Published online Jun 18, 2024. doi: 10.5500/wjt.v14.i2.92137
Revised: February 21, 2024
Accepted: April 8, 2024
Published online: June 18, 2024
Processing time: 149 Days and 22.4 Hours
Lung transplantation is a well-established treatment of end-stage lung disease. A rodent model is an inexpensive way to collect biological data from a living model after lung transplantation. However, mastering the surgical technique takes time owing to the small organ size.
To conduct rat lung transplantation using a shunt cannula (SC) or modified cannula (MC) and assess their efficacy.
Rat lung transplantation was performed in 11 animals in the SC group and 12 in the MC group. We devised a method of rat lung transplantation using a coronary SC for coronary artery bypass surgery as an anastomosis of pulmonary arteriovenous vessels and bronchioles. The same surgeon performed all surgical proce
Ten and 12 lungs were successfully transplanted in the SC and MC groups, respectively. In the SC group, one animal had cardiac arrest within 1 h after reperfusion owing to bleeding during pulmonary vein anastomosis. The opera
A hyperacute rat lung transplantation model using a coronary SC was created using a simple technique. The MC was inexpensive, easy to prepare, and simple to operate.
Core Tip: We developed a rat lung transplantation technique using a coronary shunt cannula (SC) for the pulmonary arteriovenous system and bronchial tube anastomosis. This method is simple and can be performed by a surgeon. This study evaluated the usefulness of this method by using a modified cannula (MC), which we developed by modifying the SC and improving its shortcomings. The MC is inexpensive and easy to prepare and operate. Presently, a hyperacute lung tran
- Citation: Takata M, Tanaka Y, Saito D, Yoshida S, Matsumoto I. Hyperacute experimental model of rat lung transplantation using a coronary shunt cannula. World J Transplant 2024; 14(2): 92137
- URL: https://www.wjgnet.com/2220-3230/full/v14/i2/92137.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i2.92137
Lung transplantation is a well-established treatment of end-stage lung disease. Many immune and non-immune mech
The rat lung transplantation model was first reported in 1971[1], followed by the Mizuta Cuff model[2] in 1989. Since then, various improvements in surgical techniques, cuffs, and instruments have been reported[3-7]. The advantage of using a rodent model is that it permits inexpensive collection of biological data from a living model after lung transplantation. Although trained surgeons can perform the transplantation procedure, mastering the surgical technique takes time due to the small size of the organs. The risks associated with this technique include damage to the vulnerable pulmonary artery (PA) and pulmonary vein (PV) vessel walls during anastomosis, as well as stenosis of the anastomotic site.
We developed an anastomotic technique using a coronary shunt cannula (SC) for cardiac coronary artery bypass surgery as an alternative to the previously reported cuff method[2-6]. This method enables anastomosis by inserting and ligating a cannula into the lumen of the PA, PV, and bronchus (Br), which is simpler and more reliable than conventional methods.
This study aimed to determine problems with rat lung transplantation using the SC, develop an improved cannula, and investigate its utility.
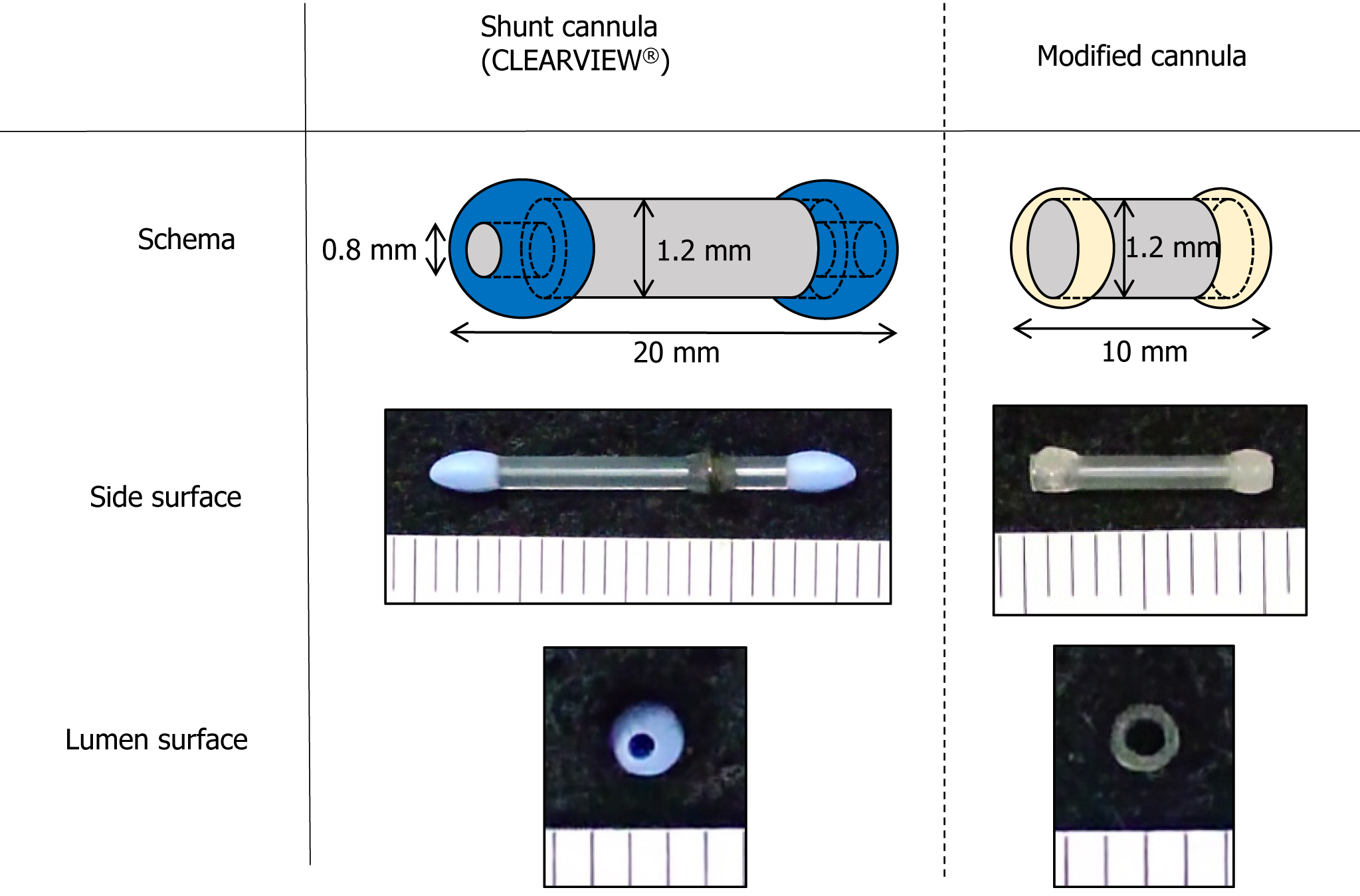
A cannula was used for anastomosis of the PA, PV, and Br during rat lung transplantation. The SC (CLEARVIEW®; Medtronic, Minneapolis, United States) used for coronary artery bypass surgery had a luminal structure with tips on both ends to prevent injury during insertion and removal from the coronary artery.
In this experiment, we divided the subjects into two groups: One using CLEARVIEW® (SC group) and the other using a cannula developed in our laboratory (modified cannula group: MC group) (Figure 1). The SC group and the MC group consisted of 11 and 12 animals, respectively, to be used as lung transplantation models. The CLEARVIEW® cannula used has a lumen diameter of 1.2 mm. The issues with the CLEARVIEW® cannula included a narrow lumen diameter of 0.8 mm at both tip ends and an excessive length of 20 mm, which posed challenges for anastomosis of the PA, PV, and Br in rats. To create a more physiologically-reliable lung transplantation model for maintaining constant blood flow in the pulmonary arteriovenous vein, we developed an MC with a constant lumen diameter. A polyamide catheter with a lumen diameter of 1.2 mm throughout its entire length (coronary artery perfusion catheter; Forte Grow Medical Corporation, Tochigi, Japan) was used as the MC. Although shorter catheters could be more physiologic and desirable for lung transplantation, the polyamide catheter was cut into a length of 10 mm in this study. Cyanoacrylate resin (Aron Alpha A; Daiichi Sankyo Company, Limited, Tokyo, Japan) was adhered to both ends to form a tip smaller than the CLEARVIEW® cannula and used for the experiment.
Male Wister rats (8–10 wk old, 240–300 g; Japan SLC, Inc., Hamamatsu, Japan) were purchased and used as donors and recipients. All experiments were conducted in the Kanazawa University Animal Testing Laboratory. Experimental animals were used following the Guidelines for the Care and Use of Laboratory Animals of Kanazawa University (approval no. AP-163743) and the Guide for the Care and Use of Laboratory Animals (8th edition) published by the National Research Council in 2011.
The same surgeon performed all surgical procedures in the donors and recipients, without using magnifying glasses. Regarding induction of anesthesia, 4%–5% isoflurane (Wako Pure Chemical Industries, Osaka, Japan) was administered with a maintenance dose of 1.5%–3%. The rats were intubated using an 18-gauge intravenous catheter through a transverse cervical incision. After intubation, the rats were ventilated using a ventilator (settings: Tidal volume, 10 mL/kg; respiratory rate, 60/min); fraction of inspired oxygen, 0.21).
The animals were supinely positioned, a median abdominal incision was made, and 250 units of heparin were injected through the inferior vena cava. A median sternotomy was made to expose the thoracic cavity. The right ventricular wall was punctured, and a 24-gauge intravenous catheter was inserted into the PA. The superior and inferior venae cavae were incised and drained while 20 mL of saline solution was injected over a period of approximately 1 min. The trachea was clamped, and the cardiopulmonary block was removed with preservation fluid after completion of perfusion.
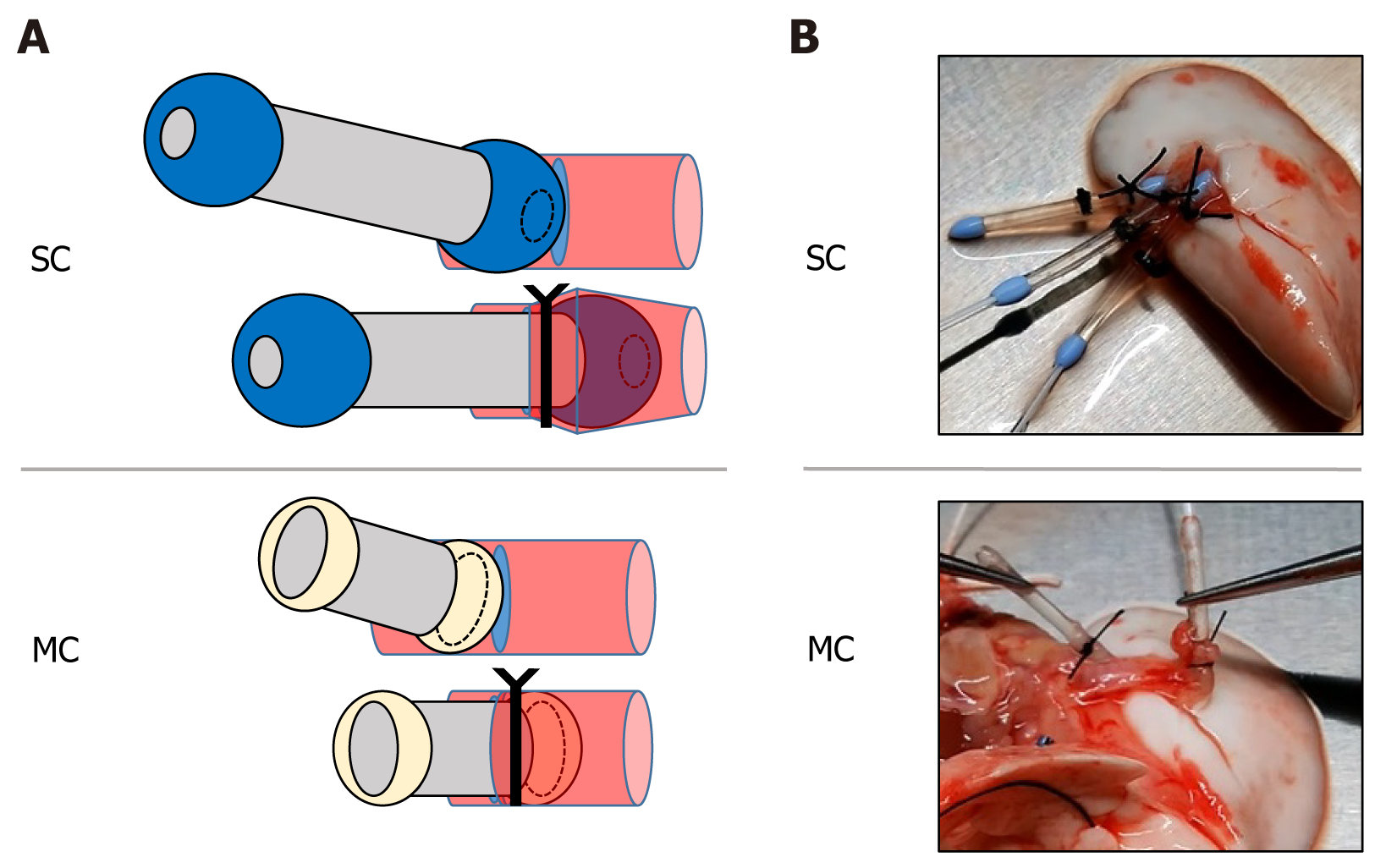
The cardiopulmonary block was placed on a Petri dish cooled to 4 °C, and the work proceeded in the cooled state. The left PA/PV/Br was detached, and a cannula was inserted into each of the vessel and bronchial walls with only a partial incision (Figure 2A). The left lung graft was ligated with a 4-0 silk suture at the center of the tip of the cannula, including the vessel and bronchial walls (Figure 2B), and separated from the cardiopulmonary block. The left lung graft was stored at 4 °C for 24 h before transplantation.
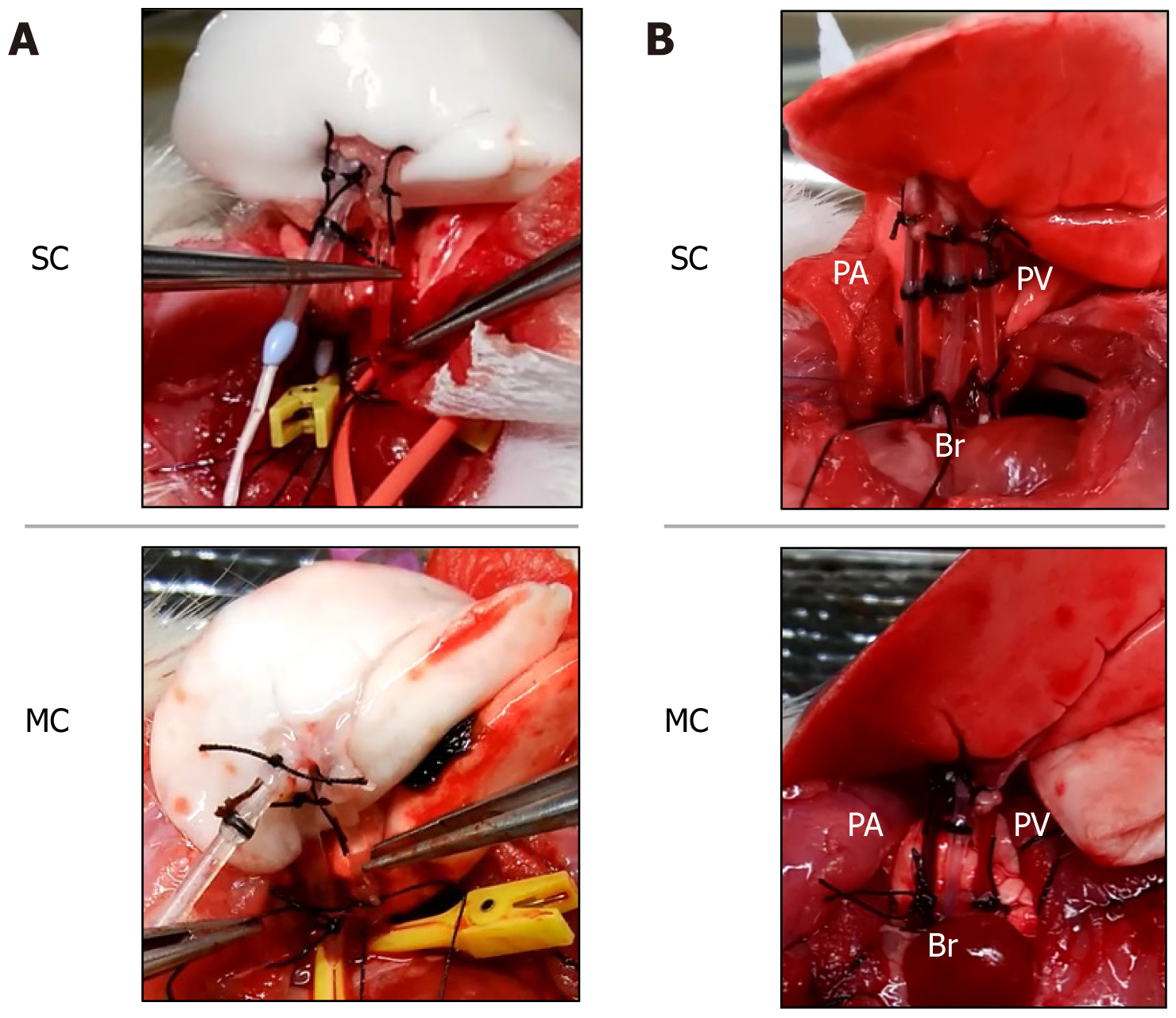
The recipient was placed in the right lateral recumbent position, and the left-side chest was opened at the intercostal space where the heartbeat was felt. Two ribs were dissected dorsally caudal to the open chest wound, and the dissected ribs were pulled caudally to secure the view. The recipient's pulmonary hilum was elevated, and the PA, Br, and PV were dissected and secured. Subsequently, 250 units of heparin were injected through the PV. The central side of all three recipient structures was clamped with a microclip. When the PA/PV/Br anastomosis was performed, an incision was made in a part of the vessel wall and bronchial walls, while the left lung of the recipient was retained without resection. The cannula tip was inserted into the left lung graft, and the anastomosis was completed by ligating the cannula following the same procedure used when the graft was created (Figure 3A). The microclip was removed, reperfusion was started, and pulmonary expansion and blood flow in the grafted lung were checked (Figure 3B). The left lung graft was removed after 2 h of continuous reperfusion at FiO2: 1.0, with general anesthesia maintained after transplantation.
The success rates of the lung transplantation, operating time, and PaO2 values were compared between the two groups. The criteria for successful lung transplantation were: (1) Maintenance of recipient's circulation; (2) visual confirmation of blood flow in the cannula of the graft lung; and (3) maintenance of graft lung coloration after 2 h of reperfusion. The recipient's circulation was determined by visual inspection of pulse and heart rates. Graft lung coloration was classified into three grades (Grades 1–3) and defined as follows: Grade 1, insufficient blood flow in the grafted lung (white tone area) being > 10% of the total surface area; Grade 2, insufficient blood flow in the grafted lung (white tone area) being < 10% of the total surface area; and Grade 3, favorable blood flow throughout the graft. Successful grafting was defined as a graft with a color grading of 2–3. Blood was collected at the PV of the graft lung to measure PaO2 (i-STAT analyzer; Abbott Point of Care, Chicago, United States).
Data were analyzed using STATMATE (ATMS Co., Ltd., Tokyo, Japan), expressed as mean ± SD, and compared with an unpaired t-test. Values of P < 0.05 were considered statistically significant.
After creating 11 lung transplantation model animals in the SC group and 12 in the MC group, all animals underwent reperfusion. One animal in the SC group had cardiac arrest 1 h after reperfusion due to hemorrhage caused by vessel wall injury during PV anastomosis. Two hours after reperfusion, we visually confirmed the maintenance of recipient hemodynamics and blood flow in the graft pulmonary cannula in 10 animals in the SC group and 12 in the MC group.
The operating time for the removal of the heart-lung block from the donor and graft lung creation was 26.8 ± 2.3 min in the SC group and 25.7 ± 1.3 min in the MC group (P = 0.21, Table 1). The duration for left lung transplantation into the recipient was 37.5 ± 2.8 min in the SC group and 35.9 ± 1.4 min (P = 0.12, Table 1) in the MC group. Although no significant difference was found between the SC and MC groups, animals in the MC group experienced a slightly shorter operating time, smoother surgical technique, and less stressful procedure for the surgeons compared with those in the SC group.
| SC group (n = 10) | MC group (n = 12) | P value | |
| Donor procedure-Graft preparation (min) | 26.8 ± 2.3 | 25.7 ± 1.3 | 0.21 |
| Recipient procedure (min) | 37.5 ± 2.8 | 35.9 ± 1.4 | 0.12 |
The graft lung coloration (Grade 1/2/3) after reperfusion was 0/2/8 (SC group) and 0/2/10 (MC group), and all grafts were reported to be successful, except in one animal in the SC group that had cardiac arrest (Table 2).
| SC group (n = 10) | MC group (n = 12) | |
| Grade 1/2/3 | 0/2/8 | 0/2/10 |
The PaO2 values after 2 h of reperfusion were 456.2 ± 25.5 mmHg in the SC group and 461.2 ± 21.5 mmHg in the MC group (P = 0.63, Table 3), showing no significant difference between the groups.
| SC group (n = 10) | MC group (n = 12) | P value | |
| PaO2 (mmHg) | 456.2 ± 25.5 | 461.2 ± 21.5 | 0.63 |
The SC technique used in this study was easy to learn and had a high success rate. In the SC group, the commercially available SC used had an inconsistent lumen diameter that could impair blood flow and was too long for use for rat lung transplantation. In the MC group, our lab-developed cannula that bypasses these limitations was used for rat lung transplantation.
The results showed no significant differences in operating time, graft success rate, or partial pressure of oxygen in the blood between the two groups, and both procedures were very easy. However, compared with those in the SC group, the procedures were easier, there were no surgical failures, and there was less stress for the surgeon in the MC group. This can be attributed to the smaller tip, enabling smoother insertion into the lumen of the blood vessel, and the overall diameter, allowing the grafted lung to be deployed in a position close to the pulmonary hilar region (anatomically correct position).
In rat lung transplantation, anastomosis of the fragile PA/PV is the most difficult[4,5]. Anastomosis using a cuff cut process from an intravenous catheter is simpler than direct anastomosis, and improvements in the cuff shape[5], anastomosis technique[3], anastomosis using the donor's descending aorta[4], and stabilizer for graft preparation[6] have been reported. However, the technique takes time to master.
In the method involving the use of a catheter in the hyperacute lung transplantation model, challenges associated with using a straight catheter include difficulties in securing the graft vasculature and catheter. Prolonged lung ventilation can also lead to misalignment at the anastomotic site. In this experiment, we used an SC for the following reasons: (1) The rounded tips could prevent damage to the PA and PV during insertion, (2) it was easy for a surgeon to secure the lumen and insert the cannula, and (3) the central side of the tip was ligated with the tissue to facilitate the procedure and prevent dislocation during revascularization and continuous lung ventilation. In addition, due to the transparent cannula body, visualizing blood flow during reperfusion was possible, which could be used as a criterion for successful transplantation. The MC is shorter in length, has a wider lumen, and is more maneuverable than existing products. Because the MC is inexpensive and easy to make, we believe that it can be used on a commercial basis in the future.
The limitations of this method include the following: (1) The cannula is long, preventing containment of the grafted lung in the body after anastomosis and prohibiting closure of the chest; and (2) the lumen of the cannula is not covered with biological tissue as in the cuff method. Therefore, considering our modified SC, this method could only provide convenience for performing hyperacute lung transplantation at this time. Therefore, evaluating the chronic phase is currently difficult using this method. Further improvement in surgical materials and protocols is needed to facilitate long-term survival of animals after transplantation.
The cannula size can be reduced by shortening the length and eliminating one of the tips, specifically on the donor side. This miniaturization allows the cannula lumen to be covered by the vessel wall, similar to the cuff method, thus reducing foreign body reactions and mechanical hemolysis. If the chest can be closed after transplantation, acute and chronic survival models can be created. However, further studies are needed to develop a chronic-phase rat lung transplantation model.
A rat lung transplantation model using a coronary artery SC could be created with a simple technique and may be a useful model for evaluating grafted lungs in the hyperacute phase after transplantation. The model using MC, which was modified in our laboratory, could be produced with easier surgical procedures and lower cost compared with those of the SC, as well as showing potential for commercialization.
The authors are deeply grateful to Dr. Hiroyuki Nakamura, for his statistical expertise.
| 1. | Asimacopoulos PJ, Molokhia FA, Pegg CA, Norman JC. Lung transplantation in the rat. Transplant Proc. 1971;3:583-585. [PubMed] |
| 2. | Mizuta T, Kawaguchi A, Nakahara K, Kawashima Y. Simplified rat lung transplantation using a cuff technique. J Thorac Cardiovasc Surg. 1989;97:578-581. [PubMed] |
| 3. | Zhai W, Ge J, Inci I, Hillinger S, Markus C, Korom S, Weder W. Simplified rat lung transplantation by using a modified cuff technique. J Invest Surg. 2008;21:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Goto T, Kohno M, Anraku M, Ohtsuka T, Izumi Y, Nomori H. Simplified rat lung transplantation using a new cuff technique. Ann Thorac Surg. 2012;93:2078-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Guo H, Nie J, Fan K, Zheng Z, Qiao X, Li J, Wang J, Jiang K. Improvements of surgical techniques in a rat model of an orthotopic single lung transplant. Eur J Med Res. 2013;18:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Tian D, Shiiya H, Sato M, Nakajima J. Rat lung transplantation model: modifications of the cuff technique. Ann Transl Med. 2020;8:407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Jin X, Kaes J, Van Slambrouck J, Inci I, Arni S, Geudens V, Heigl T, Jansen Y, Carlon MS, Vos R, Van Raemdonck D, Zhang Y, Vanaudenaerde BM, Ceulemans LJ. A Comprehensive Review on the Surgical Aspect of Lung Transplant Models in Mice and Rats. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/