INTRODUCTION
Nowadays, 43 million people are blind, with cataract, diabetic retinopathy, and glaucoma as leading causes[1]. Whole-eye transplantation holds profound significance due to its potential to revolutionize the treatment of blindness and severe visual impairments[2]. By offering the possibility of restoring sight in cases where current medical and surgical interventions fall short, this approach could drastically improve the quality of life, independence, and social integration for individuals living with vision loss[3]. The significance of whole-eye transplantation extends beyond the individuals directly impacted by these conditions, touching broader societal and economic factors. Successful whole-eye transplantation could reduce the healthcare and social support costs associated with blindness and visual impairment, contributing to greater overall productivity, and reducing the economic burden on families and communities.
The quest for whole-eye transplantation is a long-standing endeavor, marked by initial fascination and complex challenges[4]. Early experiments in the late 19th and early 20th centuries explored the feasibility of such transplants in animals, focusing on the technical and biological hurdles involved. A major obstacle identified by mid-20th-century research was the optic nerve's inability to regenerate, crucial for restoring vision in transplanted eyes[5]. This challenge, coupled with the difficulty of preventing immune rejection, steered scientific efforts towards more achievable goals like component transplantation and regenerative medicine. Corneal transplants became successful, routine procedures, while advances in stem cell therapy, gene therapy, and tissue engineering offered new hope for vision restoration without full eye transplants[6,7].
In parallel, significant advancements have been made in the development of eye prostheses and assistive technologies[8,9]. Modern ocular prosthetics not only offer aesthetic improvements but also incorporate advanced materials and designs that enhance wearer comfort and integration with the ocular socket. Beyond traditional prosthetics, cutting-edge research into electronic visual prostheses, often referred to as "bionic eyes", aims to restore sight through the direct stimulation of visual pathways in the brain or the remaining parts of the visual system[10]. These devices, which can include retinal implants and cortical visual prostheses, translate external visual information captured by cameras into electrical signals that the brain can interpret, offering a form of sight to those with certain types of blindness[11,12].
As of today, whole-eye transplantation remains a partially theoretical endeavor, with research in regenerative medicine and innovative technologies like bioengineered devices, such as retinal prosthesis and artificial cornea, holding promise for future breakthroughs[13,14]. Although the first whole-eye transplantation in human was recently performed, no functional restoration of vision has been achieved, highlighting that aesthetic improvement alone is not sufficient[4]. The foremost challenge is to restore the functional connectivity between the optic nerve and the retina. The challenge of reconnecting the optic neural axis is explained by its peculiar anatomical features where the cell bodies of the sensory neurons (ganglion cell) are located within the retina itself. Thus, regeneration of axonal populations within the optic nerve from the occipital cortex towards the retina is physiologically not feasible. Making the re-establishment of vision connections to the brain is of the highest importance for eyesight restoration. Sight is primarily a brain function rather than a purely ocular one[15,16].
On the other hand, immune rejection poses another major problem, as the body's defense mechanisms often attack the transplanted tissue, considering it foreign. Overcoming these obstacles requires breakthroughs in regenerative medicine, immunology, and surgical techniques, including promoting nerve regeneration and developing effective strategies to manage or prevent immune responses against the transplanted eye[17-22].
In this review, we assess the current state of art on whole-eye transplantation, addressing both the advancements and the future challenges, encompassing innovations in immune acceptance and neural regeneration for visual function restoration following eye transplant.
ADVANCES AND INSIGHTS
Surgical techniques and interdisciplinary insights from animal models in whole-eye transplantation
Whole-eye transplantation, a pioneering endeavor in medical science, frequently intersects with the intricacies of face transplantation, particularly regarding surgical techniques, tissue integration challenges, and the nuances of immunosuppression[23]. This confluence is particularly apparent in the comprehensive approach both fields demand, encompassing not just the transplantation of the primary organ but also the intricate reconnection of nerves, blood vessels, and surrounding tissues to reinstate function and achieve aesthetic congruence[3,24-26].
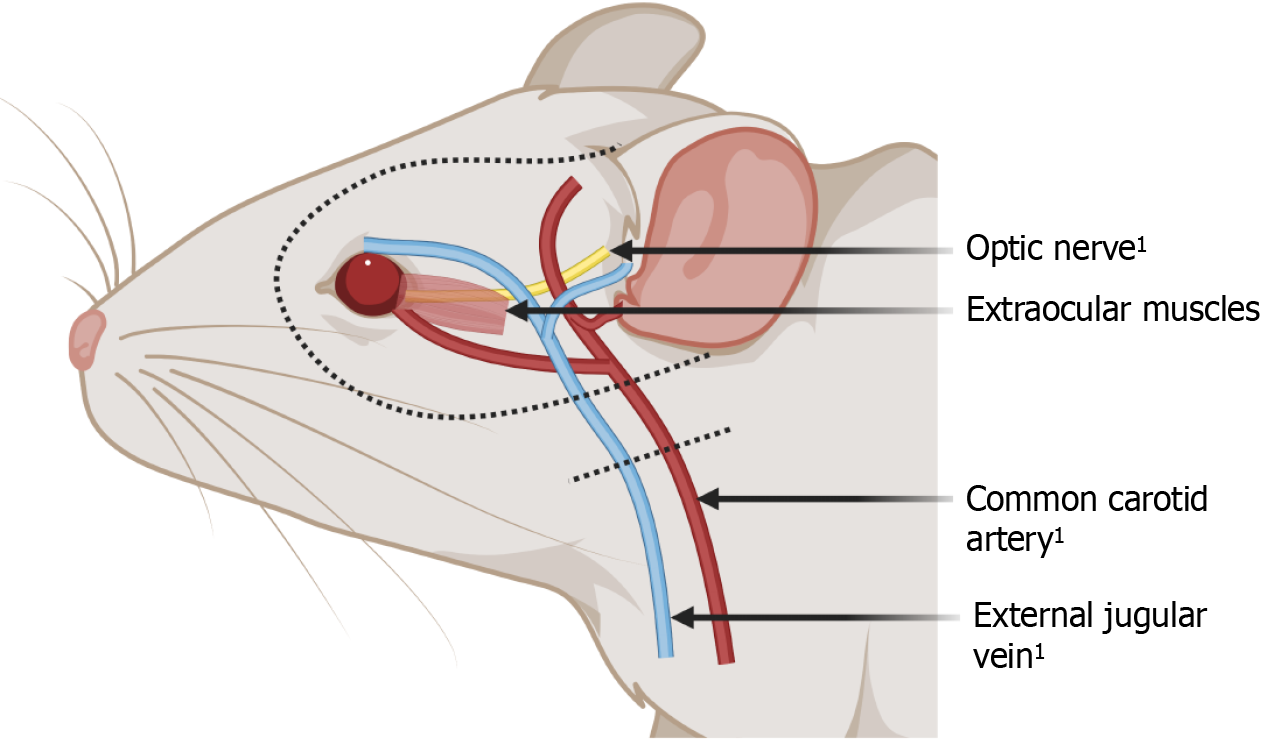
In the realm of surgical advancements, the insights garnered from face transplantation have significantly informed whole-eye transplantation efforts, especially in mastering the microsurgical techniques essential for successful tissue integration. The expertise in microvascular anastomosis, cultivated through vascularized composite allograft (VCA) transplantation, proves invaluable for whole-eye transplantation by ensuring the establishment of a functional blood supply essential for the transplanted eye's viability[27]. Furthermore, the shared challenges of immunosuppression and managing tissue rejection highlight the interdisciplinary synergy between these fields. This collaborative framework enriches the understanding and development of strategies to mitigate rejection and optimize post-transplant recovery[28-30]. The exploration of whole-eye transplantation has ventured into animal models, providing a tangible basis for assessing its feasibility and delineating the procedural complexities[31,32] (Figure 1). Notably, research has concentrated on rodents and swine, selected for their anatomical and physiological similarities in eye structure and immune response, offering a closer approximation to human applications[26,31].These animal studies have been instrumental in refining surgical techniques, such as precise optic nerve alignment and reconnection strategies, and advancing our comprehension of vascularization necessities for the transplanted eye.
Figure 1 Anatomical illustration of a rodent model for whole-eye transplantation.
1Anastamosis with the recipient. Created with BioRender.com (Supplementary material).
These endeavors in animal models have underscored the procedural feasibility of whole-eye transplantation, albeit with the caveat that restoring vision remains a formidable challenge yet to be surmounted[33]. It has been achieved in some animals (cold-blooded vertebrates), but not in mammals[34-38]. The learnings from these models are invaluable, serving as a foundation for future innovations and the eventual translation of whole-eye transplantation into a viable clinical procedure for humans[39-41]. In 2023, Laspro et al[42] highlighted the feasibility of WET, noting its safety with no recorded complications for recipients as per existing literature. They emphasized its potential for functional restoration, supported by evidence of positive retinal survival in live models. However, the capability for optic nerve regeneration still requires clarification. The surgical expertise necessary to attain our objectives has been attained; attention must now be directed towards advancing other areas.
Through this meticulous research, the goal of restoring sight to those with severe visual impairments moves closer to reality, demonstrating the power of interdisciplinary collaboration in pushing the boundaries of medical science.
Immunological background
The eye is unique as it consists of immunologically privileged and avascular structures[20]. The intricate journey towards achieving successful whole-eye transplantation is significantly influenced by the immunological landscape unique to the eye, a topic extensively explored in the literature[17,18,20,22]. This body of work sheds light on the nuanced challenges and considerations inherent in transplanting an organ as complex and immunologically distinct as the eye.
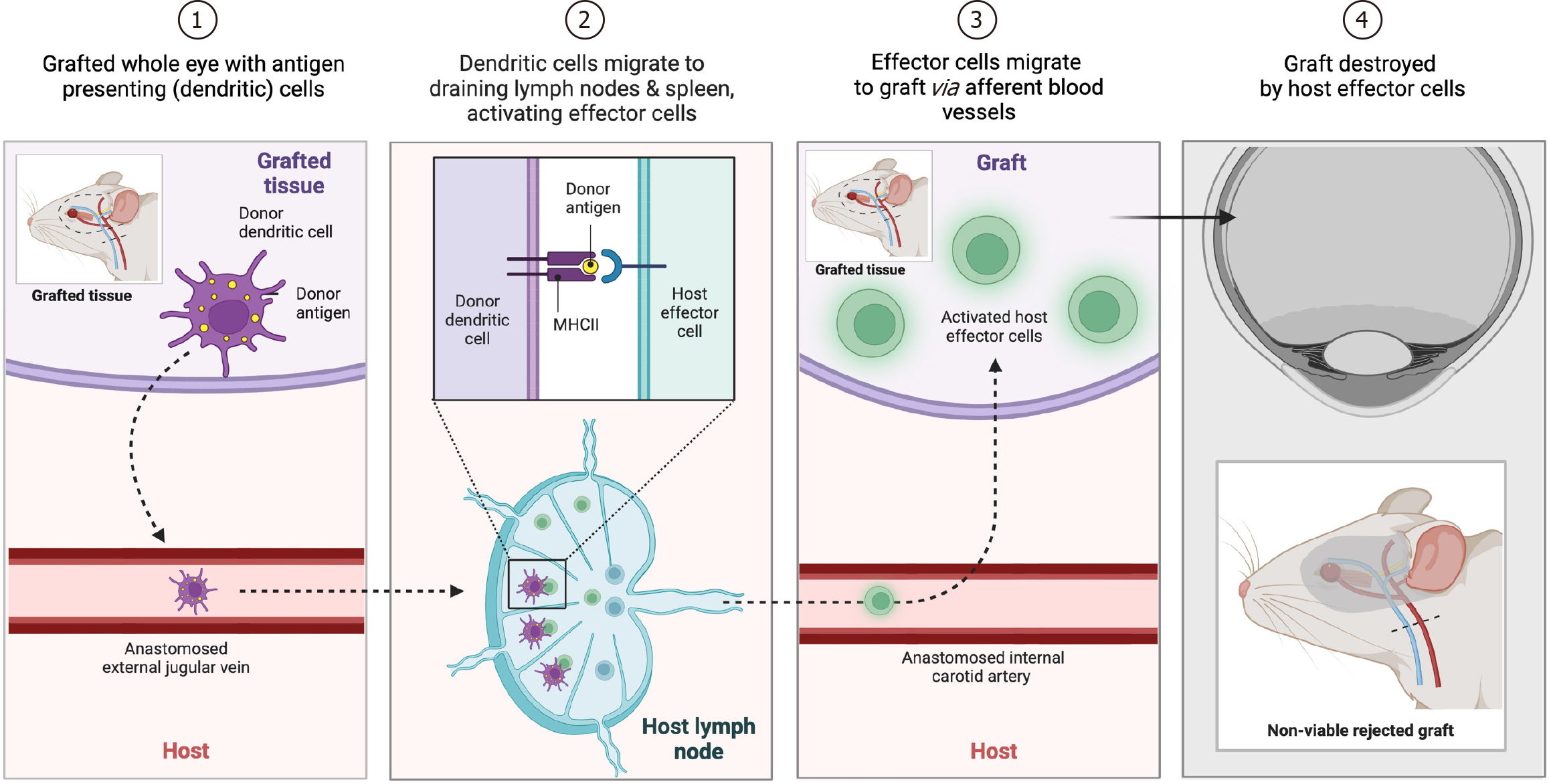
Recognizing the eye as an immune-privileged site elucidates both the advantages and complications these characteristic poses for transplantation[17-19,21]. The privilege, which under normal circumstances safeguards the eye from inflammatory damage, becomes a double-edged sword in the context of transplantation, necessitating innovative approaches to manage the immune response. The term “immune privilege” was first coined in the first half of the 18th century, when Medawar and colleagues recognized the extended survival of skin allografts placed in the anterior chamber of the eye[19,43]. Years later, Streilein and Niederkorn[44,45] demonstrated that immune privilege was in fact the result of an actively maintained and “deviant” suppressive immune response to ocular antigens, a phenomenon that was later referred to as anterior chamber-associated immune deviation. This process was mediated by antigen presenting cells (APCs), CD4+ T cells, and particularly antigen-specific regulatory T cells (Tregs) which orchestrate immune changes in the setting of autoimmune diseases and graft-rejection[46-48] (Figure 2). As a consequence of this knowledge, corneal transplantation has become one of the most successfully performed solid organ transplantation, with a more than 90% rate of success in low-risk condition, while in high-risk setting it lowers to less than 50%[49,50]. Interestingly, the impressive success rate often witnessed in low-risk corneal grafts, unlike with other solid organ transplants, can be attained without relying on HLA matching or extensive systemic immune suppression[51,52]. Following transplantation, inflammation in the eye triggers the upregulation of proinflammatory cytokines like IL-1, IL-6, and TNF-α, as well as chemokines such as MIP-1α, MIP-1β, MIP-2, and RANTES[53]. This inflammatory environment also leads to increased expression of adhesion molecules like ICAM-1 and VLA-1[54,55]. Consequently, both resident and infiltrating host APCs exhibit elevated levels of MHC class II and costimulatory molecules like CD80 (B7-1), CD86 (B7-2), and CD40[56]. This enhances the ability of donor corneal APCs, typically incapable of T cell stimulation, to prime naïve T cells into Th1 effectors, which play a crucial role in acute graft rejection[57]. When considering the retina, studies regarding the retinal pigmented epithelium transplantation demonstrated that, besides the cellular and molecular pathways mentioned before, there is a strong activation of pro-inflammatory phenotype of microglial cells which lead to an increase of TNFα, IL-1β, IL-6, nitric oxide, and ROS signaling and, ultimately, rejection and neuronal damage[58]. The critical balance between avoiding graft rejection and preserving the eye's immune privilege underscores the complexity of immunological considerations in whole-eye transplantation. This balance involves a deep understanding of the eye's specific immune responses and developing strategies that can foster graft acceptance while mitigating adverse reactions.
Figure 2 Schematic figure of different phases of the rejection process, mediated by antigen-presenting cells and activated effector cells, following whole-eye transplantation in a representative rodent model.
Created with BioRender.com (Supplementary material).
Addressing the challenges of graft rejection and the maintenance of immune privilege involves a multifaceted strategy, incorporating advances in immunosuppressive therapies and the exploration of tolerance-inducing techniques. The demand for localized immunosuppression, as opposed to systemic treatments, makes this field a promising area of investigation, potentially offering a more focused approach that minimizes broader immunosuppressive side effects.
Moreover, compatibility between donor and recipient, the exploration of novel immunomodulatory agents, and the potential role of regenerative medicine and stem cell therapies in promoting graft tolerance. In this realm, mesenchymal stem cells represent a beacon of hope given their both regenerative and immunoregulatory capacities secreting numerous substances that can promote immune-regulated environment in the setting of eye transplantation[59]. These considerations are pivotal in devising comprehensive strategies that tackle the immunological barriers to whole-eye transplantation.
Whole-eye transplantation is commonly performed in hybrid animal models with face transplant. The comprehensive immunosuppressive regimens developed for face transplant recipients, aimed at preventing the body's rejection of transplanted tissues, are of significant relevance to whole-eye transplantation. Understanding how to balance these regimens to minimize side effects while ensuring the survival of the transplanted tissue is a shared challenge that benefits from interdisciplinary research and clinical experience in both areas.
Optic nerve regeneration
The eye is the only ‘peripheral’ organ that is directly connected to the brain via the optic nerve. Unlike the nerves of the peripheral nervous system (PNS), the optic nerve do not spontaneously regenerate[60]. This lack of regeneration has been attributed primarily to differences in the molecular and cytokine environments between the two systems[61-63]. In the peripheral system, factors that stimulate regeneration are present, whereas, in the central system, inhibitory factors predominate[64]. Additionally, the reduced clearance of cellular debris in the central nervous system (CNS) further hampers regeneration efforts[64,65].
One of the distinguished features of the visual neural axis compared to the neural axis in the somatosensory system is the location of the cell bodies of the corresponding sensory neurons. While in the PNS, the sensory neurons are located in the dorsal root ganglia (DRG), which receive afferent information from distal targets like cutaneous mechanoreceptors via afferent axons. When the axonal continuity of the peripheral nerve is disrupted distally to its cell bodies in DRGs, the afferent axons regenerate anterogradely towards their targets in the periphery[66]. The anatomy of the eye and the visual neural axis differs from the peripheral somatosensory axis in that the sensory cell bodies, retinal ganglion cells (RGCs), are migrated topographically more distally and are located within the retina in direct proximity to the photoreceptors. Thus, continuity disruption of the optic nerve proximally from the retina is analogous to preganglionic brachial plexus injury in the upper extremity, where the axons fail to regenerate as the sensory neurons and signal pattern generation are not lost[67]. This underlines the major limitation in optic nerve regeneration, which does not allow for retrograde axonal growth.
In this context, Aguayo et al's[68] work stands out, demonstrating that CNS nerves may grow long distances when placed in the appropriate environment, such as within a peripheral nerve. This finding opened new avenues for optic nerve regeneration research, particularly relevant for RGCs[68]. RGC axons, which course through the optic nerve, are the sole carriers of visual information to the brain. Following injury, these axons fail to regrow, and RGC cell bodies typically die, resulting in permanent vision loss. To preserve RGCs and stimulate their axons' regeneration, researchers must overcome four major hurdles: Increasing RGC survival, navigating the inhibitory environment of the optic nerve, enhancing the intrinsic axon growth potential of RGCs, and optimizing the reestablishment of RGC connections to their targets in the brain[69,70]. The death of RGCs in post-optic nerve injury can be traced back to the disruption of axonal connections to their targets, eliminating the target-derived neurotrophic support[71]. These signals, which are retrogradely transported to the cell body, are crucial for neuronal survival. Thus, strategies to promote optic nerve regeneration must simultaneously address the post-injury death of RGCs, the inhibitory glial environment, changes in RGCs’ intrinsic axon growth potential, and the guidance of regenerating axons to their correct targets in the brain[33].
Efforts in CNS regeneration research have provided valuable insights that could be applied to optic nerve injuries. By leveraging a multi-faceted approach that includes enhancing neurotrophic support, modifying the inhibitory environment, and boosting the intrinsic growth capacity of RGCs, the field moves closer to viable strategies for optic nerve regeneration[72,73]. These strategies range from molecular and cellular therapies, such as the application of neurotrophic factors and stem cell transplantation, to innovative technologies like optogenetics and nanotechnology-based delivery systems[74-76]. Stem cells hold immense promise in the field of ophthalmology for optic nerve regeneration[77]. These versatile cells possess the capability to differentiate into various cell types, including those crucial for repairing damaged optic nerves. By harnessing the regenerative potential of stem cells, researchers aim to restore vision in conditions such as glaucoma and optic nerve injuries[78,79]. Exosomes and extracellular vesicles (EVs) play pivotal roles in stem cell-based therapies for optic nerve regeneration[80]. These tiny membrane-bound vesicles secreted by stem cells contain a cargo of bioactive molecules, including proteins, nucleic acids, and growth factors[79]. Exosomes and EVs act as messengers, facilitating intercellular communication and transferring regenerative signals to target cells within the optic nerve[81]. Through their ability to modulate cellular processes and promote tissue repair, exosomes and EVs enhance the therapeutic efficacy of stem cell treatments for optic nerve regeneration. This synergy between stem cells and their secreted vesicles offers new avenues for developing innovative regenerative therapies to restore vision and improve the quality of life for individuals with optic nerve disorders. Each approach aims not only to stimulate axon growth but also to ensure the precise navigation and integration of these axons into their brain targets, a critical step for restoring vision. As some of these approaches progress into human clinical trials for optic nerve and spinal cord injuries, the convergence of new technologies and strategies brings us closer to offering tangible hope for those affected by optic nerve diseases. The complexity of the optic nerve's environment and the need for precise axonal guidance underscore the challenges ahead. However, the ongoing advances in understanding and manipulating this environment suggest that overcoming the barriers to optic nerve regeneration may soon be within reach. By continuing to explore and integrate these diverse strategies, the goal of restoring sight through whole-eye transplantation and optic nerve regeneration becomes increasingly attainable, marking a potential paradigm shift in the treatment of blindness and visual impairment.
LIMITS TO SUCCESS
Whole-eye transplantation represents a pioneering frontier in the field of regenerative medicine and ophthalmology, holding the promise of restoring vision to those with irreversible blindness. However, this ambitious endeavor faces several formidable challenges, including maintaining: (1) Donor eye viability; (2) achieving regeneration of the neuronal networks, ensuring; (3) immunological tolerance, and navigating; and (4) ethical and legal considerations. This chapter delves into the current state of research in these critical areas, underlining both the progress and the obstacles that lie ahead.
Donor eye viability
Maintaining the viability of the donor eye post-enucleation stands as a paramount challenge in whole-eye transplantation, underpinned by three critical parameters: The recovery of visual function post-transplant, the resilience of the outer retina to ischemia, and the survival of RGCs[33,82,83]. The journey toward understanding and optimizing these factors reveals a complex interplay of biological and technical considerations.
The quest for visual function recovery post-transplant has yielded intriguing insights, particularly from studies on cold-blooded vertebrates[34-38]. These creatures demonstrate a capacity for visual function recovery that, while offering a glimmer of hope, also underscores a significant barrier due to the vastly different regenerative abilities between these species and humans. This disparity poses a formidable challenge, emphasizing the need for innovative approaches to bridge the gap in regenerative potential.
The outer retina's function, highly sensitive to ischemia, varies notably in the crucial hours following enucleation, typically ranging from 4 to 9 hours. The application of electroretinography has proven instrumental in assessing the functionality of this layer. Immediate reperfusion, ideally within a 10-min window of ischemia, has been shown to preserve its function for an extended period, suggesting that the timing and method of reperfusion are critical to maintaining the retina's viability[33,82].
Furthermore, the survival of RGCs, pivotal for the transmission of visual information from the eye to the brain, hinges on minimizing ischemic time and optimizing the point of optic nerve transection. Intracranial sections, with transections made more than 8 mm away, have shown better outcomes, potentially enhanced by the application of neurotrophic factors. This highlights the intricate balance between surgical technique and biological intervention necessary to support RGC survival[84,85].
Notably, the understanding of RGC survival has evolved over time. For instance, Scalia et al's[86] study in 1985 provided a quantitative analysis of RGC survival following optic nerve injury and regeneration, revealing that even with a full recovery of vision, only 29% of RGCs remained after 50 wk[84,86-89]. While other researchers have reported higher survival rates, these findings collectively underscore that less than 100% RGC survival might still suffice for visual recovery. This perspective, primarily derived from studies in cold-blooded vertebrates, holds promise for the enhanced ability to maintain RGC survival in mammalian systems, including humans.
Taken together, these parameters outline a landscape of challenges and opportunities in whole-eye transplantation. Each factor, from visual function recovery and outer retina resilience to RGC survival, contributes to the intricate puzzle of maintaining donor eye viability, underscoring the need for continued research and innovation in this pioneering field.
Restoration of the neural pathways
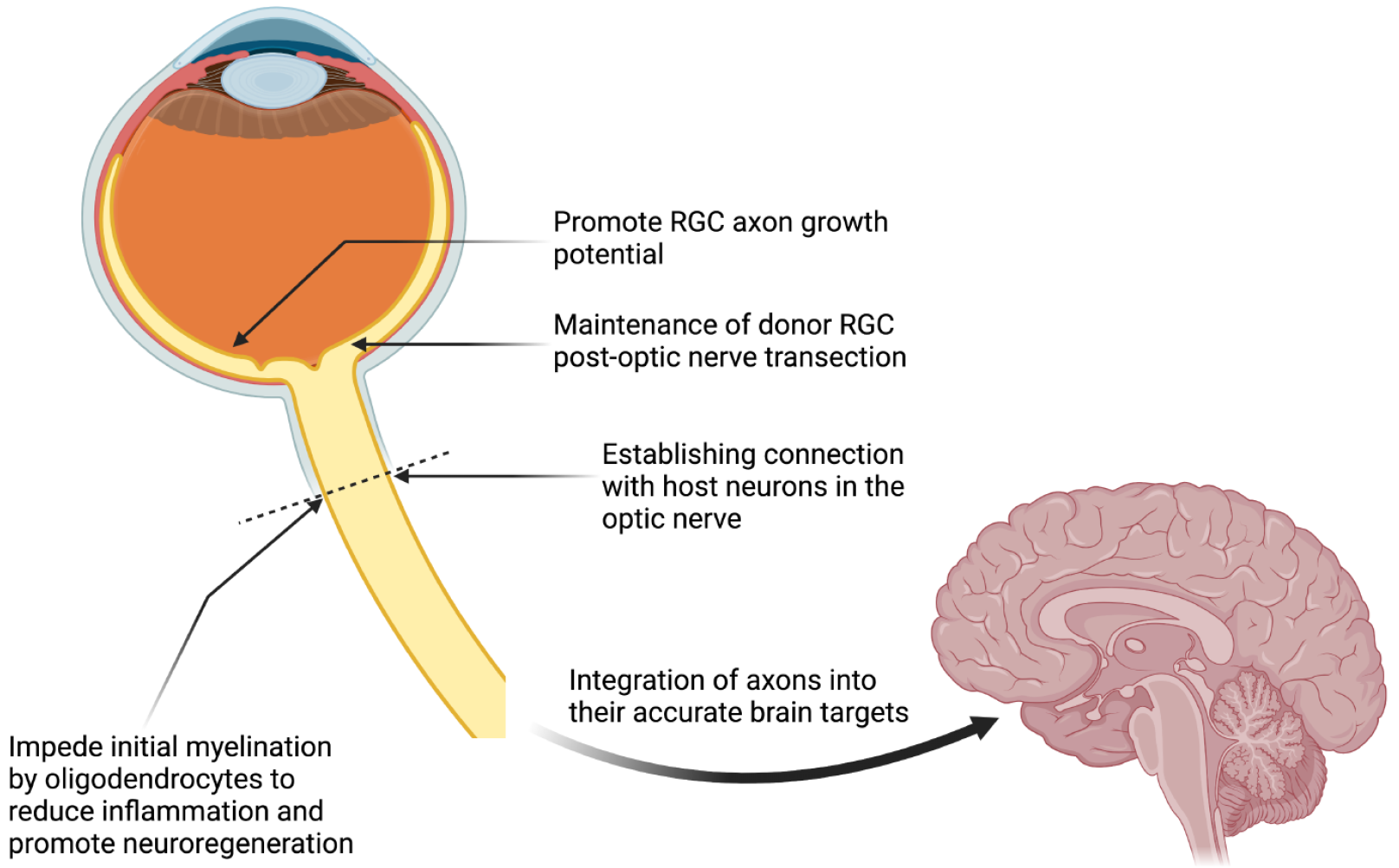
The quest for successful whole-eye transplantation is a journey through uncharted territories in medical science, particularly due to the complexity of optic nerve regeneration and its integration into the CNS. The optic nerve, comprising axons of RGCs, forms the critical link between the eye and the brain, making its regeneration essential for the restoration of vision (Figure 3). However, a fundamental hurdle in this endeavor is the mammalian CNS's inherent inability to regenerate damaged axons, a trait that starkly contrasts with the regenerative capabilities observed in many other vertebrates[40,68]. This limitation means that any damage to the RGCs results in irreversible vision loss. Despite these challenges, the rapid accumulation of knowledge regarding axonal destruction and regeneration signals a beacon of hope, edging us closer to the possibility of developing effective regenerative therapies for CNS injuries.
Figure 3 Challenges in neural pathway regeneration for restoring visual function following whole-eye transplantation.
RGC: Retinal ganglion cell. Created with BioRender.com (Supplementary material).
The regeneration of the optic nerve stands as a formidable barrier to the success of whole-eye transplantation. Achieving this feat requires not only the regeneration of damaged axons but also their successful integration and functional connection with the recipient's brain. This dual challenge implicates both regenerative and connective hurdles within the intricate environment of the CNS.
Several factors, both intrinsic and extrinsic, contribute to the optic nerve's limited regenerative capacity. Firstly, the CNS environment itself is not conducive to optic nerve regeneration, in stark contrast to the PNS, where regeneration is more readily facilitated[90,91]. Studies utilizing peripheral nerve grafts have shown promising results in enabling RGC axon regeneration through the transected rodent optic nerve, highlighting the CNS's inhibitory nature towards regeneration. Moreover, the myelin proteins within the CNS, which are crucial for insulating axons and facilitating the rapid conduction of electrical signals, play a paradoxical role in optic nerve regeneration. While myelin is beneficial for signal transmission, the myelin produced by oligodendrocytes in the CNS has an inhibitory effect on axon regeneration, a phenomenon not observed with Schwann cells in the PNS[92]. This inhibitory effect underscores the stark differences in regenerative capacities between the CNS and PNS.
In addition to the environmental and myelin-related challenges, the response to injury, including inflammation and immune reactions, significantly impacts the regenerative potential of RGCs. The cellular and molecular processes initiated by optic pathway transection can create barriers to regeneration. Immunomodulatory therapies may offer a pathway to mitigate these effects, as the astrocytes, which support synaptic development and plasticity, can form physical and molecular barriers post-injury that impede axonal regrowth.
Intrinsic factors within the RGCs themselves also play a crucial role in their regenerative capacity. As RGCs mature, they undergo significant changes in their molecular programming, including the down-regulation of growth-promoting molecules such as phosphorylated mammalian target of rapamycin (phosphor-mTOR). This down-regulation significantly hampers the axons' ability to regenerate after injury. However, research has demonstrated that targeting these intrinsic factors, such as by deleting an mTOR inhibitor like phosphatase and tensin homolog (PTEN), can markedly enhance the axonal regeneration capacity of RGCs following injury[93-95].
The challenges of optic nerve regeneration extend beyond the optic nerve itself, encompassing the need for precise reconnection of regenerated axons with the appropriate targets in the brain. Achieving such precise reintegration is crucial for restoring complex visual functions. Studies have shown some success in enhancing RGC axon regeneration into critical brain structures involved in visual processing, offering a glimpse into the potential for functional recovery. Nevertheless, the meticulous reconnection of optic nerve fibers to their designated targets in the brain, coupled with the immunological considerations inherent in transplanting an entire eye, including the optic nerve, presents significant challenges that must be addressed to ensure the long-term success and viability of whole-eye transplantation[95].
In addressing these multifaceted challenges, the exploration of biomaterials and engineered scaffolds represents a promising avenue for research. These materials can be designed to release growth factors and present cues that encourage axonal extension towards their targets, potentially overcoming some of the intrinsic and extrinsic barriers to optic nerve regeneration. Despite the advancements in understanding and experimental approaches to facilitate optic nerve regeneration, translating these findings into successful clinical applications remains an arduous task[93,94]. The journey toward whole-eye transplantation is fraught with obstacles, yet the pursuit of overcoming these barriers continues to drive forward the frontiers of ophthalmology and regenerative medicine.
Immunological tolerance
In the domain of immunological tolerance, the adaptation of protocols from those employed in face transplantation is indeed pertinent. However, it's essential to delineate specific therapies utilized for eye transplantation[96]. Current immunosuppressive regimens for face and eye transplantation typically involve agents such as tacrolimus, mycophenolate mofetil, cyclosporine, and corticosteroids[97,98]. Nevertheless, previous studies indicate that every patient who underwent face transplantation and was followed up postoperatively for one year encountered at least one episode of immune rejection[99]. A significant effort has been directed towards minimizing immunosuppressive regimens by employing lower dosages and reducing the number of medications; however, this has been associated with an increased risk of allograft rejection. Novel immunomodulatory agents, such as anti-T-cell targeting molecules[100], interleukin-2 receptor antibodies[101], and monoclonal antibodies like alemtuzumab and rituximab[102,103], have demonstrated promising outcomes in this context. The heterogeneity of ocular tissue renders eye transplantation highly immunogenic, despite its immune-privileged characteristic, and challenging to manage without robust immune suppression, which inevitably carries its own set of side effects[102,104,105]. One advantage that researchers should consider in future endeavors is the accessibility of the eye, which allows for localized therapy to be tested. Localized therapy can potentially minimize the side effects associated with immunosuppressants while simultaneously enhancing the efficacy of concomitant systemic therapy[106]. This approach capitalizes on the unique anatomical features of the eye, allowing for targeted delivery of medications to the site of action while reducing systemic exposure and associated adverse effects. By exploring localized therapies, researchers can strive to optimize the balance between effective immunosuppression and minimizing systemic side effects, ultimately improving outcomes for patients undergoing eye transplantation. The pursuit of functional eye transplants prompts a reassessment of whether a standalone eye transplantation approach might be preferable, prompting a reevaluation of immunosuppression protocols to optimize outcomes. Ensuring immunological tolerance while minimizing the risk of rejection and adverse effects is a delicate balance that requires further research.
Ethical and legal considerations
Ethical and legal considerations form an integral part of the dialogue on whole-eye transplantation. The prospect of restoring sight through such transplants raises profound ethical questions, from the allocation of donor eyes to the psychological impact on recipients and donors' families. Furthermore, legal frameworks must evolve to address the nuances of whole-eye transplantation, ensuring that these procedures are conducted with the highest ethical standards[4,107].
CHALLENGES AND FUTURE PERSPECTIVES
In the realm of eye transplantation, navigating future challenges is pivotal for advancing the field. Among these challenges, the neural component stands out as a central focus demanding thorough investigation. Currently, it acts as a tyrant, dictating the success and viability of transplantation endeavors. Therefore, minimizing neural damage during the isolation of the donor eye and reducing ischemic times, ideally below 20 min, emerge as paramount objectives. Achieving these goals necessitates a meticulously organized and finely tuned surgical technique. Efforts to mitigate neural damage and minimize ischemic times are multifaceted. Emphasizing the importance of preserving retinal cell viability, techniques leveraging cold preservation and refining surgical methodologies are imperative. By meticulously optimizing surgical procedures, researchers aim to curtail ischemic durations, safeguarding the integrity of retinal cells crucial for visual function.
Furthermore, promoting optic nerve regeneration constitutes another critical frontier. Stem cell therapies emerge as promising avenues in this endeavor, holding potential for stimulating nerve regrowth and facilitating functional recovery. These innovative approaches underscore the importance of interdisciplinary collaborations and continued exploration of regenerative medicine strategies.
In navigating these challenges, finding a delicate balance in immunosuppressive regimens poses another significant hurdle. The ideal regimen should strike a nuanced equilibrium, minimizing adverse effects while preventing rejection of the transplanted eye. This necessitates a nuanced understanding of immune responses and the development of tailored immunosuppressive protocols.
Future directions in eye transplantation necessitate a comprehensive approach, addressing not only the surgical intricacies but also the complex interplay between neural regeneration and immune responses. By leveraging advancements in surgical techniques, regenerative therapies, and immunomodulation strategies, researchers endeavor to overcome these challenges and pave the way for improved outcomes and enhanced quality of life for individuals undergoing eye transplantation.
CONCLUSION
Whole-eye transplantation stands on the cusp of revolutionizing the treatment of blindness. However, the path forward is fraught with challenges that span the biological, technical, and ethical domains. Maintaining donor eye viability, mastering optic nerve regeneration, achieving immunological tolerance, and navigating the ethical landscape are hurdles that the scientific community must overcome. The journey towards whole-eye transplantation is a testament to the resilience and innovation inherent in the quest to restore sight, promising a future where vision restoration is within reach. In summary, the current state of the art is one of cautious optimism, marked by innovative research directions that address both biological and technical challenges. Continuous advances in molecular biology, regenerative medicine, and surgical techniques fuel the hope for overcoming the current limitations, potentially paving the way for successful whole-eye transplantation in the future.