Published online Jun 18, 2024. doi: 10.5500/wjt.v14.i2.90571
Revised: February 12, 2024
Accepted: April 3, 2024
Published online: June 18, 2024
Processing time: 189 Days and 21.4 Hours
Hepatocellular carcinoma (HCC) is an aggressive malignant neoplasm that requires liver transplantation (LT). Despite patients with HCC being prioritized by most organ allocation systems worldwide, they still have to wait for long periods. Locoregional therapies (LRTs) are employed as bridging therapies in patients with HCC awaiting LT. Although largely used in the past, transarterial embolization (TAE) has been replaced by transarterial chemoembolization (TACE). However, the superiority of TACE over TAE has not been consistently shown in the literature.
To compare the outcomes of TACE and TAE in patients with HCC awaiting LT.
All consecutive patients with HCC awaiting LT between 2011 and 2020 at a single center were included. All patients underwent LRT with either TACE or TAE. Some patients also underwent percutaneous ethanol injection (PEI), concomitantly or in different treatment sessions. The choice of LRT for each HCC nodule was determined by a multidisciplinary consensus. The primary outcome was waitlist dropout due to tumor progression, and the secondary outcome was the occurrence of adverse events. In the subset of patients who underwent LT, complete pathological response and post-transplant recurrence-free survival were also assessed.
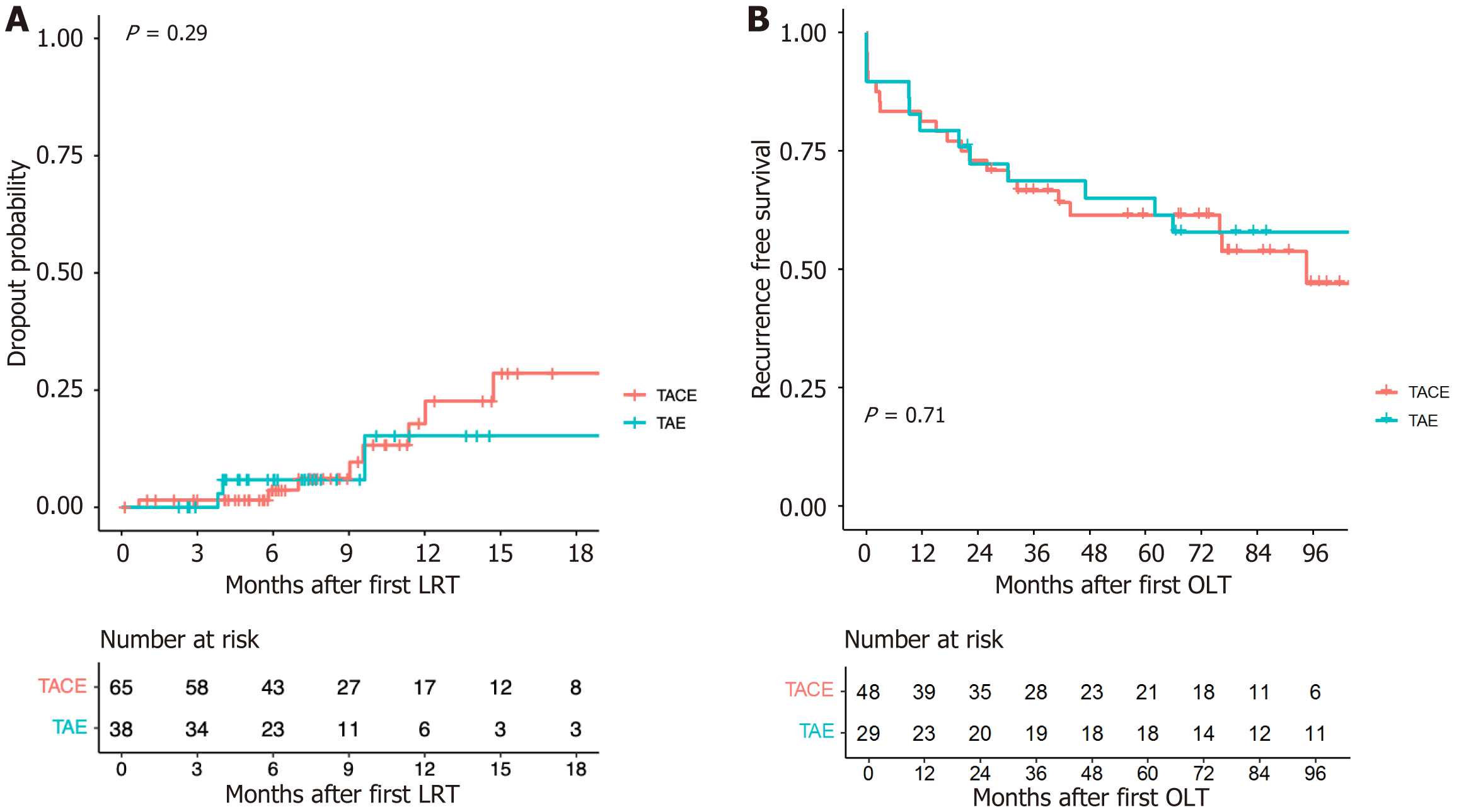
Twelve (18.5%) patients in the TACE group (only TACE and TACE + PEI; n = 65) and 3 (7.9%) patients in the TAE group (only TAE and TAE + PEI; n = 38) dropped out of the waitlist due to tumor progression (P log-rank test = 0.29). Adverse events occurred in 8 (12.3%) and 2 (5.3%) patients in the TACE and TAE groups, respectively (P = 0.316). Forty-eight (73.8%) of the 65 patients in the TACE group and 29 (76.3%) of the 38 patients in the TAE group underwent LT (P = 0.818). Among these patients, complete pathological response was detected in 7 (14.6%) and 9 (31%) patients in the TACE and TAE groups, respectively (P = 0.145). Post-LT, HCC recurred in 9 (18.8%) and 4 (13.8%) patients in the TACE and TAE groups, respectively (P = 0.756). Posttransplant recurrence-free survival was similar between the groups (P log-rank test = 0.71).
Dropout rates and posttransplant recurrence-free survival of TAE were similar to those of TACE in patients with HCC. Our study reinforces the hypothesis that TACE is not superior to TAE as a bridging therapy to LT in patients with HCC.
Core Tip: Hepatocellular carcinoma (HCC) is an aggressive malignant neoplasm, and the treatment of choice is liver transplantation (LT). Because the waiting time is often unpredictable, locoregional therapy is used to halt HCC progression until an organ is available. Although largely replaced by transarterial chemoembolization (TACE), transarterial embolization (TAE) or bland embolization is an alternative with a lower cost and safer adverse event profile. Our findings, in conjunction with those of previous studies, provide evidence of non-superiority of TACE over TAE, thereby encouraging a more liberal use of TAE for bridging HCC to LT.
- Citation: Lazzarotto-da-Silva G, Scaffaro LA, Farenzena M, Prediger L, Silva RK, Feier FH, Grezzana-Filho TJM, Rodrigues PD, de Araujo A, Alvares-da-Silva MR, Marchiori RC, Kruel CRP, Chedid MF. Transarterial embolization is an acceptable bridging therapy to hepatocellular carcinoma prior to liver transplantation. World J Transplant 2024; 14(2): 90571
- URL: https://www.wjgnet.com/2220-3230/full/v14/i2/90571.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i2.90571
Hepatocellular carcinoma (HCC) is an aggressive malignant neoplasm that arises in the presence of cirrhosis. Unless appropriate treatment is administered, HCC may progress, rupture, or metastasize[1-3]. In the presence of cirrhosis and portal hypertension, liver transplantation (LT) is the treatment of choice for HCC[4,5].
The Milan criteria is widely used to identify patients likely to benefit from LT[6,7]. Although some organ allocation systems may prioritize patients with HCC for LT[8], most of these patients face a long waiting period. Thus, locoregional therapy (LRT) is indicated for this patient population to halt tumor progression beyond the acceptable limits of the Milan criteria (bridging therapy)[9].
Although the main LRT options are transarterial chemoembolization (TACE) and radiofrequency ablation (RFA), other modalities such as transarterial radioembolization, percutaneous ethanol injection (PEI), microwave ablation, and transarterial embolization (TAE) are also employed worldwide. Recently, our group demonstrated that PEI is an acceptable bridging therapy to LT in patients with HCC[10,11]. The choice of LRT is influenced by tumor size, number, and location, liver function, and individual center experience[10-12].
Although widely used in the past, TAE has been replaced by TACE. The potential advantage of TACE over TAE may be the addition of a chemotherapeutic agent. However, because HCC expresses a multidrug resistance gene, it is resistant to most chemotherapeutic agents available[13]. Furthermore, the advantages of TACE over TAE have not been confirmed in clinical practice. A recent systematic review and meta-analysis comparing randomized control trial (RCT) data on TAE and TACE use among patients with unresectable HCC detected no superiority of TACE over TAE in terms of disease-free survival[14].
Only one study till date has compared the outcomes of TAE vs TACE in terms of dropout rates of patients with HCC on the transplant list[15]. Thus, the aim of this study was to analyze the outcomes of TAE and TACE as an LRT for patients with HCC awaiting LT. The dropout rates and post-transplant outcomes of both techniques have been compared.
This study was a retrospective analysis of a prospectively filled dataset from the Hospital de Clínicas de Porto Alegre (HCPA) Liver Transplant Program. All adults (aged > 18 years) with cirrhosis and HCC who were enlisted for orthotopic liver transplantation (OLT) between 2011 and 2020 at the authors’ institution and had undergone TACE or TAE for bridging or downstaging were included. Patients with HCC who met the Milan criteria were included in this analysis. Patients who did not meet the Milan criteria were included only after downstaging HCC using LRT to meet the Milan criteria.
The choice of LRT for each HCC nodule was determined by a consensus among LT surgeons, hepatologists, and interventional radiologists. Because RFA is not available in the Brazilian public health system, PEI was preferred for lesions ≤ 3 cm in size and accessible via percutaneous ultrasound-guided liver puncture. For tumors > 3 cm in size, TACE or TAE were preferred. Until 2013, TAE was the only modality of embolization available in the Brazilian public health system[16]. Since then, TACE is preferred over TAE. However, even after 2013, some patients underwent TAE because of contraindications to doxorubicin or unavailability of the drug. For some patients with more than one tumor, PEI was performed in addition to TACE or TAE, either in the same treatment session or in different sessions. Patients who underwent PEI only or RFA were not included in this study.
TACE and TAE were performed by one of the two experienced interventional radiologists (Scaffaro LA and Farenzena M) via the femoral route under sedation. A 5-F Cobra or Mikaelson catheter was used to achieve selective catheterization and perform an arteriogram of the celiac trunk and superior mesenteric artery. The tumor feeding artery was selectively catheterized using a 2.8-F microcatheter (Progreat; Terumo). For each TACE session, doxorubicin-lipiodol emulsion followed by polyvinyl alcohol (PVA) or microspheres with particle size 100 µm–300 µm were infused. For TAE, only PVA or microspheres with particle size 100 µm–300 µm were infused without the addition of a chemotherapeutic agent. PEI was also performed by one of the same two experienced interventional radiologists under computed tomography (CT) or ultrasound guidance. The tumor was punctured percutaneously using a 20-gauge needle under sedation.
Follow-up imaging [contrast-enhanced CT or magnetic resonance imaging (MRI)] was performed 6 wk–8 wk after each procedure. The need for subsequent therapy was decided on the basis of residual contrast enhancement in the lesion region, which indicated the presence of residual tumor. The imaging follow-up protocol remained the same throughout the study period.
Contrast-enhanced CT or MRI was used to characterize preprocedural disease extent, including the size and number of lesions. Because 74% of LIRADS 4 lesions and 94% of LIRADS 5 lesions are HCCs[17], both were considered as HCC tumors. Biopsy of the lesions was not routinely performed. Based on the tumor size and number of lesions, tumor burden was classified according to the Barcelona Liver Clinic staging system[5]. The Model for End-Stage Liver Disease (MELD) score was calculated as described in the study by Malinchoc et al[18]. Preprocedural alpha-fetoprotein (AFP) level was defined as the AFP level immediately before the first LRT. The following patient demographic data were collected: Age, sex, cirrhosis etiology, calculated MELD score, preprocedural AFP level, number of lesions, diameter of the largest tumor, and number of procedures.
According to the LRT chosen, the study patients were divided into four groups: Only TAE, only TACE, TAE + PEI and TACE + PEI. The primary study outcome was waitlist dropout due to tumor progression beyond the limits of the Milan criteria. The secondary outcomes were as follows: (1) Pathological response; (2) side effects of LRT, as graded by the Clavien–Dindo classification[19]; and (3) post-transplant HCC recurrence, as evaluated by post-transplant recurrence-free survival. Patients were followed until their death, waitlist dropout, or the end of the study on June 30, 2023.
For the main outcome measure (waitlist dropout), the date of the first LRT session of each patient enlisted for LT was defined as day zero of the follow-up. Dropout due to tumor progression was considered an event. Time to dropout due to tumor progression was defined as the number of days between the first LRT and the dropout date. The dropout rate was analyzed using the Kaplan–Meier method in a time-to-event manner. Patients who underwent LT or dropped out due to any cause other than tumor progression (e.g., clinical or psychosocial dropout) were excluded on the transplant or dropout day, respectively.
For the evaluation of post-transplant recurrence-free survival in a subset of the cohort’s patients who underwent LT, the transplant day was defined as day zero of the follow-up. The analysis of post-transplant recurrence-free survival included HCC recurrence or death due to any cause as the events. Patients lost to follow-up were censored.
The pathological response and vascular invasion by the tumor were assessed by a dedicated liver pathologist. Complete or near-complete pathological response was defined as 90% tumor necrosis on histopathological examination of the explanted liver of patients who underwent OLT.
Categorical variables were compared using the Fisher’s exact test. The normality of the continuous variables was estimated using the Shapiro–Wilk test. Continuous variables were analyzed using the Mann–Whitney test or Student’s t-test as appropriate. Time-to-event data (time to dropout due to tumor progression and recurrence-free survival) were estimated using the Kaplan–Meier method and compared using the log-rank test. For all the analyses pertaining to waitlist dropout, follow-up day zero in patients whose HCC was downstaged to meet the Milan criteria was set to when they were enlisted. All comparisons were two-sided with a level of significance of 0.05. All analyses were performed using R for microwave-assisted, continuous-flow organic synthesis (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria)[20]. The statistical methods used in the study were reviewed by a biomedical statistician from HCPA.
From 2011 to 2021, 183 patients with HCC were placed on the LT waiting list. Of these, 80 patients were excluded for the following reasons: No LRT was performed (n = 17), RFA was performed (n = 6), and PEI alone was performed (n = 57). One hundred and three patients with HCC who were enlisted for LT underwent LRT with TAE or TACE. Of these, 65 (63.1%) patients underwent TACE and 38 (36.9%) underwent TAE. There was no statistically significant difference between the groups in terms of patient, tumor, and treatment characteristics (Table 1).
| Variables | TACE | TAE | P value |
| Number | 65 | 38 | |
| Age (yr), median (IQR) | 60 (55, 65) | 61.5 (55, 64) | 0.962 |
| Male sex | 40 (61.5) | 23 (60.5) | > 0.99 |
| Diagnosis | 0.889 | ||
| HCV | 51 (78.5) | 32 (84.2) | |
| HBV | 4 (6.2) | 2 (5.3) | |
| Alcohol | 4 (6.2) | 3 (7.9) | |
| NASH | 4 (6.2) | 1 (2.6) | |
| Other | 2 (3.1) | 0 | |
| Calculated MELD score, median (IQR) | 9 (8, 12) | 11 (9, 12) | 0.122 |
| Preprocedural AFP level, median (IQR) | 22.5 (5.6, 68.3) | 15.75 (6.8, 94.5) | 0.992 |
| Number of lesions | 0.652 | ||
| 1 | 37 (56.9) | 22 (57.9) | |
| 2 | 18 (27.7) | 11 (28.9) | |
| 3 | 10 (15.4) | 4 (10.5) | |
| ≥ 4 | 0 | 1 (2.6) | |
| Largest tumor diameter, median (IQR) | 3 (2.4, 3.8) | 3.3 (2.4, 3.9) | 0.634 |
| Milan-out | 10 (15.4) | 8 (21.1) | 0.591 |
| Use of PEI | 25 (38.5) | 17 (44.7) | 0.541 |
| Number of procedures, median (IQR) | 2 (1, 3) | 2 (1, 2.75) | 0.914 |
Dropout due to tumor progression occurred in 7 (17.5%), 5 (20%), 2 (9.5%), and 1 (5.9%) patients who underwent only TACE (n = 40), TACE + PEI (n = 20), only TAE (n = 21), and TAE + PEI (n = 17), respectively (Table 2). The difference among the four treatment groups was not statistically significant (P = 0.565). The overall dropout due to tumor progression was 12 (18.5%) and 3 (7.9%) in the TACE (only TACE and TACE + PEI, n = 65) and TAE (only TAE and TAE + PEI, n = 38) groups, respectively (P = 0.162). In a time-to-event analysis using the Kaplan–Meier method (Figure 1A), no significant difference in dropout rates was detected between the TACE and TAE groups (P log-rank test = 0.29).
| Dropout due to tumor progression | |||
| No | Yes | ||
TACE | TACE only | 33 (82.5) | 7 (17.5) |
| TACE + PEI | 20 (80) | 5 (20) | |
| Overall TACE | 53 (81.5) | 12 (18.5) | |
TAE | TAE only | 19 (90.5) | 2 (9.5) |
| TAE + PEI | 16 (94.1) | 1 (5.9) | |
| Overall TAE | 35 (92.1) | 3 (7.9) | |
Of the 65 patients who underwent TACE, adverse events occurred in 8 (12.3%) patients, of which 7 were classified as Clavien–Dindo Grade 2 or lower. The remaining patient died following a combined TACE + PEI procedure due to hemorrhage. Of the 38 patients who underwent TAE, adverse events occurred in 2 (5.3%) patients. Both events were classified as Clavien–Dindo Grade 1. The difference between adverse events in the TACE and TAE groups was not statistically significant (P = 0.316).
The demographic and treatment characteristics of patients who underwent OLT are listed in Table 3. In total, 77 (74.8%) of the 103 included patients underwent LT. Forty-eight (73.8%) of the 65 patients in the TACE group and 29 (76.3%) of the 38 patients in the TAE group underwent LT (P = 0.818). No statistically significant difference in the study variables was detected between the groups.
| Variables | TACE | TAE | P value |
| Number | 48 | 29 | |
| Age (yr), median (IQR) | 60.5 (55.75, 65.25) | 62 (53, 63) | 0.458 |
| Male sex | 30 (62.5) | 19 (65.5) | 0.812 |
| Diagnosis | 0.385 | ||
| HCV | 37 (77.1) | 25 (86.2) | |
| HBV | 4 (8.3) | 1 (3.4) | |
| Alcohol | 3 (6.2) | 3 (10.3) | |
| NASH | 4 (8.3) | 0 | |
| Other | 0 | 0 | |
| Calculated MELD score, median (IQR) | 9 (8, 11.25) | 11 (9, 12) | 0.109 |
| Pretransplant AFP, median (IQR) | 11.7 (4.77, 46) | 9.1 (4.4, 31.95) | 0.668 |
| Number of lesions | 0.704 | ||
| 1 | 29 (60.4) | 16 (55.2) | |
| 2 | 12 (25) | 8 (27.6) | |
| 3 | 7 (14.6) | 4 (13.8) | |
| ≥ 4 | 0 | 1 (3.4) | |
| Largest tumor diameter, median (IQR) | 2.8 (2.3, 3.8) | 3.3 (2.5, 3.6) | 0.333 |
| Milan-out | 5 (10.4) | 8 (27.6) | 0.064 |
| Use of PEI | 19 (39.6) | 15 (51.7) | 0.348 |
| Complete pathological response | 7 (14.6) | 9 (31) | 0.145 |
| Vascular invasion | 8 (16.7) | 4 (13.8) | > 0.99 |
After transplantation, HCC recurred in 9 (18.8%) of the 48 patients in the TACE group and 4 (13.8%) of the 29 patients in the TAE group (P = 0.756). The recurrence-free survival curves are shown in Figure 1B. No statistical difference was detected in the recurrence-free survival between TACE and TAE (P log-rank test = 0.71).
The present study evaluated the outcomes of TACE and TAE in patients with HCC on the LT waitlist. Neither the proportion of patients who underwent LT nor the dropout rate due to tumor progression beyond the limits of the Milan criteria differed between the groups. Moreover, in patients who later underwent LT, recurrence-free survival was similar regardless of the bridging therapy employed. Adverse events were not statistically different between the TAE and TACE groups. However, a higher incidence of adverse events was observed in the TACE group (12.3%) than in the TAE group (5.3%).
Whether the addition of a chemotherapeutic agent to TAE has a significant clinical effect has been the subject of several studies. The first RCT on this issue suggested that TACE was superior to TAE in patients with unresectable HCC, a patient group that is different to the one analyzed in the present study[21]. However, that trial was discontinued because preliminary results demonstrated the benefit of TACE over no treatment, precluding a more precise comparison between TACE and TAE. Since then, three RCTs have failed to demonstrate improved overall or progression-free survival of TACE over TAE in patients with HCC who are unsuitable for curative treatment[22-24]. Additionally, two recent meta-analyses of RCTs suggested that there were no benefits of TACE over TAE in patients with unresectable HCC[14,25].
Only one case-control study by Kluger et al[15] directly compared TACE with TAE in patients with HCC on the LT waitlist. Similar to our findings that study also demonstrated no difference in dropout rates, complete pathological response, and recurrence-free survival between TAE and TACE as a bridging therapy to LT. Tsochatzis et al[26] demonstrated that either TACE or TAE improved post-transplant outcomes in comparison to no pre-transplant treatment. Most patients in the study by Tsochatzis et al[26] underwent TAE instead of TACE. Although a direct comparison between TACE and TAE regarding clinical outcomes was not performed, there was no difference in terms of histological response in the explanted livers. Another study found a higher rate of histological necrosis in patients who underwent TACE than in patients who underwent TAE[27]. However, that study did not report the dropout rate. Furthermore, its small sample size (n = 16) precludes a conclusion regarding post-transplant outcomes.
In our study, the rate of complete or near-complete tumor necrosis was relatively low in both groups (TACE 14.6% vs TAE 31%). This may be attributed to the fact that our pathology report only considered complete tumor necrosis when no viable tumor was observed in the entire liver explant. The rate of complete or near-complete tumor necrosis was similar between the TACE and TAE groups, with a trend toward a higher rate in the TAE group. A similar trend in complete pathological response was observed in the study by Kluger et al[15] (TAE 36% vs TAE 26%). Conversely, Nicolini et al[27] found more tumor necrosis in patients who underwent TACE than in those who underwent TAE (77% vs 27.2%). Given the conflicting results, it remains controversial whether there is a difference between TACE and TAE in terms of complete tumor necrosis.
In this study, the rate of adverse events, which included one death, was higher in the TACE group than in the TAE group (prevalence, 12.3% vs 5.3%). This difference was not statistically significant, which may be attributable to the small sample size (type II error). Two meta-analyses found increased toxicity after TACE than after TAE[14,27]. In a study evaluating the use of TAE in patients on the LT waitlist, the incidence of major complications (Clavien–Dindo Grade 3 or higher) was considerably low (2.6%)[28]. In our study, the two adverse events (5.3%) in the TAE group were minor (Clavien–Dindo Grade 1).
The ultimate goals of HCC bridging therapies are to prevent dropout due to tumor progression beyond the limits of the Milan criteria and to ensure long-term recurrence-free survival after LT. As there seems to be no superiority of TACE over TAE regarding those clinical outcomes, evidence of TACE’s superiority over TAE in this group of patients is lacking. Given the tendency of increased toxicity and the indisputable higher cost of TACE when compared with TAE, we believe that our study findings, in conjunction with those of the study by Kluger et al[15], should encourage a more liberal use of TAE for bridging therapy to LT in patients with HCC.
Our study has some limitations. It was a retrospective study. However, the data were extracted from a prospectively filled database. In addition, most patients underwent PEI in addition to TACE or TAE, which might have confounded the interpretation of the study results. Nevertheless, the proportion of patients who underwent PEI was similar between the groups. Several patients with HCC on the LT waitlist have more than one tumor with different features that render them suitable for different types of LRTs. Thus, we believe that the addition of patients who underwent an ablation procedure makes our sample more similar to “real-life” patients, thereby improving the external validity of the study.
In conclusion, the use of TAE in patients with HCC who are on the LT waitlist produced similar outcomes as the use TACE in terms of dropout rate, transplant rate, pathological necrosis, and post-transplant recurrence-free survival. Our study further reinforces that TACE is not superior to TAE for the treatment of HCC. Thus, TAE may be employed in scenarios in which the use of chemotherapeutic agents is contraindicated, such as intolerance to antineoplastic drugs, and in frail patients in whom its concomitant use with PEI or RFA is required.
| 1. | Zhang Y. International agency for research on cancer (IARC). In: Encyclopedia of Global Health. New York: SAGE Publications, 2008: 928-928. |
| 2. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3126] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 3. | Ebara M, Hatano R, Fukuda H, Yoshikawa M, Sugiura N, Saisho H. Natural course of small hepatocellular carcinoma with underlying cirrhosis. A study of 30 patients. Hepatogastroenterology. 1998;45 Suppl 3:1214-1220. [PubMed] |
| 4. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3292] [Article Influence: 143.1] [Reference Citation Analysis (1)] |
| 5. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3082] [Article Influence: 770.5] [Reference Citation Analysis (61)] |
| 6. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5384] [Article Influence: 179.5] [Reference Citation Analysis (7)] |
| 7. | Heimbach JK, Hirose R, Stock PG, Schladt DP, Xiong H, Liu J, Olthoff KM, Harper A, Snyder JJ, Israni AK, Kasiske BL, Kim WR. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant 2010; 10:1643–8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 793] [Article Influence: 56.6] [Reference Citation Analysis (2)] |
| 10. | Lazzarotto-da-Silva G, Grezzana-Filho TJM, Scaffaro LA, Farenzena M, Silva RK, de Araujo A, Arruda S, Feier FH, Prediger L, Lazzaretti GS, Alvares-da-Silva MR, Chedid AD, Kruel CRP, Chedid MF. Percutaneous ethanol injection is an acceptable bridging therapy to hepatocellular carcinoma prior to liver transplantation. Langenbecks Arch Surg. 2023;408:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, Murad MH, Mohammed K. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology. 2018;67:381-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 12. | Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, Covino M, Ravaioli M, Fagiuoli S, Gasbarrini G, Rapaccini GL. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Li Y, Tan X, Liu X, Liu L, Fang Y, Rao R, Ren Y, Yang X, Liu W. Enhanced anticancer effect of doxorubicin by TPGS-coated liposomes with Bcl-2 siRNA-corona for dual suppression of drug resistance. Asian J Pharm Sci. 2020;15:646-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Lawson A, Kamarajah SK, Parente A, Pufal K, Sundareyan R, Pawlik TM, Ma YT, Shah T, Kharkhanis S, Dasari BVM. Outcomes of Transarterial Embolisation (TAE) vs. Transarterial Chemoembolisation (TACE) for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Kluger MD, Halazun KJ, Barroso RT, Fox AN, Olsen SK, Madoff DC, Siegel AB, Weintraub JL, Sussman J, Brown RS Jr, Cherqui D, Emond JC. Bland embolization versus chemoembolization of hepatocellular carcinoma before transplantation. Liver Transpl. 2014;20:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Chedid MF, Scaffaro LA, Chedid AD, Maciel AC, Cerski CT, Reis MJ, Grezzana-Filho TJ, de Araujo A, Leipnitz I, Kruel CD, Alvares-da-Silva MR, Kruel CR. Transarterial Embolization and Percutaneous Ethanol Injection as an Effective Bridge Therapy before Liver Transplantation for Hepatitis C-Related Hepatocellular Carcinoma. Gastroenterol Res Pract. 2016;2016:9420274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Lee YT, Wang JJ, Zhu Y, Agopian VG, Tseng HR, Yang JD. Diagnostic Criteria and LI-RADS for Hepatocellular Carcinoma. Clin Liver Dis (Hoboken). 2021;17:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2104] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 19. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26035] [Article Influence: 1183.4] [Reference Citation Analysis (1)] |
| 20. | Dam HK, Ghose A, Gilbert N, Singh MP. Understanding Responsible Computing via Project Management for Sustainability. IEEE Internet Comput. 2023;27:37-42. [DOI] [Full Text] |
| 21. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2652] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 22. | Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, Moschouris H, Emmanouil E, Rizos S, Kelekis D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 23. | Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, Davies N, Williams E, Pereira SP, Hochhauser D, Mayer A, Gillmore R, O'Beirne J, Patch D, Burroughs AK. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer. 2013;108:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, Jarnagin WR, D'Angelica MI, Allen PJ, Erinjeri JP, Brody LA, O'Neill GP, Johnson KN, Garcia AR, Beattie C, Zhao B, Solomon SB, Schwartz LH, DeMatteo R, Abou-Alfa GK. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol. 2016;34:2046-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 25. | Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, Vendemiale G, Serviddio G. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United European Gastroenterol J. 2017;5:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Tsochatzis E, Garcovich M, Marelli L, Papastergiou V, Fatourou E, Rodriguez-Peralvarez ML, Germani G, Davies N, Yu D, Luong TV, Dhillon AP, Thorburn D, Patch D, O'Beirne J, Meyer T, Burroughs AK. Transarterial embolization as neo-adjuvant therapy pretransplantation in patients with hepatocellular carcinoma. Liver Int. 2013;33:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Hodavance MS, Vikingstad EM, Griffin AS, Pabon-Ramos WM, Berg CL, Suhocki PV, Kim CY. Effectiveness of Transarterial Embolization of Hepatocellular Carcinoma as a Bridge to Transplantation. J Vasc Interv Radiol. 2016;27:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0