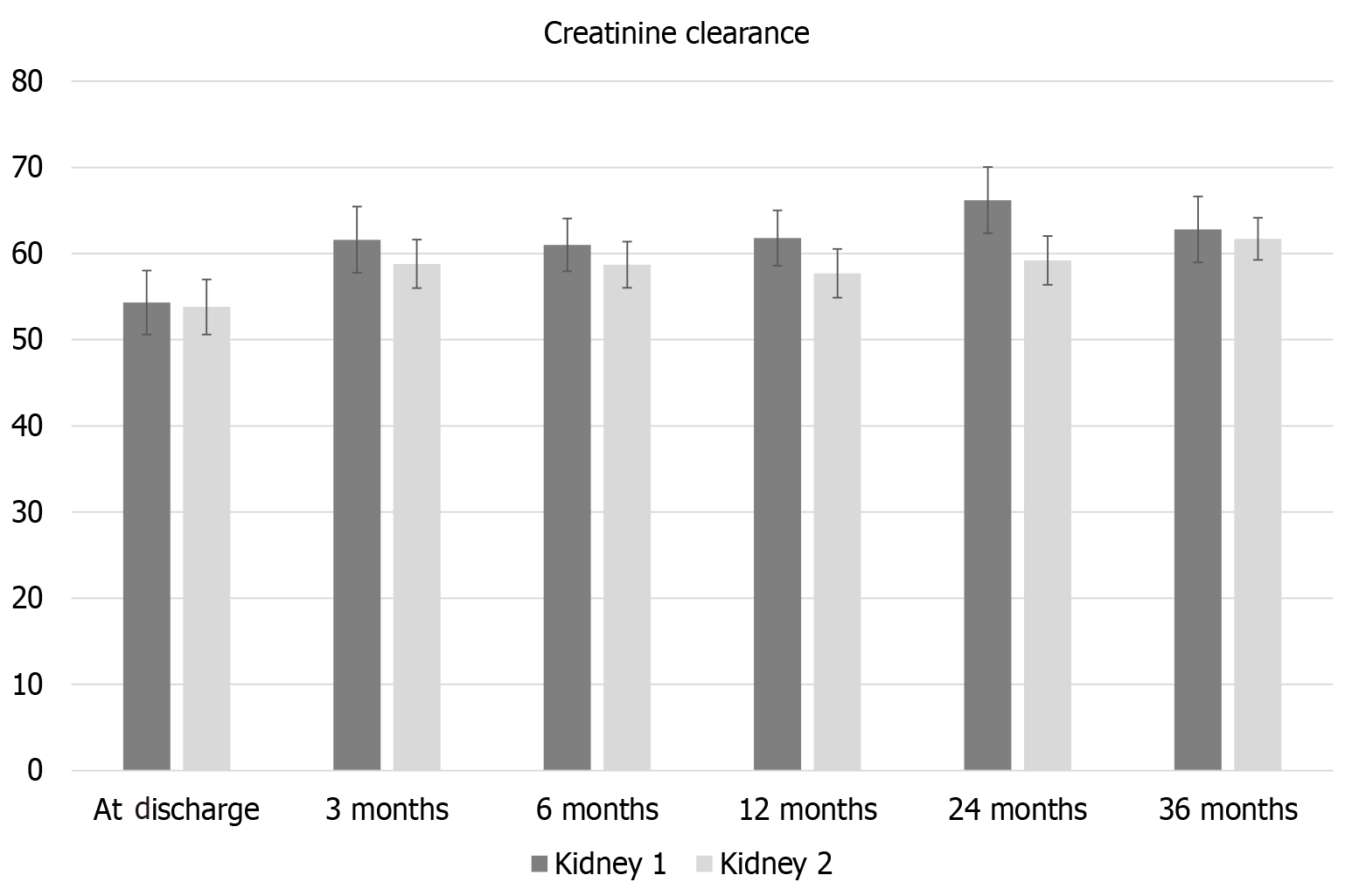
There was a borderline higher incidence of DGF in K1 compared to K2 (20/61 (33%) vs 12/61 (20%), P = 0.045. Creatinine clearance at discharge, 3, 6, 12, 24, and 36 months is shown in Figure 1. There was no significant difference in creatinine clearance at any of the time points.
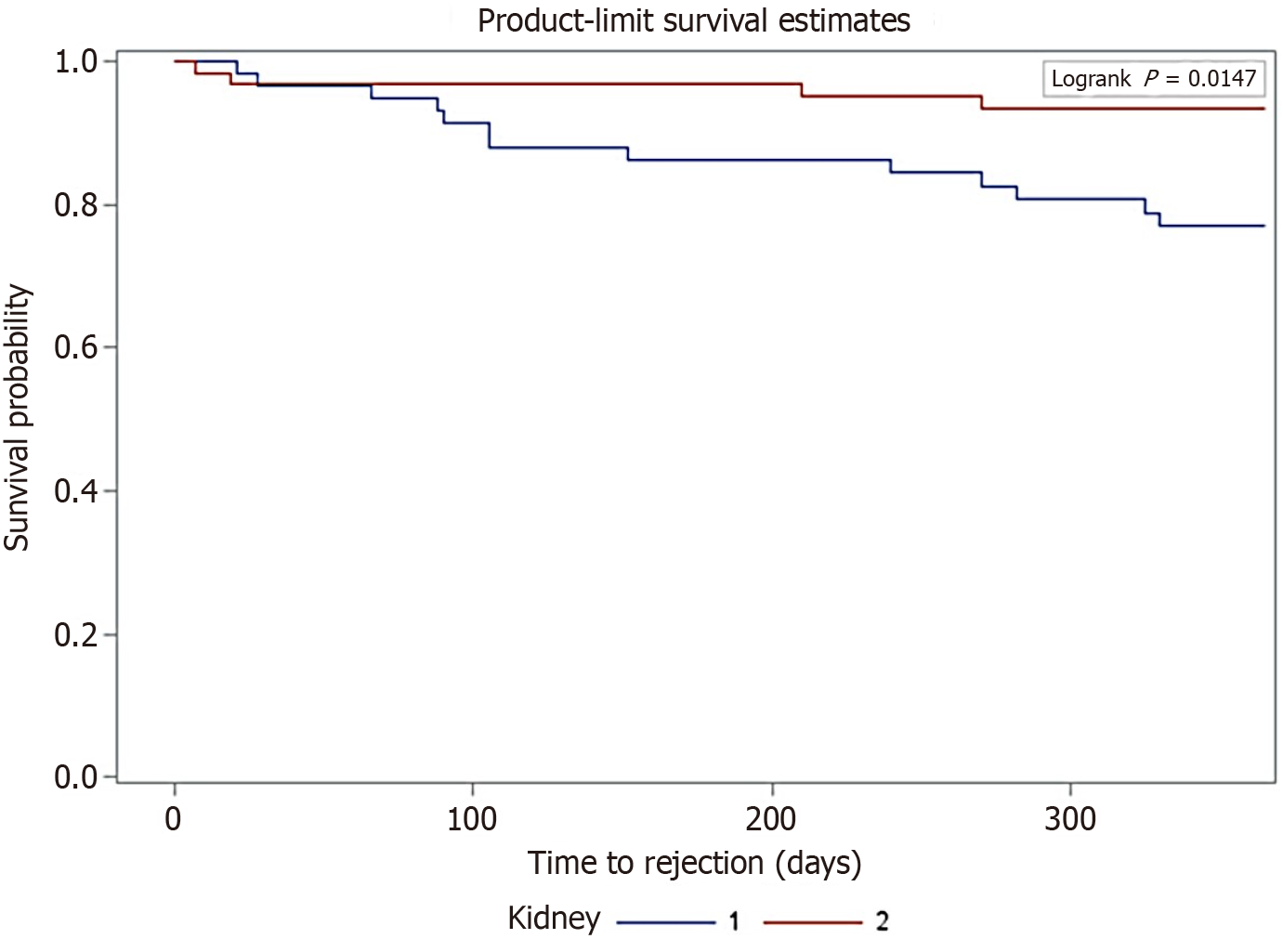
Using Kaplan-Meyer survival analysis, freedom from BPAR in the first year was observed with a higher incidence in K2 recipients compared to K1 recipients, with a P-value of 0.015 (Figure 2). In the longer term, there was no significant difference between the two groups in terms of freedom from BPAR.
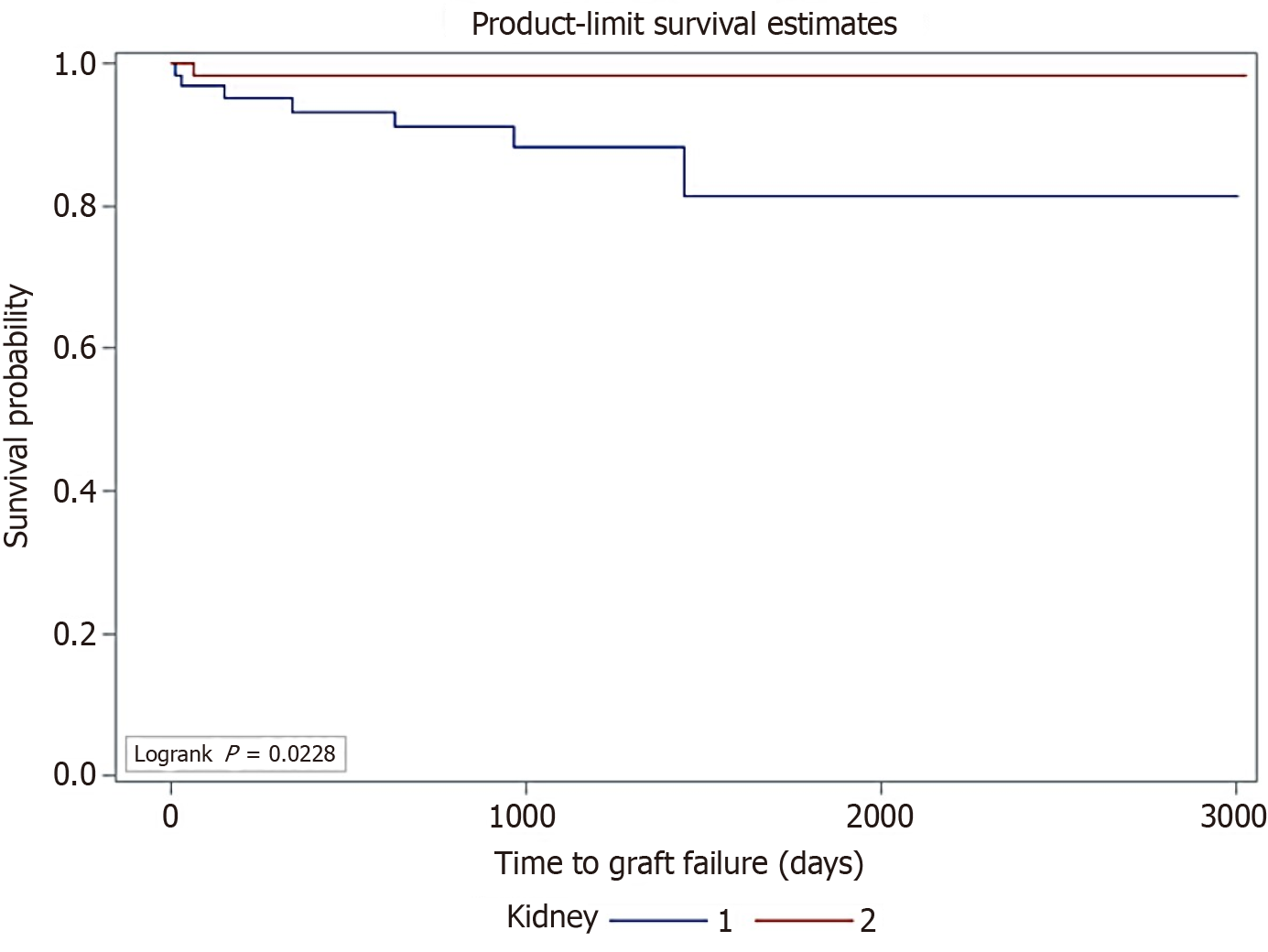
Using Kaplan-Meyer survival analysis, graft survival for K2 was longer than for K1 (P = 0.023) (Figure 3). The median time to graft loss, when it occurred, was estimated at 1072 days for K1 vs 1276 days for K2.
We analyzed 61 pairs of kidneys from the same donor to revisit a previous paired kidney study. All the kidneys in our study were transplanted in the same institution and by the same surgeons using a standard transplant procedure and immunosuppression. Our results were consistent with the unexpected findings observed in the previous study: the longer HMP times of the second kidneys were associated with a reduced risk of BPAR in the first year. The second kidneys in a pair appeared to have outcomes that were at least as good as those of the first and seemed slightly better in some respects. Yet longer CIT has been widely documented to increase the risk of kidney injury, leading to higher rates of DGF and BPAR.
A study which included over 79000 (unpaired) patients from the UNOS database investigated the potential immunological benefits of HMP (used for 42% of the kidneys in the study)[8]. Adjusting for eight variables, including donor and recipient factors and DCDD, they concluded that HMP was associated with a significant reduction in the incidence of early acute rejection at one year (OR: 0.92, P = 0.002).
A study looking at pairs of kidneys transplanted between 1989 and 1995, without the use of HMP, using cyclosporine immunosuppression, showed a significant decrease in graft survival (but not patient survival) for the second kidney[9]. Two other paired kidney studies (not specifically looking at HMP) documented an increase in DGF in the second kidneys but no difference in graft survival[10,11]. In all three studies, the incidence of early acute rejection was not measured.
The first studies found in the literature that examined the impact of longer HMP time on rejection in paired kidneys were by Ciancio et al[5]. Their earlier work suggested a decrease in early acute rejection, and they hoped to confirm this by studying recipients who received paired kidneys in three separate prospective trials of immunosuppression. Their second study included a single DCDD donor pair. They documented a trend towards higher freedom from BPAR in the second kidney in paired analysis, which became significant (P = 0.04) with Kaplan-Meier analysis, in keeping with our findings. A meta-analysis of studies of liver transplants managed with HMP versus static cold storage showed a reduction in acute rejection when machine perfusion was used (OR: 0.55, P = 0.02)[12].
Our study is unique in that the transplants were all retrieved and performed in the same program, using a standardized surgical technique and immunosuppression, including a significant proportion of DCDD donor kidneys. Our CIT was lower than those reported in the other studies summarized above; we averaged 9 and 15 hours for K1 and K2, while other studies noted around 16-17 hours and around 23 hours for the first and second kidney, respectively. Ciancio's study had the highest CITs in our literature review, averaging 28 and 36 hours for the first and second kidney, respectively[6]. Only 10 patients out of 122 in our study received kidneys with more than 18 hours of CIT.
It is currently not clear why longer pump times seem to have a beneficial effect on acute rejection rates. It is possible that longer pump times contribute to reducing inflammatory factors and vasoconstricting peptides, allowing cells and tubules to recover better from ischemic injury. However, more research is needed to confirm this. The optimal duration of machine cold perfusion to achieve anti-inflammatory effects without allowing the ischemic injury to outweigh the benefits remains unclear. One study investigated the effects of oxygenated machine cold perfusion of expanded criteria donation kidneys for at least 2 hours (median 4 hours) at the end of preservation[13]. This study did not demonstrate benefits in terms of DGF, renal function, or acute rejection within this time frame. While HMP may have anti-inflammatory benefits, there is undoubtedly a point beyond which increasing CIT, whether the kidney is pumped or not, will lead to adverse effects that outweigh the anti-inflammatory benefits. Our paired data, with relatively short CITs, support this.
A strength of our study was the uniformity and consistency of the study population and the paired nature of the comparisons. All the kidneys were retrieved and transplanted in our program using the same standardized transplant operation and immunosuppression regimens. By the nature of our program and catchment area, the follow-up is thorough. However, there are a few limitations to the current study. The data was collected retrospectively, and the assignment to K1 or K2 was not randomized. Even though the two groups of recipients look very similar in terms of the factors that were compared, there may have been other factors, medical and surgical, that made it more likely for a patient to receive the first kidney in a pair. With a sample size of 61 pairs, similar to the 66 pairs in the previous study[7], the generalizability of our results is somewhat limited. However, the paired nature of both studies compensates for this small sample size to some extent, and the consistency of findings across both studies enhances confidence in the results.
This retrospective analysis of donor-matched kidney pairs suggests that longer HMP times may not have an entirely negative impact on transplanted kidneys. In fact, there appears to be a trend towards improved outcomes, such as a lower incidence of BPAR. These findings are consistent with those of a previous study and, at the very least, may provide reassurance to patients receiving the second kidney in a pair. The consistency of these findings with a large-scale UNOS database study involving 79000 patient records and published results from a major American program underscores the potential for a paradigm shift in organ preservation strategies: HMP time (within limits) may be beneficial in reducing inflammation and early acute rejection.
Further studies are needed, including studies involving more donor-matched pairs of recipients. Other studies should also examine inflammatory peptides in the perfusate, which might help explain the improved outcomes of longer time on HMP.
| 1. | van der Vliet JA, Warlé MC, Cheung CL, Teerenstra S, Hoitsma AJ. Influence of prolonged cold ischemia in renal transplantation. Clin Transplant. 2011;25:E612-E616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Halloran PF, Homik J, Goes N, Lui SL, Urmson J, Ramassar V, Cockfield SM. The "injury response": a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc. 1997;29:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E, Kirste GR, Rahmel A, Leuvenink HG, Paul A, Pirenne J, Ploeg RJ. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 815] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 4. | Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, Rahmel A, Squifflet JP, van Heurn E, Monbaliu D, Ploeg RJ, Pirenne J. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Ciancio G, Gaynor JJ, Sageshima J, Chen L, Roth D, Kupin W, Guerra G, Tueros L, Zarak A, Hanson L, Ganz S, Ruiz P, O'Neill WW, Livingstone AS, Burke GW 3rd. Favorable outcomes with machine perfusion and longer pump times in kidney transplantation: a single-center, observational study. Transplantation. 2010;90:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Ciancio G, Gaynor JJ, Sageshima J, Roth D, Kupin W, Guerra G, Tueros L, Zarak A, Hanson L, Ganz S, Chen L, Ruiz P, Livingstone AS, Burke GW 3rd. Machine perfusion following static cold storage preservation in kidney transplantation: donor-matched pair analysis of the prognostic impact of longer pump time. Transpl Int. 2012;25:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Samoylova ML, Nash A, Kuchibhatla M, Barbas AS, Brennan TV. Machine perfusion of donor kidneys may reduce graft rejection. Clin Transplant. 2019;33:e13716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Mikhalski D, Wissing KM, Ghisdal L, Broeders N, Touly M, Hoang AD, Loi P, Mboti F, Donckier V, Vereerstraeten P, Abramowicz D. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation. 2008;85:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Giblin L, O'Kelly P, Little D, Hickey D, Donohue J, Walshe JJ, Spencer S, Conlon PJ. A comparison of long-term graft survival rates between the first and second donor kidney transplanted--the effect of a longer cold ischaemic time for the second kidney. Am J Transplant. 2005;5:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Pérez-Canga JL, Martín Penagos L, Ballestero Diego R, Valero San Cecilio R, Rodrigo Calabia E, Belmar Vega L, Serrano Soto M, Ruiz Martínez L, Lopez Del Moral Cuesta C, Ruiz San Millán JC. Effect of Cold Ischemia Time on Kidney Graft Function and Survival: Differences Between Paired Kidney Transplants From the Same Donor. Transplant Proc. 2019;51:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11:2647-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Maspero M, Ali K, Cazzaniga B, Yilmaz S, Raj R, Liu Q, Quintini C, Miller C, Hashimoto K, Fairchild RL, Schlegel A. Acute rejection after liver transplantation with machine perfusion versus static cold storage: A systematic review and meta-analysis. Hepatology. 2023;78:835-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Husen P, Boffa C, Jochmans I, Krikke C, Davies L, Mazilescu L, Brat A, Knight S, Wettstein D, Cseprekal O, Banga N, Bellini MI, Szabo L, Ablorsu E, Darius T, Quiroga I, Mourad M, Pratschke J, Papalois V, Mathe Z, Leuvenink HGD, Minor T, Pirenne J, Ploeg RJ, Paul A. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant: A Randomized Clinical Trial. JAMA Surg. 2021;156:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/