Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.89255
Peer-review started: October 25, 2023
First decision: January 12, 2024
Revised: January 18, 2024
Accepted: February 27, 2024
Article in press: February 27, 2024
Published online: March 18, 2024
Processing time: 141 Days and 19.9 Hours
Detection of early chronic changes in the kidney allograft is important for timely intervention and long-term survival. Conventional and novel ultrasound-based investigations are being increasingly used for this purpose with variable results.
To compare the diagnostic performance of resistive index (RI) and shear wave elastography (SWE) in the diagnosis of chronic fibrosing changes of kidney allograft with histopathological results.
This is a cross-sectional and comparative study. A total of 154 kidney transplant recipients were included in this study, which was conducted at the Departments of Transplantation and Radiology, Sindh Institute of Urology and Transplan
The mean age of all patients was 35.32 ± 11.08 years. Among these, 126 (81.8%) were males and 28 (18.2%) were females. The mean serum creatinine in all patients was 2.86 ± 1.68 mg/dL and the mean estimated GFR was 35.38 ± 17.27 mL/min/1.73 m2. Kidney allograft biopsy results showed chronic changes in 55 (37.66%) biopsies. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of SWE for the detection of chronic allograft damage were 93.10%, 96.87%%, 94.73%, and 95.87%, respectively, and the diagnostic accuracy was 95.45%. For RI, the sensitivity, specificity, PPV, and NPV were 76.92%, 83.33%, 70.17%, and 87.62%, respectively, and the diagnostic accuracy was 81.16%.
The results from this study show that SWE is more sensitive and specific as compared to RI in the evaluation of chronic allograft damage. It can be of great help during the routine follow-up of kidney transplant recipients for screening and early detection of chronic changes and selecting patients for allograft biopsy.
Core Tip: Kidney transplantation is the treatment of choice for patients with end-stage kidney disease. Although short-term outcomes have improved markedly, chronic allograft damage remains a formidable challenge. Early detection of chronic changes is crucial for the optimal well-being of the graft. Biopsy is the gold standard but is invasive, and prone to sampling error and interobserver variation. The resistive index on Doppler is routinely used for the assessment of renal allograft status but its value in chronic renal allograft dysfunction is unclear. Shear wave sonoelastography is a novel imaging technique that has shown promising results in a number of studies.
- Citation: Jesrani AK, Faiq SM, Rashid R, Kalwar TA, Mohsin R, Aziz T, Khan NA, Mubarak M. Comparison of resistive index and shear-wave elastography in the evaluation of chronic kidney allograft dysfunction. World J Transplant 2024; 14(1): 89255
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/89255.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.89255
Kidney transplantation is the treatment of choice for patients with end-stage kidney disease. However, the recipients of kidney transplants have to be continually monitored both clinically and by radiological and laboratory tests to ensure the proper functioning of the allograft and to detect any damage to the allograft at an early and reversible stage. In this regard, it is to be noted that allograft dysfunction can occur at any time post-transplantation. It is variously categorized as acute and chronic allograft dysfunction and the causes vary accordingly. An early and accurate diagnosis of the underlying causes is essential for optimal management and better long-term outcomes. Any damage to the graft parenchyma may result in chronic sclerosing changes in the parenchyma if not treated promptly. In spite of a comprehensive approach toward the allograft’s well-being adopted in most transplantation centers, kidney graft damage often sets in and goes undiagnosed as early abnormalities are either undetected or the laboratory or radiological investigations and clinical presentation are insensitive to early changes in the graft parenchyma[1-3].
A number of diagnostic modalities including imaging and laboratory-based tools are used in practice to detect graft damage at an early stage. Conventionally, structural assessment of the allografts is done by the greyscale and Doppler ultrasounds (US), computed tomography scans, and magnetic resonance imaging, some of which, now provide added information regarding the function of the allograft[4-9]. US is a very useful and often the first-line non-invasive tool for the early diagnosis of reversible surgical complications and is used routinely during the follow-up of kidney transplant recipients (KTRs). The role of Doppler US in the assessment of vascular pathologies in transplanted kidneys can not be overemphasized[6,7]. Currently, several transplantation centers utilize the intrarenal resistive index (RI), which is calculated using Doppler ultrasonography, to evaluate the functional status of the renal allografts, particularly in the early post-transplant period. The RI is a hemodynamic index commonly used to measure blood flow resistance in organs to assess vascular disease[6]. Several studies have reported that an increased RI is diagnostic of acute transplant dysfunction. Naesens et al[7] in their seminal paper studied the usefulness of RI in protocol and graft dysfunction settings in 321 KTRs[7]. A total of 1124 kidney allograft RI measurements were included in the analysis. At protocol-specified biopsy time points, the RI was not associated with kidney allograft histologic features. Older recipient age was the strongest determinant of a higher RI. However, the RI was significantly higher in cases of antibody-mediated rejection or acute tubular necrosis, as compared with normal biopsy results, in allograft biopsies performed because of graft dysfunction[7]. They concluded that the routinely performed RI at pre-specified time points after transplantation reflects characteristics of recipient but not those of the graft[7]. Radermacher and Haller commented on the study by Naesens et al[7] and noted that the findings of their study differ from most previous studies, in which an increase in RI was associated with graft deterioration[8]. They suggested possible explanations for these discrepant results. Naesens et al[7] studied interlobar arteries, whereas the previous studies investigated segmental arteries, and RI values are lower in the former arteries. The use of a lower cutoff value for the RI (i.e., one considered abnormal) might have been more accurate in the study by Naesens et al[7] In addition, peripheral vessels are more prone to sampling bias, and the Doppler signal quality is poorer[8]. Timing of RI measurement was also a minor factor. The length of follow-up period is also a contributory factor to the discrepant results. According to Radermacher and Haller, a consensus on a single vessel area for study might provide a single cutoff value for the RI. This should allow an assessment of whether the RI predicts graft loss, recipient death, or both, and the results of which would define the role of the RI in the assessment of transplant patients[8]. The usefulness of the RI after kidney transplantation, particularly in chronic allograft dysfunction, remains controversial. RI as an investigation suffers from certain pitfalls, particularly in extended criteria donors or old recipients. Most importantly, its assessment is not uniformly standardized. It is a non-specific prognostic marker of vascular diseases that affect the kidney. The RI is thought to reflect central hemodynamic (cardiac or aortic) characteristics rather than properties of the kidney or kidney allograft. There is little correlation between the RIs and the quantitative extent of kidney allograft dysfunction.
More recently, another emerging technology of US, i.e., sonoelastography, is increasingly being used to assess and visually display tissue stiffness by US probes[10-14]. Elasticity imaging or elastography is an imaging modality based on tissue stiffness or hardness, rather than anatomy. US elastography can be considered the imaging equivalent of palpation, being able to quantify the stiffness of a lesion, which was previously judged only subjectively by physical examination[10,11]. Palpation has been used to evaluate malignancy for a very long time. Sonoelastography has mainly been used in the diagnosis of cancers in both superficial and deep organs like the breast, thyroid, and prostate gland[15-21].
Recent studies have suggested that quantitative elastography is a reliable non-invasive tool to assess chronic fibrosing changes in organs like the liver[22-26] and kidney[27-32] at early stages. A few studies have investigated the usefulness of sonoelastography in the assessment of chronic fibrosing changes in the kidney allograft[33-36]. In the first clinical pilot study by Arndt et al[33], parenchymal stiffness measured by sonoelastography was found to be suitable for assessing the progression of kidney allograft fibrosis. They concluded that a longitudinal assessment of parenchymal stiffness might be a powerful tool to identify patients with chronic allograft damage who benefit from biopsy and consequent adaptation of the immunosuppressive treatment[33]. Subsequently, many more studies have reported the diagnostic utility of sonoelastography in the assessment of chronic kidney allograft dysfunction[34-36]. However, only a few studies have compared the diagnostic performance of RI vs shear-wave elastography (SWE) in the assessment of chronic sclerosing changes in the kidney allograft. The aim of this study was to compare the diagnostic performance of RI and SWE in the early detection of chronic fibrosing changes in kidney allograft against the findings of renal allograft biopsy.
This cross-sectional, observational study was conducted at the Radiology, Histopathology, and Transplantation departments, Sindh Institute of Urology and Transplantation, Karachi, Pakistan from August 2022 to February 2023. A formal approval was sought from the research and ethical committees of the institution before starting the study. All consecutive adult KTRs who fulfilled the inclusion criteria were included. The inclusion criteria included patients presenting with kidney allograft dysfunction occurring any time after the first three months of transplantation and manifesting as a rise in serum creatinine > 20% from the baseline or reduced estimated glomerular filtration rate (eGFR) < 50 mL/min, as determined by Cockcroft-Gault (C-G) formula and a normal allograft size (≥ 9 cm). Kidney transplant patients with a skin-to-allograft distance of > 3 cm, cortex thickness < 1 cm, kidney allograft dysfunction within first three months after transplantation, small graft size (< 9 cm), and perigraft fluid collection were excluded.
Written informed consent was taken from all eligible patients. The patients were either referred from the outpatient department of transplant services or they were admitted in the transplant ward. All patients participating in this study received kidney transplants from a living-related donor.
All consecutive adult patients (≥ 20 years) of either gender were investigated by all three methods, i.e., Doppler US, SWE, and kidney allograft biopsy.
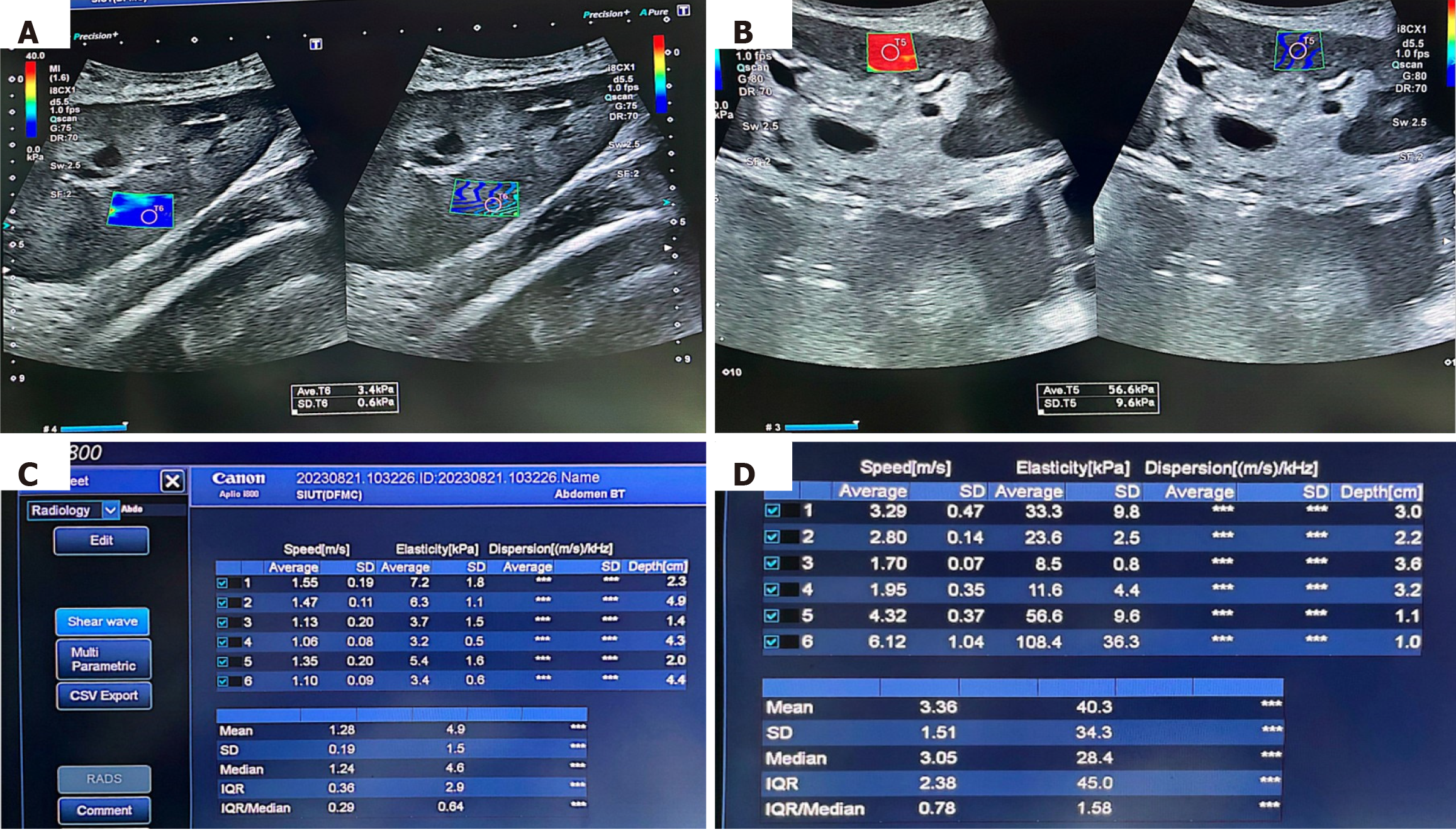
All US assessments including SWE measurements were performed by the two experienced radiologists with > 10 years of experience in the abdominal US, including 5 years of experience with SWE and Doppler sonography. One of these performed RI measurements first on all included patients independently followed by the other radiologist, who performed SWE and allograft biopsy, also independently, such that no duplicate measurements of the radiological tests were performed. Both were blinded to the patient data and each other’s sonographic findings. A “check” US examination was performed first to assess the morphologic characteristics of the allograft and its vascularity, perigraft collection, and skin-to-allograft distance. SWE measurements were then undertaken with the patient lying in a supine position. The sampling for point-based SWE was performed with the patient holding his or her breath. A total of six measurements of SWE (US systems (CANON; APLIO i800) in kPa were made with two measurements each from the upper pole, lower pole, and mid-polar regions. The mean of these six values of parenchymal stiffness was calculated for each patient and was analyzed. The representative SWE visual displays and the quantitative parameters in a case of stable graft function and another case with chronic allograft changes are shown in Figure 1. In Figure 1B and D, the elastography demonstrates the non-homogeneous color coding of the area in renal allograft with multiple colors with red color predominating which represents a significant loss of elasticity and increased stiffness of the renal allograft parenchyma. In addition, both the speed and elasticity columns are very heterogeneous in Figure 1B and D, reflecting patchy distribution of early fibrosis. Most severely affected area was chosen for sampling for the allograft biopsy. The sonoelastography findings were correlated with histopathology of the same renal allografts showing variable degrees of chronic changes (Figure 2). Kidney allograft biopsies were interpreted according to the updated Banff classifications. Two cores of kidney allograft biopsies were performed routinely and processed according to standard guidelines. As noted above, the most abnormal area of allograft parenchyma on SWE was selected for biopsy purpose.
The same procedure was repeated for measuring the RI on the same US system as was used for SWE. A single reading was recorded for each pole and the mean value was calculated for each patient.
The findings of the SWE and RI were then compared with the histopathological findings of the allografts on renal allograft biopsy in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. The results of histopathology were considered the gold standard for this purpose. The average of the semi-quantitative scores of chronic changes affecting the two cores were considered for final analysis.
Statistical analysis was performed by using Statistical Package for Social Sciences (SPSS 21.0). Descriptive statistics were applied. Mean ± SD was computed for the quantitative variables distributed normally, i.e. age of patients and serum creatinine. For non-normally distributed data, such as posttransplant duration of biopsies, median ± interquartile range (IQR) were used. Frequencies and percentages were calculated for qualitative variables, i.e., presenting complaints and histopathological findings.
Taking histopathological findings as the gold standard, all statistical parameters (sensitivity, specificity, PPV, NPV) were calculated to obtain diagnostic accuracy of SWE and RI.
In this study, a total of 154 KTRs of both genders were included. The mean age of all patients was 35.32 ± 11.08 years (range: 20-60 years). Among these, 126 (81.8%) were males and 28 (18.2%) were females. The US-based investigations and allograft biopsies were performed at a median posttransplant duration of 24 months (IQR: 7 to 61.5 months). Around 50% of biopsies were performed within 24 months after transplantation. The mean serum creatinine at the time of biopsy was 2.86 ± 1.68 mg/dL and the mean eGFR was 35.38 ± 17.27 mL/min/1.73 m2. Histopathological confirmation of chronic allograft changes was obtained in 55 (37.66%) biopsies. However, SWE results were positive for chronic changes in 57 (37.01%) of cases, as shown in Table 1. The sensitivity, specificity, PPV, NPV, and diagnostic accuracy of SWE for the detection of chronic changes were 93.10%, 96.87%, 94.73%, and 95.87% and the overall diagnostic accuracy was 95.45% (Table 1). On the other hand, the sensitivity, specificity, PPV, NPV, and diagnostic accuracy of RI for the detection of chronic changes were 76.92%, 83.33%, 70.17%, 87.62%, and the diagnostic accuracy 81.16% (Table 2).
| Shear-wave elastography results | Histopathological results | Total | |
| Positive | Negative | ||
| Positive | 54 (TP) | 3 (FP) | 57 (37.01) |
| Negative | 4 (FN) | 93 (TN) | 97 (62.98) |
| Total | 58 (37.66) | 96 (62.33) | 154 (100) |
| Sensitivity | 54/58 | 93.10 | |
| Specificity | 93/96 | 96.87 | |
| Positive predictive value | 54/57 | 94.73 | |
| Negative predictive value | 93/97 | 95.87 | |
| Diagnostic accuracy | (54 + 93)/154 | 95.45 | |
| Resistive index results | Histopathological results | Total | |
| Positive | Negative | ||
| Positive | 40 (TP) | 17 (FP) | 57 (37.01) |
| Negative | 12 (FN) | 85 (TN) | 97 (62.98) |
| Total | 52 (33.76) | 102 (66.23) | 154 (100) |
| Sensitivity | 40/52 | 76.92 | |
| Specificity | 85/102 | 83.33 | |
| Positive predictive value | 40/57 | 70.17 | |
| Negative predictive value | 85/97 | 87.62 | |
| Diagnostic accuracy | (40 + 85)/154 | 81.16 | |
The sonoelastography clearly performed better than RI in predicting the chronic allograft changes with superior sensitivity, specificity, and positive and NPV as shown in Table 2.
Chronic sclerosing changes in kidney allografts have been categorized in different ways. In the pre-Banff era, these were labeled as “chronic rejection” irrespective of the underlying etiopathogenesis. The Banff classification introduced the term chronic allograft nephropathy (CAN)[37]. In 2005, the term CAN was replaced by interstitial fibrosis/tubular atrophy (IFTA). The changes of IFTA are highly prevalent in kidney allografts. A study by Nankivell et al[38] found chronic changes in 24.7% of renal transplant recipients 1 year post-transplant and the percentage increased to 89.9% in recipients after 10 years of a kidney transplant making CAN the most frequent reason for kidney graft failure[38]. The chronic changes are thought to be the result of chronic subclinical injury, either immune-mediated or non-immune, that progresses to kidney allograft failure[39].
An early and accurate diagnosis of chronic changes is imperative for salvaging the kidney allograft from failure. Protocol biopsies represent the gold standard for detecting chronic changes in the allograft parenchyma at an early stage. However, these are associated with certain complications and drawbacks related to the invasive nature, sampling error, and subjectivity of their interpretation[40]. The current approaches for diagnosis of suspected IFTA include serum creatinine and eGFR measurements and vascular perfusion assessment by RI using Doppler US. When abnormalities are detected in the above-mentioned parameters, the next step in evaluation is kidney allograft biopsy for tissue diagnosis[41,42]. Various formulas are used for calculating eGFR in the kidney transplant patients and all give comparable results[43,44]. Hence, we used C-G formula in our study, as it is relatively straightforward in calculation.
Very few studies are available in the literature on the detection of early fibrosing changes in transplanted kidneys using sonoelastography, which assesses stiffness as a measure of fibrosis[34-36,45-50]. A large number of studies are available for superficial organs like the breast and thyroid gland[15-26]. The native kidneys are deep-seated and hence, have been little investigated by this technique[27-32]. In a study done to determine the elasticity of various tissues, Arda et al[45] studied normal elasticity values within the kidney cortex along with many other internal organs in 127 healthy volunteers aged 17-63 years. The mean elasticity values were 5.2 ± 2.9 kPa (range: 1-13 kPa) in men and 4.9 ± 2.9 kPa (range: 1-26 kPa) in women of renal cortex[45]. Some studies conducted previously have reported that renal parenchymal elasticity values differ with anisotropy, and vascular and urinary pressures[46]. According to these authors, intrarenal elasticity values fluctuate with tissue anisotropy and, with vascular and urinary pressure levels. These parameters must be taken into account for the interpretation of tissue changes[47].
Exploiting the superficial location of the kidney allograft, several studies have been conducted to determine the diagnostic utility of SWE in the evaluation of kidney allograft dysfunction and compared it with various clinical, laboratory, or imaging parameters[34-36,48-55]. The mean parenchymal stiffness on SWE was 24.5 + 7.34 kPa (range: 17-32 kPa) in patients with allograft dysfunction in this study. Parenchymal stiffness showed a positive correlation with serum creatinine level (r = 0.714; P < 0.001) and a negative correlation with eGFR (r = 20.725; P < 0.001). Lukenda et al[48] studied transient elastography (TE) in 52 KTRs and reported a highly significant negative correlation of kidney allograft stiffness on SWE with eGFR in 52 KTRs (r = -0.640; P < 0.0001). The kidney allograft stiffness showed a positive correlation with allograft fibrosis on biopsy (r = 0.727; P = 0.0001). They concluded that parenchymal stiffness obtained by elastography reflects interstitial fibrosis[48]. Therefore, elastography provides the opportunity for noninvasive screening of CAN. Similarly, Ozkan et al[47] studied 42 patients by real-time sonoelastography to investigate the relationship of tissue stiffness with RI and eGFR. Allograft parenchymal stiffness demonstrated a significant positive correlation with RI (r: 0.41, P = 0.007). They did not find a significant correlation between parenchymal stiffness and eGFR (P = 0.42). Interobserver agreement, expressed as intraclass correlation coefficient, was fair at 0.47 (95%CI: 0.05- 0.70). They concluded that parenchymal stiffness showed a significant positive correlation with RI but sonoelastography has also a wide range of intra- and low interobserver agreement in kidney transplants warranting further studies[47].
Arndt et al[33] studied TE in 57 KTRs and found that parenchymal stiffness was significantly and positively correlated to the extent of interstitial fibrosis (r = 0.67, P = 0.002) and inversely related to eGFR (r = 0.47, P = 0.0003). Parenchymal stiffness values of patients with an eGFR > 50 mL/min were significantly lower than in patients with an eGFR 50 mL/min (22.2 ± 11.0 vs 37.1 ± 14.2 kPa, P = 0.0005). The parenchymal stiffness values of Chronic allograft injury Banff grades 0-1 differed significantly from grade 2 (P = 0.008) and grade 3 (P = 0.046). Parenchymal stiffness measured by TE reflects interstitial fibrosis in kidney allografts. They concluded that a longitudinal assessment of parenchymal stiffness might be a potent tool to identify patients with chronic allograft changes who benefit from biopsy and consequent alteration of the immunosuppressive regime[33].
More recently, Barsoum et al[54] studied 36 KTRs with SWE with biopsy-proven CAN. All patients underwent a B-mode US examination followed by US SWE in the same sitting, as in our study. They compared the results of SWE measurements with the histopathological results. They found that the mean parenchymal stiffness was directly correlated with time post-transplantation. With a longer post-transplantation period, parenchymal stiffness and IF/TA percentages increased with r = 0.72, 0.90, and P value < 0.001. Antero-posterior (AP) diameter of the kidney allograft was significantly correlated with mean parenchymal stiffness as the larger the AP diameter, the higher the mean parenchymal stiffness with r = 0.47, 0.73, and P value 0.001. Sensitivity analysis showed that US SWE can significantly predict moderate Banff score of renal fibrosis using a cutoff value of 28.67 kPa with sensitivity of 87.5%, specificity of 90%, area under the curve (AUC) of 0.91, and P value < 0.001. SWE may be useful for the prediction of fibrosis in KTRs, especially in the case of a moderate Banff score, where the accuracy reached 87.5% using a cutoff value of 28.67 kPa. They concluded that US SWE may be of great help in the regular follow-up of KTRs. It can act as a screening tool to identify patients with early parenchymal fibrosis, eventually helping in the early diagnosis and management and helping in selecting patients who are candidates for biopsy and in avoiding repeated unnecessary biopsies for others[54].
We found a sensitivity of 93.10% and specificity of 96.87% of SWE for the detection of chronic fibrosing changes in the allograft biopsy. These results are marginally better than RI on Doppler studies. Our results are also slighter better as compared to those of Barsoum et al[54] in terms of overall sensitivity and specificity[54]. In our study, the parenchymal stiffness measurement correlated with histopathological diagnosis.
Although histopathology is considered the gold standard for the detection of chronic renal allograft changes, there are a few drawbacks related to this invasive method. These drawbacks include sampling errors, traumatic complications, and interobserver variations among histopathologists. Hence, a search for non-invasive techniques for the early diagnosis of kidney allograft damage has always been a dream of researchers. The best attribute of sonoelastography as a modality is its noninvasive nature making it a safe screening tool for serial evaluation of kidney allograft. In addition to being non-invasive, SWE enables us to assess a much larger area of the tissue under study as compared to biopsy. On the basis of the results of the present study, it would not be wrong to state that this study will help in building confidence among clinicians regarding non-invasive modalities for the diagnosis of chronic allograft dysfunction. However, we do recognize that allograft biopsy will retain the status of the gold standard in cases with equivocal or ambiguous findings, or in synchrony with sonoelastography. In addition, if used judicially, this technique will help in decreasing the bulk of invasive procedures making the investigative process less risky for the patients.
There are certain limitations to this study. Firstly, it is a single-center study. No follow-up data was collected for this study. We did not calculate the AUC for SWE regarding its diagnostic utility. There is a need for multicenter studies to add more strength to the observations made in this study. Certain artifacts are associated with increased thickness of the patient which renders it appropriate in patients of a certain body habitus.
In conclusion, SWE is more sensitive and specific as compared with RI and can serve as a reliable noninvasive imaging modality for the detection of early chronic changes in the kidney allograft. On the basis of these results, we propose to use SWE routinely for serial evaluation of kidney allograft during follow-up for early detection of chronic changes and selecting patients for allograft biopsy.
Kidney transplantation is the treatment of choice for patients with end-stage kidney disease. Although, short-term outcomes have improved but long-term graft survival remains a formidable challenge. Detection of early chronic changes in the kidney allograft is important for timely intervention and long-term survival. Conventional and novel ultrasound (US)-based investigations are being increasingly used for this purpose with variable results. This study aims to compare the diagnostic performance of two US-based tests with biopsy results.
The main aim is to determine the diagnostic performance of a non-invasive US-based investigation in the assessment of early chronic changes in the kidney allograft. This will help avoid or minimize the invasive procedure of kidney allograft biopsy.
The main objective was to assess the diagnositc performance of shear-wave elastography (SWE) on US of the allograft kidney for detection of early chronic changes in the kidney allograft. It was found that SWE performs better than resistive index (RI) and this can be a useful addition to the diagnostic armamenterium for post-transplant follow-up.
All consecutive kidney transplant patients with increased serum creatinine levels and reduced glomerular filtration rate three months after transplantation were assessed by SWE and RI tools and the findings of these were analyzed against the kidney allograft biopsy results to determine their diagnostic performance.
The sensitivity, specificity, positive predictive value, and negative predictive value of SWE for the detection of chronic allograft damage were better as compared to RI results. These results indicate that SWE test is more sensitive for the detection of early chronic changes in the kidney allograft and this should be routinely used in the assessment of kidney allograft during post-transplant follow-up.
Novel US-based techniques offer promising new tools for non-invasive monitoring of early chronic kidney allograft damage. These can be used for screening the kidney transplant patients during routine follow-up visits followed by biopsies.
Further improvements in US-based techniques for non-invasive monitoring of kidney allograft status are needed.
| 1. | Langewisch E, Mannon RB. Chronic Allograft Injury. Clin J Am Soc Nephrol. 2021;16:1723-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Riella LV, Djamali A, Pascual J. Chronic allograft injury: Mechanisms and potential treatment targets. Transplant Rev (Orlando). 2017;31:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Hara S. The Chronology of Renal Allograft Dysfunction: The Pathological Perspectives. Nephron. 2023;147:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Schutter R, Lantinga VA, Borra RJH, Moers C. MRI for diagnosis of post-renal transplant complications: current state-of-the-art and future perspectives. MAGMA. 2020;33:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Volkan-Salanci B, Erbas B. Imaging in Renal Transplants: An Update. Semin Nucl Med. 2021;51:364-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Gómez V, Orosa A, Rivera M, Diez-Nicolás V, Hevia V, Alvarez S, Carracedo D, Ramos E, Burgos FJ. Resistance index determination in the pre and post kidney transplantation time points in graft dysfunction diagnosis. Transplant Proc. 2015;47:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Naesens M, Heylen L, Lerut E, Claes K, De Wever L, Claus F, Oyen R, Kuypers D, Evenepoel P, Bammens B, Sprangers B, Meijers B, Pirenne J, Monbaliu D, de Jonge H, Metalidis C, De Vusser K, Vanrenterghem Y. Intrarenal resistive index after renal transplantation. N Engl J Med. 2013;369:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 8. | Radermacher J, Haller H. The role of the intrarenal resistive index in kidney transplantation. N Engl J Med. 2013;369:1853-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Yu YM, Ni QQ, Wang ZJ, Chen ML, Zhang LJ. Multiparametric Functional Magnetic Resonance Imaging for Evaluating Renal Allograft Injury. Korean J Radiol. 2019;20:894-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 646] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 11. | Sporea I. Clinical elastography. Med Ultrason. 2018;20:263-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 12. | Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 393] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 13. | Yuan S, Magarik M, Lex AM, Fleischer AC. Clinical applications of sonoelastography. Expert Rev Med Devices. 2016;13:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Clevert DA, Beyer G, Nieß H, Schlenker B. Ultrasound—New Techniques Are Extending the Applications. Dtsch Arztebl Int. 2023;120:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Mesurolle B, El Khoury M, Chammings F, Zhang M, Sun S. Breast sonoelastography: Now and in the future. Diagn Interv Imaging. 2019;100:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Wang RY, Zhang YW, Gao ZM, Wang XM. Role of sonoelastography in assessment of axillary lymph nodes in breast cancer: a systematic review and meta-analysis. Clin Radiol. 2020;75:320.e1-320.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Anvari A, Barr RG, Dhyani M, Samir AE. Clinical application of sonoelastography in thyroid, prostate, kidney, pancreas, and deep venous thrombosis. Abdom Imaging. 2015;40:709-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Zhang G, Yu J, Lei YM, Hu JR, Hu HM, Harput S, Guo ZZ, Cui XW, Ye HR. Ultrasound super-resolution imaging for the differential diagnosis of thyroid nodules: A pilot study. Front Oncol. 2022;12:978164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Yang H, Xu Y, Zhao Y, Yin J, Chen Z, Huang P. The role of tissue elasticity in the differential diagnosis of benign and malignant breast lesions using shear wave elastography. BMC Cancer. 2020;20:930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Turnaoğlu H, Haberal KM, Arslan S, Yavuz Çolak M, Ulu Öztürk F, Uslu N. Interobserver and intermethod variability in data interpretation of breast strain elastography in suspicious breast lesions. Turk J Med Sci. 2021;51:547-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Nakaoka K, Hashimoto S, Miyahara R, Kawashima H, Ohno E, Ishikawa T, Kuwahara T, Tanaka H, Hirooka Y. Current status of the diagnosis of chronic pancreatitis by ultrasonographic elastography. Korean J Intern Med. 2022;37:27-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020;296:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (1)] |
| 23. | Sande JA, Verjee S, Vinayak S, Amersi F, Ghesani M. Ultrasound shear wave elastography and liver fibrosis: A Prospective Multicenter Study. World J Hepatol. 2017;9:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 24. | Luo QT, Zhu Q, Zong XD, Li MK, Yu HS, Jiang CY, Liao X. Diagnostic Performance of Transient Elastography Versus Two-Dimensional Shear Wave Elastography for Liver Fibrosis in Chronic Viral Hepatitis: Direct Comparison and a Meta-Analysis. Biomed Res Int. 2022;2022:1960244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Kim JH, Yoon JH, Joo I, Lee JM. Intra-individual comparison of two-dimensional shear wave elastography techniques using plane wave imaging and the multi-beam technique: are they interchangeable in measuring liver fibrosis? Ultrasonography. 2023;42:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Samir AE, Allegretti AS, Zhu Q, Dhyani M, Anvari A, Sullivan DA, Trottier CA, Dougherty S, Williams WW, Babitt JL, Wenger J, Thadhani RI, Lin HY. Shear wave elastography in chronic kidney disease: a pilot experience in native kidneys. BMC Nephrol. 2015;16:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Peride I, Rădulescu D, Niculae A, Ene V, Bratu OG, Checheriță IA. Value of ultrasound elastography in the diagnosis of native kidney fibrosis. Med Ultrason. 2016;18:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Islamoglu MS, Gulcicek S, Seyahi N. Kidney tissue elastography and interstitial fibrosis observed in kidney biopsy. Ren Fail. 2022;44:314-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Leong SS, Wong JHD, Md Shah MN, Vijayananthan A, Jalalonmuhali M, Chow TK, Sharif NHM, Ng KH. Shear wave elastography accurately detects chronic changes in renal histopathology. Nephrology (Carlton). 2021;26:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Leong SS, Jalalonmuhali M, Md Shah MN, Ng KH, Vijayananthan A, Hisham R, Wong JHD. Ultrasound shear wave elastography for the evaluation of renal pathological changes in adult patients. Br J Radiol. 2023;96:20220288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Cè M, Felisaz PF, Alì M, Re Sartò GV, Cellina M. Ultrasound elastography in chronic kidney disease: a systematic review and meta-analysis. J Med Ultrason (2001). 2023;50:381-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Arndt R, Schmidt S, Loddenkemper C, Grünbaum M, Zidek W, van der Giet M, Westhoff TH. Noninvasive evaluation of renal allograft fibrosis by transient elastography--a pilot study. Transpl Int. 2010;23:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Ghonge NP, Mohan M, Kashyap V, Jasuja S. Renal Allograft Dysfunction: Evaluation with Shear-wave Sonoelastography. Radiology. 2018;288:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Wang Z, Yang H, Suo C, Wei J, Tan R, Gu M. Application of Ultrasound Elastography for Chronic Allograft Dysfunction in Kidney Transplantation. J Ultrasound Med. 2017;36:1759-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Soudmand A, Ulu Ozturk F, Uslu N, Haberal N, Boyvat F, Moray G, Haberal M. Efficacy of the Sonoelastography Method for Diagnosis of Fibrosis in Renal Transplant Patients. Exp Clin Transplant. 2022;20:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 37. | Khan H, Mubarak M, Aziz T, Ahmed E, Fazal Akhter S, Kazi J, Aa Naqvi S, Ah Rizvi S. Prevalence and risk factors for early chronic allograft nephropathy in a live related renal transplant program. J Nephropathol. 2014;3:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1500] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 39. | Yates PJ, Nicholson ML. The aetiology and pathogenesis of chronic allograft nephropathy. Transpl Immunol. 2006;16:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Seron D. Early diagnosis of chronic allograft nephropathy by means of protocol biopsies. Transplant Proc. 2004;36:763-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Pascual J, Pérez-Sáez MJ, Mir M, Crespo M. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev (Orlando). 2012;26:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Lai X, Zheng X, Mathew JM, Gallon L, Leventhal JR, Zhang ZJ. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Front Immunol. 2021;12:661643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Santos J, Martins LS. Estimating glomerular filtration rate in kidney transplantation: Still searching for the best marker. World J Nephrol. 2015;4:345-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 44. | White CA, Huang D, Akbari A, Garland J, Knoll GA. Performance of creatinine-based estimates of GFR in kidney transplant recipients: a systematic review. Am J Kidney Dis. 2008;51:1005-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011;197:532-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 46. | Gennisson JL, Grenier N, Combe C, Tanter M. Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol. 2012;38:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 47. | Ozkan F, Yavuz YC, Inci MF, Altunoluk B, Ozcan N, Yuksel M, Sayarlioglu H, Dogan E. Interobserver variability of ultrasound elastography in transplant kidneys: correlations with clinical-Doppler parameters. Ultrasound Med Biol. 2013;39:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Lukenda V, Mikolasevic I, Racki S, Jelic I, Stimac D, Orlic L. Transient elastography: a new noninvasive diagnostic tool for assessment of chronic allograft nephropathy. Int Urol Nephrol. 2014;46:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Qi R, Yang C, Zhu T. Advances of Contrast-Enhanced Ultrasonography and Elastography in Kidney Transplantation: From Microscopic to Microcosmic. Ultrasound Med Biol. 2021;47:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Ma MK, Law HK, Tse KS, Chan KW, Chan GC, Yap DY, Mok MM, Kwan LP, Tang SC, Choy BY, Chan TM. Non-invasive assessment of kidney allograft fibrosis with shear wave elastography: A radiological-pathological correlation analysis. Int J Urol. 2018;25:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Early HM, Cheang EC, Aguilera JM, Hirschbein JSW, Fananapazir G, Wilson MD, McGahan JP. Utility of Shear Wave Elastography for Assessing Allograft Fibrosis in Renal Transplant Recipients: A Pilot Study. J Ultrasound Med. 2018;37:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Chhajer G, Arunachalam VK, Ramasamy R, Mehta P, Cherian M. Elastography: a surrogate marker of renal allograft fibrosis - quantification by shear-wave technique. Pol J Radiol. 2021;86:e151-e156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 53. | Early H, Aguilera J, Cheang E, McGahan J. Challenges and Considerations When Using Shear Wave Elastography to Evaluate the Transplanted Kidney, With Pictorial Review. J Ultrasound Med. 2017;36:1771-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Barsoum NR, Elsisy AE, Mohamed MF, Hassan AA. Role of shear wave elastography in assessment of chronic allograft nephropathy. Egypt J Radiol Nucl Med. 2022;53:100. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Zhang TY, Yan J, Wu J, Yang W, Zhang S, Xia J, Che X, Li H, Li D, Ying L, Yuan X, Zhou Y, Zhang M, Mou S. Shear wave elastography parameters adds prognostic value to adverse outcome in kidney transplantation recipients. Ren Fail. 2023;45:2235015. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gonzalez FM, Chile S-Editor: Li L L-Editor: A P-Editor: Zhao S