Published online Sep 18, 2024. doi: 10.5500/wjt.v14.i3.92335
Revised: March 4, 2024
Accepted: July 2, 2024
Published online: September 18, 2024
Processing time: 190 Days and 9 Hours
Mineral bone disease is associated with chronic kidney disease and persists after kidney transplantation. Immunosuppressive treatment contributes to the patho
To evaluate the effectiveness and safety of bisphosphonate treatment on post kidney transplantation bone mineral density (BMD).
We included kidney transplant recipients (KTRs) whose BMD was measured after the operation but before the initiation of treatment and their BMD was measured at least one year later. We also evaluated the BMD of KTRs using two valid mea
Out of 254 KTRs, 62 (39 men) were included in the study. Bisphosphonates were initiated in 35 KTRs in total (20 men), 1.1 ± 2.4 years after operation and for a period of 3.9 ± 2.3 years while 27 (19 men) received no treatment. BMD improved significantly in KTRs who received bisphosphonate treatments (from -2.29 ± 1.07 to -1.66 ± 1.09, P < 0.0001). The control group showed a non-significant decrease in BMD after 4.2 ± 1.4 years of follow-up after surgery. Kidney function was not affected by bisphosphonate treatment. In KTRs with established osteoporosis, active treatment had a similar and significant effect on those with osteopenia or normal bone mass.
In this retrospective study of KTRs receiving bisphosphonate treatment, we showed that active treatment is effective in preventing bone loss irrespective of baseline BMD.
Core Tip: Mineral bone disease can occur in patients with chronic kidney disease and is highly prevalent among patients after kidney transplantation. In this study, antiresorptive treatment with bisphosphonates had a beneficial effect on bone mineral density (BMD) in kidney transplant recipients (KTRs) irrespective of baseline BMD values. KTRs who received no treatment showed a non-significant decrease in BMD.
- Citation: Georgopoulou GA, Papasotiriou M, Ntrinias T, Savvidaki E, Goumenos DS, Papachristou E. Impact of bisphosphonate treatment on bone mineral density after kidney transplant. World J Transplant 2024; 14(3): 92335
- URL: https://www.wjgnet.com/2220-3230/full/v14/i3/92335.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i3.92335
Mineral bone disease is common among patients with chronic kidney disease, especially in those with end-stage kidney disease (ESKD), which often persists after kidney transplantation. Following kidney transplantation, despite imp
The pathogenesis of bone disease after transplantation is based on several factors, including preprocedural bone density abnormalities caused by ESKD, high or low bone turnover disease, or a combination of both. In most instances, ESKD is associated with low bone mineral density (BMD)[3,4]. Moreover, immunosuppressive drugs, such as corticosteroids, reduce bone mass by inhibiting osteoblasts and stimulating osteoclast activity or by decreasing gastrointestinal calcium absorption and increasing renal calcium excretion[4]. Finally, calcineurin inhibitors (tacrolimus and cyclosporine) also cause bone loss[5]. Most bone loss occurs within the first 6 months after kidney transplantation, mostly in trabecular bone tissue[6], and it stabilizes or even tends to recover to baseline after 12 months[7]. This loss can reach 10% during the first year after surgery, as shown in older cohorts[8], while bone loss is reported to be in the range of 0.1%-5.7% in the lumbar spine in more recent publications of relevant trials[9]. The abovementioned data show a great difference in fracture risk rates during the years after kidney transplantation. The latter is due to several factors, such as fewer comorbidities in KTRs, lower preferences for corticosteroid treatment, limited follow-up time, and the variety of fracture locations[10]. Independent of the degree of bone loss, KTRs are at increased risk for fractures[11]. In a cohort study from Canada, the 10-year cumulative incidence of hip fractures among KTRs was 1.7%. Moreover, KTRs had a greater 3-year cumulative incidence of nonvertebral fractures than did the general population who had no previous nonvertebral fractures[10].
Several treatment options have been implemented for reducing bone loss in KTRs, and these options have been thoroughly reviewed elsewhere[12]. The administration of bisphosphonates, either orally or intravenously (i.v.), vitamin D analogs and calcitonin seems to improve the BMD of the lumbar spine, while bisphosphonates and vitamin D analogs can increase the BMD at the femoral neck[13]. Previous guidelines recommend that treatment with bisphosphonates can be effective in postmenopausal women with osteoporosis and in those with glucocorticoidinduced osteoporosis[14,15]. Nevertheless, the overall net benefit of such a therapeutic approach with bisphosphonates is still not clear in KTRs, while a recent meta-analysis did not show that bisphosphonates reduce the risk for fracture[16].
Thus, the aim of this retrospective study was to evaluate the effectiveness of bisphosphonate treatment in preventing post-kidney transplantation BMD loss as well as its safety.
In total, 254 successful KTRs with a functioning graft one month after surgery were treated between 1997 and 2018 at the University Hospital of Patras. In this study, we retrospectively examined the BMD of recipients to evaluate the impact of bisphosphonate treatment on BMD. The inclusion criteria included 18 years of age or older at the time of surgery, availability of a BMD measurement after surgery and before the initiation of bisphosphonate treatment, and at least one BMD measurement one year or more after the initiation of treatment. Bone mineral density measurements were carried out either by hip or spine dual energy X-ray absorptiometry imaging. All available repeat measurements were performed at the same location as the original examination. All patients were prescribed supplemental calcium and vitamin D or vitamin D analogs unless contraindicated, as in those with corrected serum calcium levels greater than 10 mg/dL. For the control group, we used KTRs who had two valid BMD measurements after transplantation, and they received no antiresorptive treatment. Bone mineral density was evaluated using the average T score at the examined site of interest. Kidney function (estimated glomerular filtration rate: eGFR) was evaluated using the Chronic Kidney Disease Epidemiology Collaboration formula at the initiation of treatment and at the time of the repeat BMD measurements. Other collected data included time from surgery to the initiation of treatment, treatment duration, body mass index (BMI), and serum intact parathormone.
All KTRs received an interleukin-2 receptor blocker (basiliximab) and 500 mg of methylprednisolone intravenously as part of immunosuppression induction treatment. Corticosteroids were gradually reduced to an oral dose of 16 mg on Day 30. Standard treatment also included a calcineurin inhibitor (cyclosporine or tacrolimus) and mycophenolate mofetil (1.5-2 g/d).
The diagnostic thresholds for BMD measurements were as follows: normal with a T score ≥ -1 standard deviation (SD); osteopenia (low bone mass) with a T score < -1 and > -2.5 SD; and osteoporosis with a T score less than or equal to -2.5 SD[17].
This retrospective cohort study was approved by the hospital’s Ethics Committee (No. 181/14.03.2024) and was performed in accordance with the 1975 Helsinki Declaration. Written informed consent was waived due to the retrospective nature of the study.
Continuous and qualitative variables are presented as the means with SDs or percentages and frequencies, respectively. Normally distributed continuous variables were examined using the Kolmogorov-Smirnov test. Differences in the means between baseline and final biochemical or clinical indices of KTRs who received bisphosphonates or no treatment were examined with a simple t test (normally distributed data) or the Mann-Whitney test (skewed data). Paired t tests or Wilcoxon tests (for normally distributed continuous data and skewed continuous data, respectively) were used to investigate the differences among the means of baseline and repeated BMD measurements in actively treated KTRs and patients in the control group.
All tests were 2-tailed, and P < 0.05 indicated statistical significance. For the statistical analyses, we used SPSS for Windows (version 16.0 SPSS Inc. Chicago II, United States) and GraphPad Prism (version 8.0.2 for Windows, GraphPad Software, San Diego California, United States).
Overall, out of 254 KTRs 62 (39 men) met the aforementioned criteria and were included in the study. Among these patients, three had received a kidney transplant from a living relative, and all others had received a kidney transplant from a deceased donor. Treatment with bisphosphonates was prescribed for 35 patients (20 men, mean age 55.2 ± 11 years) and 27 patients (19 men, mean age 53.4 ± 15 years) received no such treatment. Other baseline clinical and biochemical characteristics of the patients are presented in Table 1. There were no differences in baseline kidney function (eGFR), BMI, or other clinical characteristics between the two groups, with the exception of BMD, which was significantly lower in the treatment cohort. Treatment was initiated 1.1 ± 2.4 years after surgery except for 9 patients who received bisphosphonates in the early posttransplant period (one month after the operation). Overall, treatment was administered for 3.9 ± 2.3 years.
| Study group (n = 35) | Control group (n = 27) | P value | |
| Age in years (mean ± SD) | 55.22 ± 10.99 | 53.44 ± 15.03 | 0.6 |
| Gender (male/female) | 20/15 | 19/8 | NS |
| eGFR (mL/min/1.73 m2) | 61.93 ± 15.8 | 59.9 ± 14.9 | 0.6 |
| Time on dialysis (years ± SD) | 6.22 ± 2.75 | 6.07 ± 3.02 | NS |
| Cadaver donor | 33 | 26 | NS |
| Diabetes | 8 | 6 | NS |
| BMI (mean ± SD) | 24.32 ± 3.91 | 25.29 ± 3.02 | 0.29 |
| iPTH (ng/mL) | 109.9 ± 102.5 | 107.7 ± 77.7 | 0.9 |
| Baseline T score (mean ± SD) | -2.28 ± 1.06 | -1.43 ± 1.13 | 0.0031 |
| Follow-up (years ± SD) | 3.78 ± 1.76 | 4.64 ± 2.96 | NS |
All patients received bisphosphonate oral dosings. The agents used were alendronate sodium (n = 20, 57%), risedronate sodium (n = 10, 29%), and ibandronic acid (n = 5, 14%). Treatment was prescribed for patients with osteopenia or established osteoporosis, except for two patients who received treatment without pathological BMD measurements.
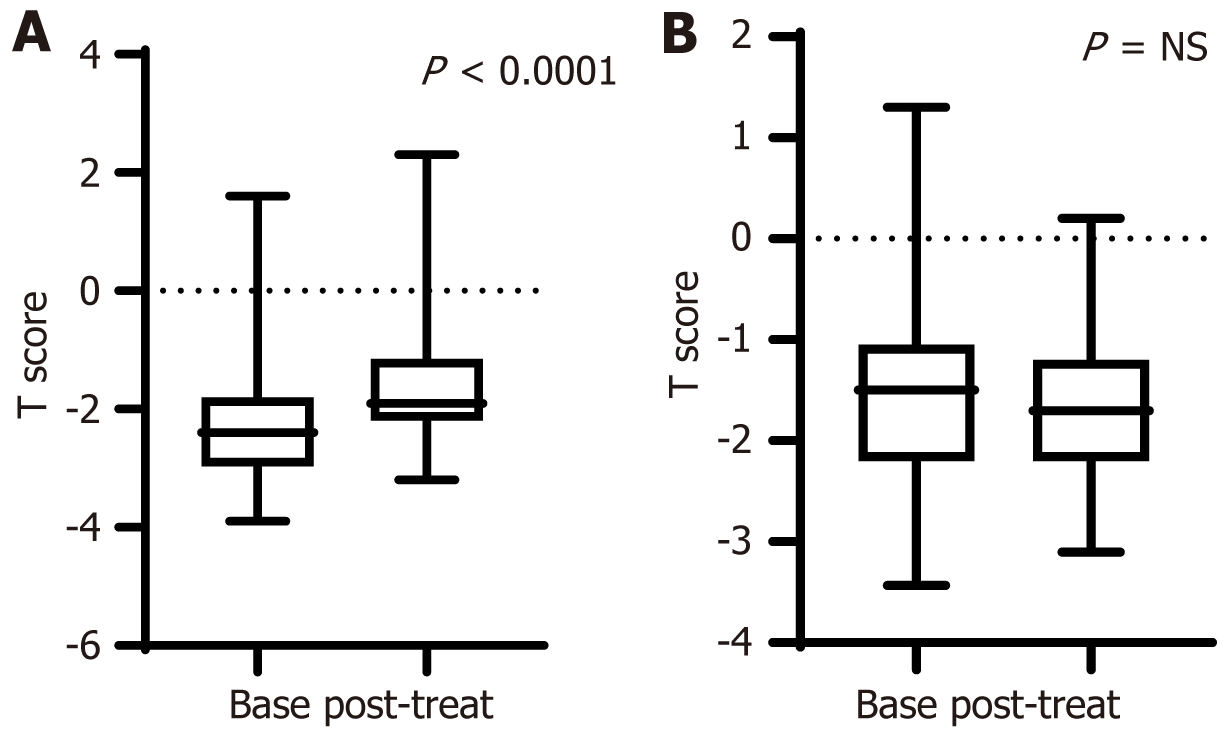
Overall, patients who received bisphosphonates showed a significant improvement in BMD (from -2.29 ± 1.07 to -1.66 ± 1.09, P < 0.0001). The control group showed a non-significant decrease in BMD (from -1.43 ± 1.1 to -1.68 ± 0.8, P = 0.17) after 4.2 ± 1.4 years of follow-up (Figure 1). Delta BMD was also greater in treated patients (treated, 0.63 ± 0.64 vs -0.26 ± 0.96 in controls, P < 0.0001).
When we examined the impact of bisphosphonates on vertebral (n = 13) and femoral (n = 22) BMD separately, we found that vertebral BMD increased significantly from a T score of -2.01 ± 1.39 to -1.55 ± 1.44 SD (ΔT score = 0.46 ± 0.5, P = 0.006) and that femoral BMD increased significantly from a T score of -2.46 ± 0.82 to -1.73 ± 0.85 SD (ΔT score = 0.73 ± 0.69, P < 0.0001).
Kidney function was not affected by bisphosphonate treatment (baseline eGFR vs end of follow-up eGFR, 61.9 ± 15.8 vs 62.4 ± 16.6 mL/min/1.73 m2, P = 0.76, respectively) and remained unchanged in those who received no treatment (baseline eGFR vs end of follow-up eGFR, 59.9 ± 14.9 vs 59.7 ± 17 mL/min/1.73 m2, P = 0.89, respectively). Moreover, tacrolimus or cyclosporine levels were not significantly affected after the initiation of treatment, and no patient stopped active treatment.
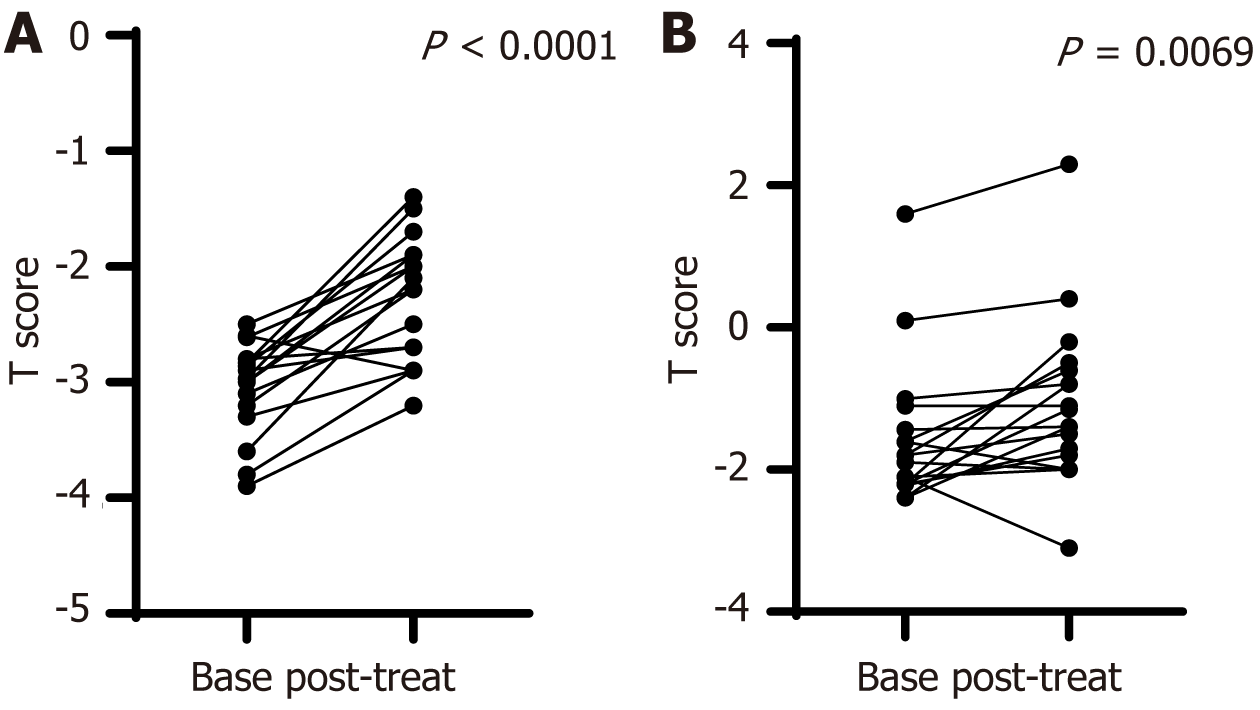
When we examined the impact of bisphosphonates on BMD in patients with diagnosed osteoporosis (BMD T score < -2.5 SD) in comparison to those with osteopenia or normal bone mass, we found that active treatment had a similar and significant effect in both groups (Figure 2).
In the control group, we noticed a significant effect of the absence of treatment, mostly in those with osteopenia (BMD T score < -1 and > -2.5) as their BMD decreased significantly during follow-up. In the osteoporotic patients in the control group, BMD was not significantly affected by the absence of treatment; nevertheless, this group consisted of only five KTRs (Table 2).
| Baseline T score | Follow-up T score | P value | |
| Treated with bisphosphonates | |||
| BMD T score < -2.5 SD (n = 17) | -3.05 ± 0.4 | -2.28 ± 0.54 | < 0.0001 |
| BMD T score > -2.5 SD (n = 18) | -1.58 ± 1 | -1.08 ± 1.16 | 0.0069 |
| Controls | |||
| BMD T score < -2.5 SD (n = 5) | -2.77 ± 0.38 | -2.43 ± 0.64 | 0.26 |
| BMD T score > -2.5 SD (n = 22) | -1.12 ± 1 | -1.5 ± 0.8 | 0.049 |
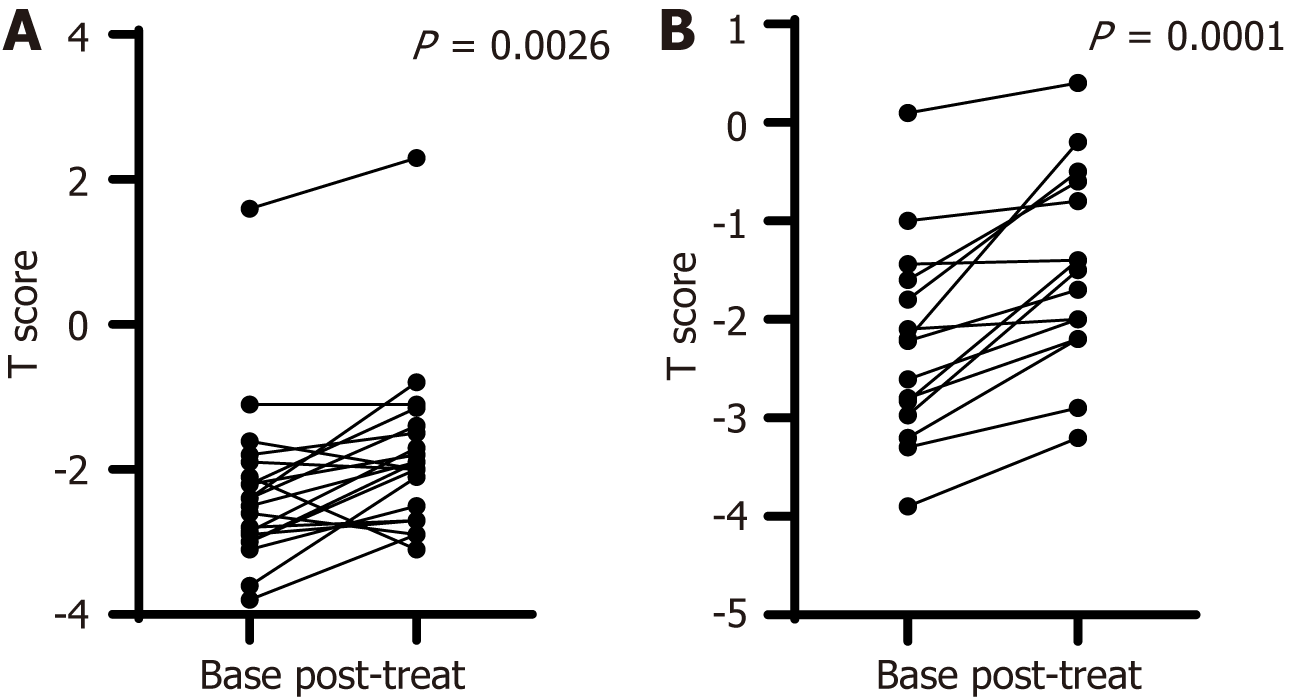
When we examined the impact of bisphosphonates on BMD in male and female patients separately, we found that active treatment improved the T score in both sexes (Figure 3). This improvement, though insignificant, was similar to that shown by the Δt score (0.38 ± 0.72 for males vs 0.77 ± 0.62 for females, P = 0.14). Subgroup analysis of controls according to sex revealed that the T score deteriorated non-significantly during follow-up in men and women (Table 3).
| Baseline T score | Follow up T score | P value | |
| Treated with bisphosphonates | |||
| Males (n = 20) | -2.3 ± 1.13 | -1.8 ± 1.16 | 0.0026 |
| Females (n = 15) | -2.26 ± 1.01 | -1.48 ± 0.99 | 0.0001 |
| Controls | |||
| Males (n = 19) | -1.45 ± 1.1 | -1.64 ± 0.9 | 0.32 |
| Females (n = 8) | -1.37 ± 1.2 | -1.79 ± 0.72 | 0.38 |
In this retrospective study on the safety and efficacy of bisphosphonate treatment in KTRs, we showed that active treatment has a favorable effect on BMD in patients with established osteoporosis as well as those with osteopenia. Moreover, this effect was not associated with sex. Finally, we have shown that KTRs who received no active treatment had a minor decline in BMD overall, while osteopenic patients who were not treated experienced a significant deterioration in BMD.
Studies on the use of bisphosphonate treatment for the prevention of bone disease in KTRs include either parenteral preparations[9,18] or agents administered orally on a daily[19] or weekly bases[20]. Although parenteral administration is more copious and may discourage KTRs from persisting with treatment, it is, in most cases, ideal for maintaining treatment compliance. Oral treatment is more convenient for patients, especially weekly dosing; nevertheless, it comes at the cost of reducing compliance and increasing the rate of common adverse effects, such as inflammation and erosions of the upper gastrointestinal tract. In this study, only weekly or monthly dosing of orally administered bisphosphonates were used. The dosage was 70 mg of alendronate sodium (once weekly), 50 mg of ibandronic acid (daily), and 75 mg of risedronate sodium (for two consecutive days every month). No serious adverse effects were recorded; however, although all patients who received active treatment were educated on proper and steady use of prescribed bisphosphonate, compliance could not be otherwise confirmed.
Concerning the impact of bisphosphonates on vertebral and femoral BMD, there are several reports that show a positive effect, though of different magnitudes. The vertebral BMD was shown to remain stable in a cohort of KTRs with osteopenia who received pamidronate for 12 months, while it was reduced in control groups[21]. In a randomized placebo-controlled study of intravenous ibandronate, KTRs who received active treatment showed a significant increase in BMD; nevertheless, this increase was not significantly different from that in the control group[9]. In another study comprising 35 KTRs, those who received alendronate plus 1000 mg of elemental calcium daily exhibited an increase of 8.2% in vertebral BMD at 12 months after the initiation of treatment[19]. A meta-analysis of eight studies that evaluated the impact of bisphosphonates on vertebral BMD revealed that treatment significantly increased vertebral BMD by 5.98% (3.77-8.18)[3]. Regarding the BMD of the femoral neck, certain studies have shown that it is significantly improved after two years of follow-up[22], while others have shown a marginal positive effect of 0.5%[23]. In a double-blind placebo-controlled study on the effect of zoledronic acid (4 mg) on hip geometry, active treatment appeared to enhance the subperiosteal diameter, endocortical diameter, and cross-sectional moment of inertia at the narrow neck after 6 months[24]. Overall, a meta-analysis of five studies revealed a significant improvement in femoral BMD, reaching 5.57% at 12 months of treatment[3]. In this study, both the vertebral and femoral BMD improved significantly, with a marginally greater increase in the femur. A new therapeutic option consisting of an anti-receptor activator of nuclear factor-kappaB ligand antibody (denosumab) has shown better results than bisphosphonates in both lumbar spine and femoral BMD[25].
A favorable effect of bisphosphonates on BMD was observed in this study among all patients, irrespective of the baseline T score, osteoporosis diagnosis, and those with either osteopenia or even near-normal BMD. Data from other studies show the same pattern of treatment effect, although this pattern has not been thoroughly examined in different subgroups of KTRs according to the baseline T score. Nevertheless, the mean baseline T score in these studies was near the normal threshold (over -1 SD)[9] or in the osteopenia region[21]. Active treatment in these groups of patients has been shown to stabilize the BMD in the short term and marginally improve it at 12 months[9,21]. Concerning those KTRs who had baseline osteopenia and received no active treatment, they showed a significant deterioration in BMD, which was also shown in another study[21]. Although this finding is not consistent, as in another group of patients who received placebo, BMD actually improved[9]. This last finding, although unexpected, is in line with our finding that KTRs with established osteoporosis did not experience an increase in BMD due to the absence of treatment. Nevertheless, this finding must be interpreted with caution because it only involved five patients.
Concerning the safety and long-term impact of bisphosphonate treatment on graft survival, most studies have shown a neutral effect[7,18]. In a study by Grotz et al[23] on the effect of ibandronate on bone loss and kidney function, it was shown that treatment correlated with fewer acute rejection episodes, but graft function 12 months after transplantation was comparable to that of the control group KTRs. Overall, in a Cochrane database systematic review, bisphosphonate treatment was shown to have no significant impact on graft survival or function, although most included studies had a short follow-up period of 12 months[12]. Nevertheless, bisphosphonates were shown to significantly reduce the risk of graft failure in a nested case control study involving more than 3800 KTRs[26]. More importantly, this study revealed an association between an increased cumulative duration of bisphosphonate administration of more than a year and a reduced risk of graft failure[26]. Moreover, bisphosphonate use has been associated with fewer acute graft rejection episodes[12]. In our study, after a mean follow-up of almost 4 years, kidney function was similar between controls and those who received active treatment, while no acute rejection episodes were recorded in any group.
Subgroup analysis of the effect of sex on bisphosphonate use in KTRs has not been performed. In most studies of kidney transplant recipients, males comprise the majority[7,9,18,19,21,23], while there is one study in which female patients predominated[27] and one with equal numbers of sexes[2]. Moreover, there are no data on the different effects of treatment or placebo on BMD between males and females in randomized or open-label trials. As most participants and actively treated KTRs are males, we can assume that the favorable effects of bisphosphonates, as shown in single studies, are mainly attributed to the effects of treatment on males. In this study, we have shown that bisphosphonates have an equal positive effect on both sexes. There was a greater increase in BMD in female KTRs than in male KTRs, while ΔBMD did not significantly differ between males and females.
Limitations of our study include its single-center and retrospective design. There was no randomization of patients, and those who were treated with bisphosphonates had a significantly lower BMD value than controls. Moreover, allocation of treatment was not performed homogeneously at the early posttransplant period when the higher dose of corticosteroids was administered and the highest degree of bone mass loss occurred. Nevertheless, this feature of the study can also be interpreted in view of the favorable effects of bisphosphonates even with later initiation of treatment. Finally, no long-term fracture assessment was performed, and thus, the effectiveness of treatment in preventing fractures could not be determined. Studies on the effectiveness and long-term safety of bisphosphonates should be conducted by increasing the number of patients and determining control groups and subgroups.
In conclusion, in this retrospective study of bisphosphonate treatment in KTRs, we showed that active treatment is effective in preventing bone loss in both male and female patients irrespective of baseline BMD.
| 1. | Nair SS, Lenihan CR, Montez-Rath ME, Lowenberg DW, Chertow GM, Winkelmayer WC. Temporal trends in the incidence, treatment and outcomes of hip fracture after first kidney transplantation in the United States. Am J Transplant. 2014;14:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Torregrosa JV, Fuster D, Pedroso S, Diekmann F, Campistol JM, Rubí S, Oppenheimer F. Weekly risedronate in kidney transplant patients with osteopenia. Transpl Int. 2007;20:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Versele EB, Van Laecke S, Dhondt AW, Verbeke F, Vanholder R, Van Biesen W, Nagler EV. Bisphosphonates for preventing bone disease in kidney transplant recipients: a meta-analysis of randomized controlled trials. Transpl Int. 2016;29:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis. 2013;61:310-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Sprague SM, Josephson MA. Bone disease after kidney transplantation. Semin Nephrol. 2004;24:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Horber FF, Casez JP, Steiger U, Czerniak A, Montandon A, Jaeger P. Changes in bone mass early after kidney transplantation. J Bone Miner Res. 1994;9:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Torregrosa JV, Fuster D, Gentil MA, Marcen R, Guirado L, Zarraga S, Bravo J, Burgos D, Monegal A, Muxí A, García S. Open-label trial: effect of weekly risedronate immediately after transplantation in kidney recipients. Transplantation. 2010;89:1476-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Brandenburg VM, Westenfeld R, Ketteler M. The fate of bone after renal transplantation. J Nephrol. 2004;17:190-204. [PubMed] |
| 9. | Smerud KT, Dolgos S, Olsen IC, Åsberg A, Sagedal S, Reisæter AV, Midtvedt K, Pfeffer P, Ueland T, Godang K, Bollerslev J, Hartmann A. A 1-year randomized, double-blind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant. 2012;12:3316-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Naylor KL, Jamal SA, Zou G, McArthur E, Lam NN, Leslie WD, Hodsman AB, Kim SJ, Knoll GA, Fraser LA, Adachi JD, Garg AX. Fracture Incidence in Adult Kidney Transplant Recipients. Transplantation. 2016;100:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Chiu MY, Sprague SM, Bruce DS, Woodle ES, Thistlethwaite JR Jr, Josephson MA. Analysis of fracture prevalence in kidney-pancreas allograft recipients. J Am Soc Nephrol. 1998;9:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Palmer SC, Chung EY, McGregor DO, Bachmann F, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev. 2019;10:CD005015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Palmer S, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev. 2005;18:CD005015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J Clin Endocrinol Metab. 2020;105:dgaa048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 269] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 15. | Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Byun Robinson A, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 16. | Leng Y, Yu X, Yang Y, Xia Y. Efficacy and safety of medications for osteoporosis in kidney transplant recipients or patients with chronic kidney disease: A meta-analysis. J Investig Med. 2023;71:760-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 862] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 18. | Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, Tellis V, Greenstein S, Schechner R, Figueroa K, McDonough P, Wang G, Malluche H. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;14:2669-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Koc M, Tuglular S, Arikan H, Ozener C, Akoglu E. Alendronate increases bone mineral density in long-term renal transplant recipients. Transplant Proc. 2002;34:2111-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Nayak B, Guleria S, Varma M, Tandon N, Aggarwal S, Bhowmick D, Agarwal SK, Mahajan S, Gupta S, Tiwari SC. Effect of bisphosphonates on bone mineral density after renal transplantation as assessed by bone mineral densitometry. Transplant Proc. 2007;39:750-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Torregrosa JV, Fuster D, Monegal A, Gentil MA, Bravo J, Guirado L, Muxí A, Cubero J. Efficacy of low doses of pamidronate in osteopenic patients administered in the early post-renal transplant. Osteoporos Int. 2011;22:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Uçar ZA, Sinangil A, Koç Y, Barlas IS, Ecder ST, Akin EB. The Effect of Alendronate on Bone Mineral Disorder in Renal Transplant Patients. Transplant Proc. 2022;54:658-662. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, Strey C, Kirste G, Olschewski M, Reichelt A, Rump LC. Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol. 2001;12:1530-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Dabbaghmanesh A, Bakhshayeshkaram M, Roshanzamir S, Naseri A, Dabbaghmanesh MM, Heydari ST, Talehzadeh P, Dabbaghmanesh MH, Jahromi SE. The effect of zoledronic acid on hip geometry in renal transplant recipients: a double-blind placebo-controlled randomized study. BMC Nephrol. 2023;24:331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | McKee H, Ioannidis G, Lau A, Treleaven D, Gangji A, Ribic C, Wong-Pack M, Papaioannou A, Adachi JD. Correction to: Comparison of the clinical effectiveness and safety between the use of denosumab vs bisphosphonates in renal transplant patients. Osteoporos Int. 2020;31:981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Song SH, Choi HY, Kim HY, Nam CM, Jeong HJ, Kim MS, Kim SII, Kim YS, Huh KH, Kim BS. Effects of bisphosphonates on long-term kidney transplantation outcomes. Nephrol Dial Transplant. 2021;36:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Torregrosa JV, Moreno A, Gutierrez A, Vidal S, Oppenheimer F. Alendronate for treatment of renal transplant patients with osteoporosis. Transplant Proc. 2003;35:1393-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/