Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.88133
Peer-review started: October 3, 2023
First decision: October 17, 2023
Revised: November 1, 2023
Accepted: December 11, 2023
Article in press: December 11, 2023
Published online: March 18, 2024
Processing time: 163 Days and 16.4 Hours
Data examining the impact of sex on liver transplant (LT) outcomes are limited. It is clear that further research into sex-related differences in transplant patients is necessary to identify areas for improvement. Elucidation of these differences may help to identify specific areas of focus to improve on the organ matching process, as well as the peri- and post-operative care of these patients.
To utilize data from a high-volume Eurotransplant center to compare characteristics of male and female patients undergoing liver transplant and assess asso
A retrospective review of the University of Essen’s transplant database was performed with collection of baseline patient characteristics, transplant-related data, and short-term outcomes. Comparisons of these data were made with Shapiro-Wilk, Mann-Whitney U, χ2 and Bonferroni tests applied where app
Of the total 779 LT recipients, 261 (33.5%) were female. Female patients suffered higher incidences of acute liver failure and lower incidences of alcohol-related or viremic liver disease (P = 0.001). Female patients were more likely to have received an organ from a female donor with a higher donor risk index score, and as a high urgency offer (all P < 0.05). Baseline characteristics of male and female recipients were also significantly different. In multivariate hazard regression analysis, recipient lab-Model for End-Stage Liver Disease score and donor cause of death were associated with long-term outcomes in females. Pre-operative diagnosis of hepatocellular carcinoma, age at time of listing, duration of surgery, and units transfused during surgery, were associated with long-term outcomes in males. Severity of complications was associated with long-term outcomes in both groups. Overall survival was similar in both males and females; however, when stratified by age, females < 50 years of age had the best survival.
Female and male LT recipients have different baseline and transplant-related characteristics, with sex-specific variables which are associated with long-term outcomes. Female recipients < 50 years of age demonstrated the best long-term outcomes. Pre- and post-transplant practices should be individualized based on sex-specific variables to optimize long-term outcomes.
Core Tip: Within this retrospective review, we evaluated baseline and transplant-related features of both male and female liver transplant recipients. Our results identify several sex-specific variables that affect long-term outcomes of liver transplantation, including statistically significant survival outcomes seen in females under the age of 50.
- Citation: Andacoglu OM, Dennahy IS, Mountz NC, Wilschrey L, Oezcelik A. Impact of sex on the outcomes of deceased donor liver transplantation. World J Transplant 2024; 14(1): 88133
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/88133.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.88133
Since the advent of liver transplantation in 1967, significant efforts have been made by the transplant community to refine not only the technical aspects of the procedure and medical management of patients, but also the equity of graft allocation. The current system prioritizes patients based on severity of disease, with a major landmark in its evolution being the adoption of the Model for End-stage Liver Disease (MELD; 2002) as a way to predict individual pre-transplant mortality. This was quickly recognized as a potential way to stratify patients according to medical urgency of liver transplant (LT) and has been noted to have a significant impact on waitlist mortality and number of transplants per
As of 2020, 60.9% of patients on the liver transplant waitlist were male, as were 63.2% of recipients[4]. Females are known to be disadvantaged due to certain MELD components, namely creatinine and sodium[2,5,6]. Females also experience longer waitlist times and higher pre-transplant mortality as well as impaired access to transplant[2,3,5,7,8]. Renal transplant data has shown that female patients are less likely to be referred for transplant and that there may be biases in their evaluation for fitness to undergo surgery, which may contribute to this[9-11]. Females are generally considered to be disadvantaged in all aspects of the process, including referrals for evaluation qualification for transplant and receipt of a matched organ[12]. MELD 3.0, which is pending adoption by UNOS, aims to reduce this discrepancy and has been shown to afford females a significantly higher chance of transplant[13].
Female liver transplant recipients demonstrate comparable, if not better, outcomes than males across a number of etiologies; however, as their access to liver transplant is limited, female patients are getting progressively sicker while waiting and risk being removed from the transplant list while their male counterparts undergo successful transplant[14-18]. It is clear that more research into sex-related differences in transplant patients is needed to identify areas for improvement. Elucidation of these differences may help to identify specific areas of focus to improve on the organ matching process, as well as the peri- and post-operative care of these patients.
The aim of this study was to utilize data from a high-volume Eurotransplant center to compare characteristics of male and female patients undergoing liver transplant and assess association between sex-specific variables with short- and long-term post-transplant outcomes.
We performed a retrospective review of the University of Essen’s transplant database, which included pre-collected and deidentified data. All adult liver transplant recipients between January 2010 and December 2020 were included. We reviewed patient baseline characteristics including sex, age, body mass index (BMI), and underlying etiology of liver disease. These were categorized as acute liver failure (ALF), alcohol-related liver disease (ALD), hepatitis B- or hepatitis C-related liver disease, non-alcoholic steatohepatitis (NASH) and primary sclerosing cholangitis (PSC). We also collected data on additional risk factors including: Smoking history, medical comorbidities such as chronic obstructive pulmonary disease, diabetes mellitus, peripheral vascular disease, coronary artery disease, diagnosis of hepatocellular carcinoma (HCC) and MELD score[19]. Waitlist times were reported in the form of days from listing until transplant. Transplant-related characteristics including donor age, high urgency transplant status, donor risk index (DRI), operative time cold and warm ischemic times (WIT), intraoperative transfusion requirements and perioperative death were reviewed[20]. Short-term postoperative outcomes were assessed in terms of both intensive care unit (ICU) stay and overall length of hospital stay in days. The comprehensive complication index (CCI) was used to assess and record the severity of post-operative complications[21]. Finally, overall survival was also recorded to a limit of 140 mo post-operatively.
The normality of all data was tested using the Shapiro-Wilk test. Non-normally distributed data were compared using the Mann-Whitney U test. χ2 and Bonferroni tests were applied to draw comparisons between categorical data points. Relationships between numerical variables were assessed using Spearman’s rank correlation. Mean and median survival times and overall survival rates were estimated using the Kaplan Meier method. The Log-rank test was then applied to compare overall survival rates between groups. For determination of risk factor-association with overall survival, multivariate cox proportional hazard regression analysis was performed, and hazard ratios (HRs) and 95% confidence intervals (CIs) were assigned to each independent variable. For determination of risk factors for perioperative death, a multivariate binary logistic regression model was built, and odds ratios (ORs) and 95%CIs were generated for each independent variable. For length of hospital stay, ICU stay, waitlist time, CCI, and MELD score at time of transplantation, generalized linear models were applied, and Beta coefficients and 95%CIs were derived for each independent variable. Multi-collinearity was confirmed by calculating variance inflation factor (VIF) scores. Collinear variables (VIF scores > 2) were not included in multivariate analysis to avoid problems with multi-collinearity. Des
This study was deemed exempt by the Institutional Review Board of Essen University. All research referenced in this manuscript was conducted in accordance with institutional processes as well as both the Declarations of Helsinki and Istanbul.
Data from 779 LT recipients was collected. 518 (66.5%) patients were male, and 261 (33.5%) were female. Female patients were on average younger at the time of transplant (median 52 vs 54 years, P = 0.04) and had lower BMI (median 24.38 vs 26.3, P = 0.001) compared to males. Lab- and match-MELD scores were similar between females and males. Female patients overall had fewer comorbidities at baseline compared to male LT recipients (Table 1). Female recipients had higher incidences of acute liver failure and lower incidences of alcohol-related or viremic liver disease (P = 0.001). Female patients were more likely to have received an organ from a female donor, with a higher donor risk index score (1.71 vs 1.84), and as a high urgency offer (all P < 0.05). Median wait time was similar between 2 groups (Tables 1 and 2).
| Male | Female | P value | |
| 518 (66.5) | 261 (33.5) | ||
| Donor sex | 0.001 | ||
| Male | 326 (62.9) | 59 (22.6) | |
| Female | 192 (37.1) | 202 (77.4) | |
| Etiology | 0.001 | ||
| Acute liver failure | 18 (3.5) | 34 (13.0) | |
| Alcohol | 149 (28.8) | 46 (17.6) | |
| HBV/HCV | 160 (30.9) | 53 (20.3) | |
| HCC | 152 (29.3) | 45 (17.2) | 0.001 |
| NASH | 58 (11.2) | 20 (7.7) | |
| PSC | 53 (10.2) | 12 (4.6) | |
| Others1 | 80 (15.4) | 96 (36.8) | |
| Milan criteria | |||
| HCC within Milan | 132 (86.8) | 39 (86.7) | 0.9756 |
| HCC beyond Milan | 20 (13.2) | 6 (13.3) | |
| Comorbidities | 364 (70.3) | 162 (62.1) | 0.021 |
| Coronary artery disease | 83 (16.0) | 25 (9.6) | 0.014 |
| Diabetes | 140 (27.0) | 41 (15.7) | 0.001 |
| Peripheral vascular disease | 5 (1.0) | 2 (0.8) | 0.781 |
| COPD | 75 (14.5) | 33 (12.6) | 0.484 |
| Smoker | 131 (25.3) | 49 (18.8) | 0.042 |
| High urgency transplant | 25 (4.8) | 41 (15.7) | 0.001 |
| Intraoperative blood transfusion | 217 (41.9) | 115 (44.1) | 0.563 |
| Perioperative death | 89 (17.2) | 39 (14.9) | 0.426 |
| Male | Female | P value | |
| Median [25%-75%] | Median [25%-75%] | ||
| Age at time of listing | 54 [47-59] | 51 [43-59] | 0.019 |
| BMI (kg/m²) | 26.3 [23.46-29.41] | 24.38 [21.72-28.7] | 0.001 |
| Comorbidity Index | 33.5 [0-63.8] | 33.5 [0-58.1] | 0.84 |
| Lab MELD | 15 [11-21] | 16 [12-24] | 0.052 |
| Wait list time (d) | 78 [23-206] | 61 [7-220] | 0.094 |
| Match MELD | 25 [22-28] | 25 [22-28] | 0.598 |
| Age at time of transplant | 54 [48-60] | 52 [44-59] | 0.039 |
| Donor age | 58 [49-70] | 61 [46-73] | 0.781 |
| DRI | 1.706 [1.432-1.962] | 1.837 [1.528-2.078] | 0.001 |
| CIT (min) | 450 [370-530] | 445 [382-521] | 0.741 |
| WIT (min) | 30 [26-36] | 28 [25-32] | 0.001 |
| Duration of surgery (min) | 249 [209-302] | 229 [190-286] | 0.001 |
| ICU stay (d) | 5 [3-10] | 5 [3-9] | 0.571 |
| Hospital stay (d) | 19 [15-26] | 19 [15-29] | 0.317 |
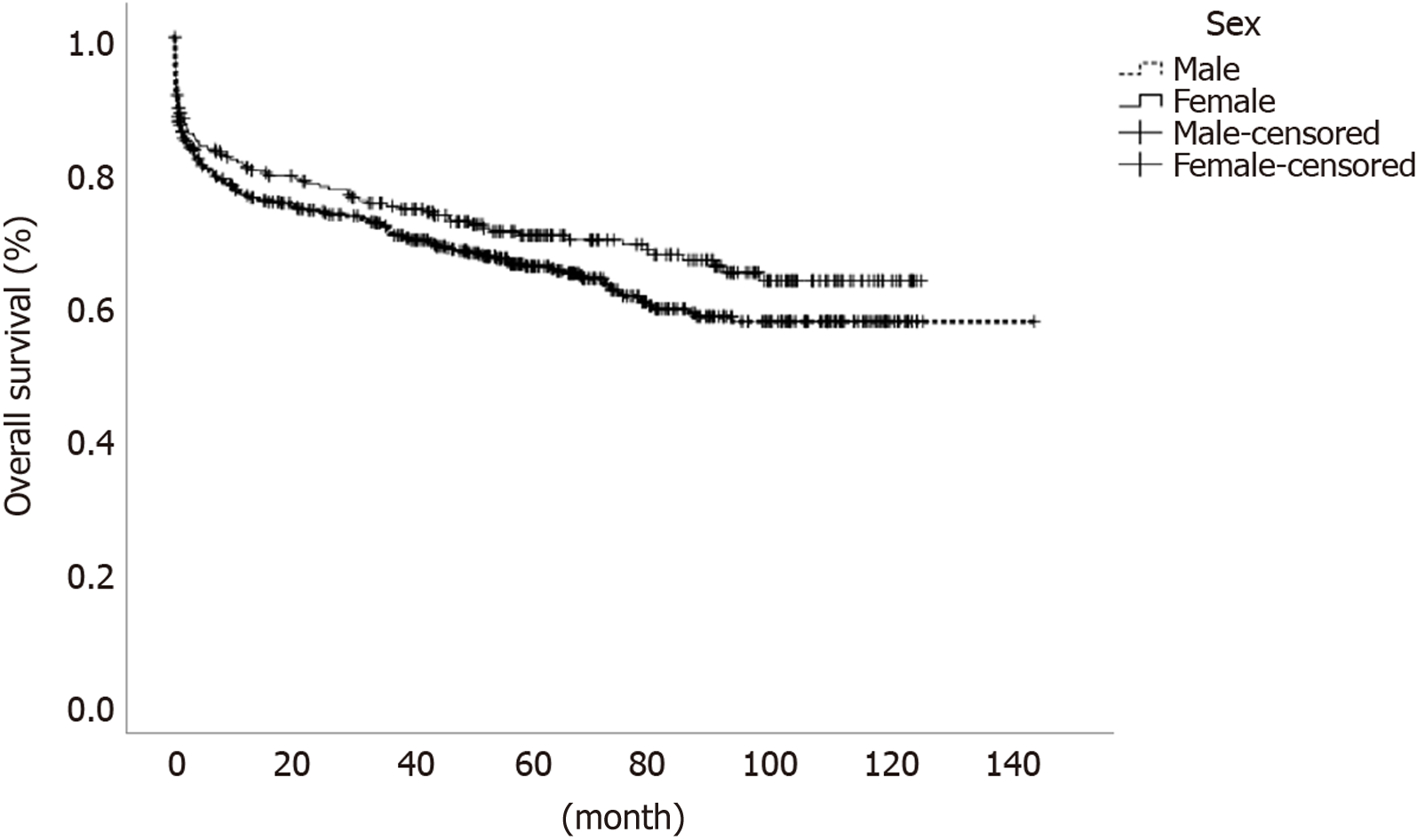
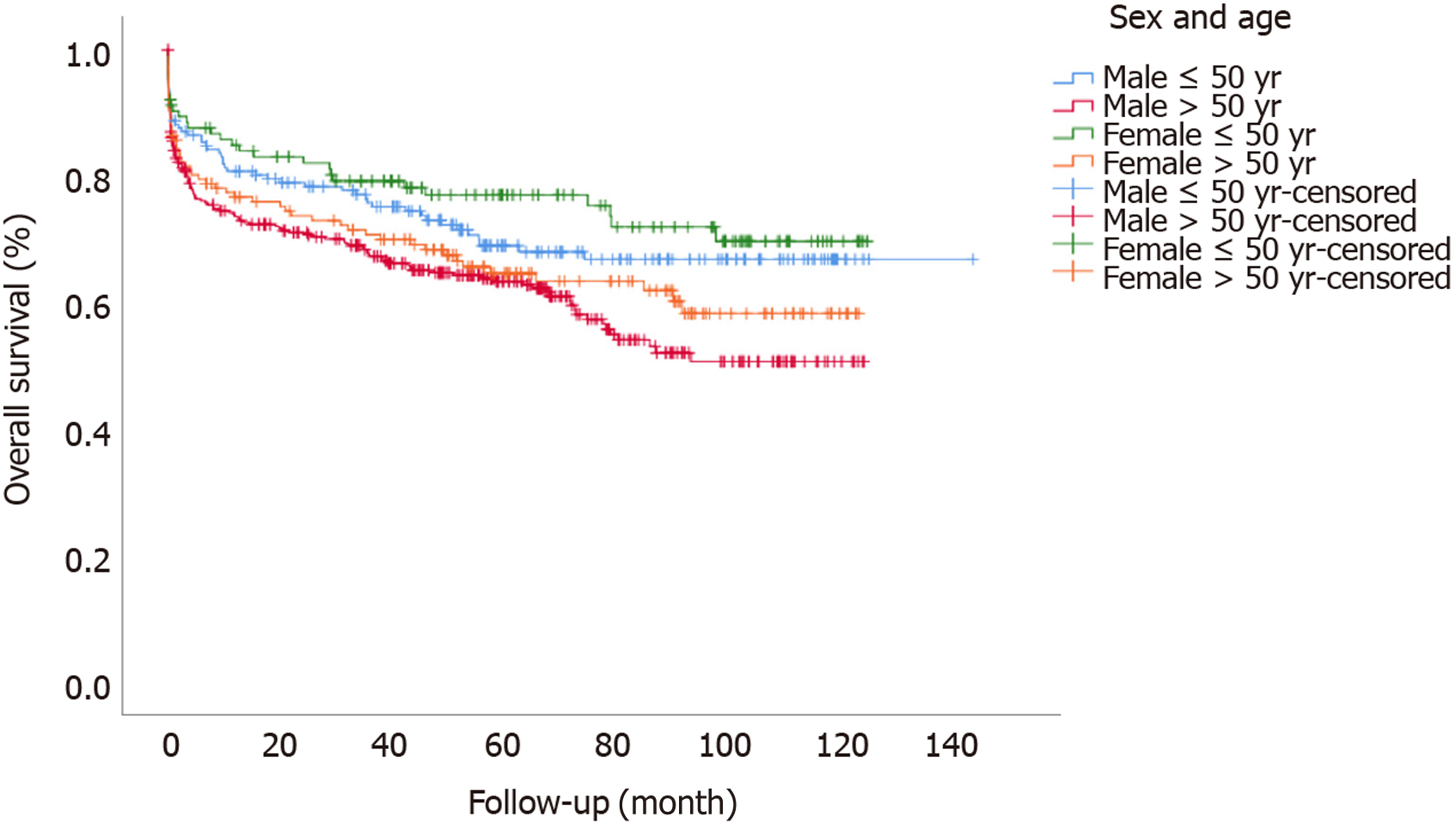
Regarding intra- and post-operative data, females had shorter WIT and shorter duration surgery; however, length of ICU or total stay, complication indexes and perioperative death rates were similar between males and females (Tables 1 and 2). On multivariate hazard regression analysis, higher lab-MELD score of the recipient and donor cause of death were associated with differences in long-term outcomes for female patients. A pre-operative diagnosis of HCC, increased age at time of listing, high urgency status of transplant, longer duration of surgery, and a higher number of units transfused during surgery were all associated with differences in long-term outcomes for males. Complication index grade was associated with differences in long-term outcomes for both groups (Table 3). One-, 3- and 5-year patient survival rates were similar between females and males [80.2%, 74.4% and 70% for females and 76.1%, 70.5% and 65.3% for males, (P = 0.12)] (Figure 1). When we performed sub-group analyses according to sex and age-related categorization, female patients younger than 50 had the best overall survival (P = 0.003) (Figure 2).
| Male | Female | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| HCC | 1.6 (1.11-2.3) | 0.011 | 1.71 (0.81-3.64) | 0.161 |
| Age at time of listing | 1.02 (1-1.04) | 0.014 | 1.02 (0.99-1.05) | 0.116 |
| Comorbidity Index | 1.04 (1.03-1.04) | 0.001 | 1.04 (1.03-1.05) | 0.001 |
| Lab MELD | 1.01 (0.99-1.04) | 0.302 | 1.05 (1.02-1.08) | 0.004 |
| High urgency | 2.87 (1.48-5.57) | 0.002 | 0.84 (0.39-1.8) | 0.656 |
| Trauma (cause of death, donor) | 1.32 (0.71-2.45) | 0.384 | 3.05 (1.05-8.85) | 0.04 |
| Duration of surgery (min) | 1 (1-1) | 0.013 | 1 (1-1.01) | 0.2 |
| Units transfused | 1.04 (1-1.09) | 0.043 | 1.04 (0.95-1.13) | 0.439 |
Overall, characteristics of our study population were similar to known demographics of transplant patients in Germany. Proportions of male and female transplant recipients (66.5% vs 33.5%) were consistent with what is generally seen throughout the region[22]. Differences in the etiology of chronic liver disease according to sex in our study were also consistent with known predominance of ALD, viral hepatitis, NASH and PSC in males[23,24]. In our study, we report that significantly larger number of transplants for ALF were performed in female patients, which is in accordance with existing literature[25,26]. Females are known to be more susceptible to certain causes of ALF than males, including acetaminophen overdose and other drug toxicities, as well as acute-on-chronic liver failure associated with alcohol use[27-29]. It is possible that our findings represent selection bias associated with use of single-institution data; however, we suspect that our findings may demonstrate increasing incidences of alcohol-related liver disease, particularly in females[30]. This is a trend which has especially been seen in relation to the recent pandemic[31]. Unfortunately, we did not have data for the underlying etiology of ALF in our cohort. As a result, further information would be required to make solid conclusions.
The baseline characteristics of our study cohort are similar to those previously reported[15]. In our study, females were on average younger than males both at time of listing and at time of transplant. They also had significantly lower BMIs. Interestingly, although previous studies have suggested that higher BMIs in male recipients may contribute to worse survival in these patients, the impact of BMI on overall survival was not found to be significant in our multivariate analysis[32]. Male patients had significantly higher overall rates of comorbidity consistent with previous data[33]. However, we found that the comorbidity index was associated with long-term survival in both male and female transplant patients, as expected. Consideration of pre-transplant comorbidities during listing and allocation is crucial, and pre-operative risk should be managed, where possible, to maximize chances of the best possible outcome. Currently there is no specific risk calculator for transplant surgery, and the only surgical risk calculator which considers sex is the ACS Surgical risk calculator[34].
Despite recent concerns that MELD may significantly disadvantage females in terms of waitlist times and pre-transplant mortality, in our study, we saw that waiting times were similar between males and females, with a trend towards shorter waitlist times for females[2,3]. Though the female patients in our cohort did not experience longer waitlist times, the fact that this pattern has been demonstrated in a number of other recent studies is concerning. MELD is thought to underestimate the severity of liver disease and its complications in females, in part due to sex-related differences in muscle mass (female patients typically demonstrate a lower glomerular filtration rate per given creatinine level)[3,5]. In our report, female patients had higher lab-MELD scores, although this was not statistically significant. We believe this finding is still important to mention because it requires a more severe disease process to reach the same or higher MELD scores in female patients as male patients. This may be reflected in the increased number of females receiving high-urgency transplants as compared to males in our study, which is almost double that of similar database studies[35]. High-urgency transplants are considered to have comparable outcomes to those performed in patients who have demonstrated more stable disease[36]. However, this does not eliminate the fact that female patients are placed at higher risk of pre-transplant mortality by the current system[2]. Furthermore, in this study, lab-MELD was found to differentially impact the overall survival of female patients after liver transplant. Taken together, sex-specific adjustments to scoring as well as allocation systems are necessary.
Male patients were also statistically more likely to have HCC, which correlates with larger database studies[5]. This translates into sex bias in transplant prioritization, as exception points are awarded to patients with HCC after 6 mo on the waitlist. In our study, transplant in the setting of known HCC in male patients was found to be associated with poorer outcomes, but not for female patients. This may partially reflect the fact that male patients with HCC demonstrate poorer long-term survival independent of transplantation[37]. However, it has also been shown through a retrospective analysis of the UNOS database that females have a 25% lower recurrence rate after transplant. Lastly, some literature suggested donor/recipient sex match may also play a role in HCC recurrence after LT[38,39]. Unfortunately we had limited data on tumor-specific variables in our study; for instance, we did not have tumor grade, AFP levels, or downstage data. However, we report similar numbers of within and beyond Milan criteria HCC in both groups. We were also not able to analyze interactions between donor sex and HCC-specific outcomes due to the small sample size. Regardless, we believe our findings merit attention that sex-specific factors may impact LT outcomes, specifically for HCC. Further analysis is necessary regarding the impact of sex on LT after HCC.
Whether there are sex differences in post-transplant survival remains controversial based on underlying disease and/or age classification or MELD scores[35,40,41]. In this study, we report similar overall survival rates between male and female patients; however, we found significantly better survival for females younger than 50 years of age as compared to all other groups[42]. Given the retrospective nature of our study, limited sample size, and existing donor differences in both groups, we agree that prospective randomized studies with more granular data would be necessary to determine the impact of sex on LT outcomes.
It is well known that females experience more problems with donor-recipient matching than males. Part of this issue is due to concerns for large-for-size transplants in smaller female patients. Aside from just technical difficulty associated with transplanting a size-mismatched organ, it is thought that large discrepancies in this area can lead to increased risk of graft failure[43]. Donor mismatch is often cited as one of the top causes of offer denial[44]. Our study showed sig
Limitations of our study are largely related to the fact that this is a single-institution, retrospective study, and therefore assessment of baseline characteristics of patients is not generalizable to the wider population. However, variables identified in multivariate analysis, which are associated with worse outcomes according to sex, remain translatable to other population groups. We also have limited baseline data on our HCC patients, making it difficult to ascertain the exact impact of cancer on outcomes. Our study included a small transplant population and was underpowered to detect smaller differences that may still be clinically significant. Lastly, due to a lack of anatomical data, we are not able to make conclusions based on WIT or duration of surgery.
Overall, female and male transplant candidates demonstrate different characteristics, which have a complex interplay to influence access to liver transplant as well as transplant outcomes. Despite global improvements in the allocation and technique of liver transplantation over recent years, female patients are still significantly disadvantaged in terms of access to transplant, underscored disease severity, longer wait times, more difficulty to have proper or timely organ offers and longer hospital stay in the post-operative period as described in the literature. Herein, we demonstrate sex-based differences in disease etiology, comorbidity profile and donor characteristics. In addition, we demonstrated specific factors with differential impact on the survival of each sex after liver transplant. These should be considered as tools to improve the system, and adjustments to the allocation process could reduce the disparities between males and females. Lastly, perioperative care of females with chronic liver disease may differ from males. Thus, management and follow up of liver transplant patients should be individualized, with consideration of sex-specific variables. This may further optimize long-term outcomes, and further prospective studies are warranted.
Female liver transplant recipients generally demonstrate comparable, if not better, outcomes than males across a number of etiologies. However, due to lack of access, female patients are getting progressively sicker while waiting and risk being removed from the transplant list while their male counterparts undergo successful transplant (14-18). Further research into sex-based differences in transplant patients is paramount in identifying areas of improvement. Defining these differences may lead to focused improvement on the organ-matching process and more specific management of peri- and post-operative care of male and female recipients.
Female and male transplant candidates demonstrate different characteristics, which have a complex interplay to influence access to liver transplant as well as transplant outcomes. Herein, we demonstrate sex-based differences in disease etiology, comorbidity profile and donor characteristics. In addition, we demonstrated specific factors with differential impact on the survival of each sex after liver transplant. These should be considered as tools to improve the system, and adjustments to the allocation process could reduce the disparities between males and females.
The aim of this study was to utilize data from a high-volume Eurotransplant center to compare characteristics of male and female patients undergoing liver transplant and assess association between sex-specific variables with short- and long-term post-transplant outcomes.
A retrospective review of the University of Essen’s transplant database was performed with collection of baseline patient characteristics, transplant-related data, and short-term outcomes. Comparisons of these data were made with Shapiro-Wilk, Mann-Whitney U, χ2 and Bonferroni tests applied where appropriate. A P value of < 0.05 was accepted as statistically significant.
There were significant differences in baseline characteristics between male and female recipients. Female patients suffered more from acute liver failure and less from alcohol-related or viremic liver disease (P = 0.001). Female patients were more likely to receive an organ from a female donor, with a higher donor risk index score, and as a high urgency offer (all P < 0.05). On multivariate hazard regression analysis, patient lab-MELD score and donor cause of death were associated with differences in long-term outcomes for females. A pre-operative diagnosis of hepatocellular carcinoma, increased age at time of listing, high urgency status of transplant, duration of surgery, and higher number of units transfused during surgery were all associated with differences in long-term outcomes for males.
Through this retrospective review, we have demonstrated sex-based differences in disease etiology, comorbidity profile and donor characteristics as well as specific factors with differential impact on the survival of each sex after liver transplant. These should be considered as tools to improve the system, and adjustments to the allocation process could reduce the disparities between males and females. Lastly, perioperative care of females with chronic liver disease may differ from males. Thus, management and follow up of liver transplant patients should be individualized, with consideration of sex-specific variables.
Further research should aim to focus to optimize long-term outcomes between male and female liver transplant reci
Assistance with the study: Authors acknowledge statistical analysis by Seval Kul, PhD. Presentation: This study was presented at the International Liver Transplantation Society (ILTS) Meeting, 2023, and at the American Transplant Congress (ATC) Meeting, 2023.
| 1. | Bernardi M, Gitto S, Biselli M. The MELD score in patients awaiting liver transplant: strengths and weaknesses. J Hepatol. 2011;54:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Myers RP, Shaheen AA, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol. 2011;54:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Kasiske BL, Lentine KL, Ahn Y, Skeans MA, Eberhard T, Folken C, Wainright J, Larkin L, Nystedt C. OPTN/SRTR 2020 Annual Data Report: Living Donor Collective. Am J Transplant. 2022;22 Suppl 2:553-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation. 2018;102:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 6. | Sawinski D, Lai JC, Pinney S, Gray AL, Jackson AM, Stewart D, Levine DJ, Locke JE, Pomposelli JJ, Hartwig MG, Hall SA, Dadhania DM, Cogswell R, Perez RV, Schold JD, Turgeon NA, Kobashigawa J, Kukreja J, Magee JC, Friedewald J, Gill JS, Loor G, Heimbach JK, Verna EC, Walsh MN, Terrault N, Testa G, Diamond JM, Reese PP, Brown K, Orloff S, Farr MA, Olthoff KM, Siegler M, Ascher N, Feng S, Kaplan B, Pomfret E. Addressing sex-based disparities in solid organ transplantation in the United States - a conference report. Am J Transplant. 2023;23:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 7. | Bryce CL, Angus DC, Arnold RM, Chang CC, Farrell MH, Manzarbeitia C, Marino IR, Roberts MS. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9:2092-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Oloruntoba OO, Moylan CA. Gender-based disparities in access to and outcomes of liver transplantation. World J Hepatol. 2015;7:460-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Thamer M, Hwang W, Fink NE, Sadler JH, Bass EB, Levey AS, Brookmeyer R, Powe NR; CHOICE Study. Choices for Healthy Outcomes in Caring for ESRD. U.S. nephrologists' attitudes towards renal transplantation: results from a national survey. Transplantation. 2001;71:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Ojo A, Port FK. Influence of race and gender on related donor renal transplantation rates. Am J Kidney Dis. 1993;22:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Kefalakes H. Women Are Disadvantaged During Deceased Donor Liver Transplant Allocation: Strategies to Overcome Inequities. Gastroenterology. 2022;162:2110-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, Wood NL, Gentry SE, Kwong AJ. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021;161:1887-1895.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 14. | Chauhan M, Zhang T, Thuluvath PJ. Gender Differences in Liver Transplantation Outcomes in Polycystic Liver Disease. Dig Dis Sci. 2022;67:3445-3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Matsuoka L, Izzy M, Feurer ID, Rega SA, Ziogas IA, Alexopoulos SP. Sex and Gender Disparities in Pretransplant Characteristics and Relationships with Postoperative Outcomes in Liver Transplant Recipients with Alcoholic Liver Disease. Exp Clin Transplant. 2020;18:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Berenguer M, Di Maira T, Baumann U, Mirza DF, Heneghan MA, Klempnauer JL, Bennet W, Ericzon BG, Line PD, Lodge PA, Zieniewicz K, Watson CJE, Metselaar HJ, Adam R, Karam V, Aguilera V; all the other contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Characteristics, Trends, and Outcomes of Liver Transplantation for Primary Sclerosing Cholangitis in Female Versus Male Patients: An Analysis From the European Liver Transplant Registry. Transplantation. 2021;105:2255-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Rubin JB, Cullaro G, Ge J, Lai JC. Women who undergo liver transplant have longer length of stay post-transplant compared with men. Liver Int. 2020;40:1725-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1894] [Article Influence: 82.3] [Reference Citation Analysis (1)] |
| 20. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1522] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 21. | Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1429] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 22. | Loosen SH, Bock HH, Hellmich M, Knoefel WT, Trautwein C, Keitel V, Bode JG, Neumann UP, Luedde T. Hospital Mortality and Current Trends in Liver Transplantation in Germany—a Systematic Analysis of Standardized Hospital Discharge Data, 2008–2017. Dtsch Arztebl Int. 2021;118:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Rodríguez-Castro KI, De Martin E, Gambato M, Lazzaro S, Villa E, Burra P. Female gender in the setting of liver transplantation. World J Transplant. 2014;4:229-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Invernizzi F, Cilla M, Trapani S, Guarino M, Cossiga V, Gambato M, Morelli MC, Morisco F, Burra P, Floreani A; Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF). Gender and Autoimmune Liver Diseases: Relevant Aspects in Clinical Practice. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Weiler N, Schlotmann A, Schnitzbauer AA, Zeuzem S, Welker MW. The Epidemiology of Acute Liver Failure. Dtsch Arztebl Int. 2020;117:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. |
Karvellas CJ, Leventhal TM, Rakela JL, et al Outcomes of patients with acute liver failure listed for liver transplantation: A multicenter prospective cohort analysis.
|
| 27. | Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 537] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 28. | Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol (N Y). 2013;9:633-639. [PubMed] |
| 29. | Singal AK, Arora S, Wong RJ, Satapathy SK, Shah VH, Kuo YF, Kamath PS. Increasing Burden of Acute-On-Chronic Liver Failure Among Alcohol-Associated Liver Disease in the Young Population in the United States. Am J Gastroenterol. 2020;115:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 604] [Article Influence: 75.5] [Reference Citation Analysis (1)] |
| 31. | Deutsch-Link S, Curtis B, Singal AK. Covid-19 and alcohol associated liver disease. Dig Liver Dis. 2022;54:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Thuluvath PJ, Wagennar RR, Verma S. Gender and ethnic differences in the post-liver transplant outcomes of patients with autoimmune hepatitis with acute liver failure at initial presentation: a case-control study. Arch Med Sci. 2015;11:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Li Q, Wang Y, Ma T, Liu X, Wang B, Wu Z, Lv Y, Wu R. Impact of cigarette smoking on early complications after liver transplantation: A single-center experience and a meta-analysis. PLoS One. 2017;12:e0178570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-42.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1445] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 35. | Mathur AK, Schaubel DE, Zhang H, Guidinger MK, Merion RM. Disparities in liver transplantation: the association between donor quality and recipient race/ethnicity and sex. Transplantation. 2014;97:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Finkenstedt A, Nachbaur K, Zoller H, Joannidis M, Pratschke J, Graziadei IW, Vogel W. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 37. | Dohmen K, Shigematsu H, Irie K, Ishibashi H. Longer survival in female than male with hepatocellular carcinoma. J Gastroenterol Hepatol. 2003;18:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Nakamura T, Sasaki K, Kojima L, Teo R, Inaba Y, Yamamoto T, Kimura S, Dageforde LA, Yeh H, Elias N, Bozorgzadeh A, Kawai T, Markmann JF. Impact of donor sex on hepatocellular carcinoma recurrence in liver transplantation after brain death. Clin Transplant. 2023;37:e14989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Han S, Yang JD, Sinn DH, Kim JM, Choi GS, Jung G, Ahn JH, Kim S, Ko JS, Gwak MS, Kwon CHD, Leise MD, Gwak GY, Heimbach JK, Kim GS. Risk of Post-transplant Hepatocellular Carcinoma Recurrence Is Higher in Recipients of Livers From Male Than Female Living Donors. Ann Surg. 2018;268:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62:946-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Gabbay U, Issachar A, Cohen-Naftaly M, Brown M, Nesher E. Gender specific survival rates after deceased donor liver transplantation: A retrospective cohort. Ann Med Surg (Lond). 2022;79:103933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Bruns H, Lozanovski VJ, Schultze D, Hillebrand N, Hinz U, Büchler MW, Schemmer P. Prediction of postoperative mortality in liver transplantation in the era of MELD-based liver allocation: a multivariate analysis. PLoS One. 2014;9:e98782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Reyes J, Perkins J, Kling C, Montenovo M. Size mismatch in deceased donor liver transplantation and its impact on graft survival. Clin Transplant. 2019;33:e13662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Nephew LD, Goldberg DS, Lewis JD, Abt P, Bryan M, Forde KA. Exception Points and Body Size Contribute to Gender Disparity in Liver Transplantation. Clin Gastroenterol Hepatol. 2017;15:1286-1293.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl. 2021;27:900-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 46. | Addeo P, Noblet V, Naegel B, Bachellier P. Large-for-Size Orthotopic Liver Transplantation: a Systematic Review of Definitions, Outcomes, and Solutions. J Gastrointest Surg. 2020;24:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, Porte RJ, Rogiers X, Ringers J; European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC). The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 48. | Blok JJ, Braat AE, Adam R, Burroughs AK, Putter H, Kooreman NG, Rahmel AO, Porte RJ, Rogiers X, Ringers J; European Liver Intestine Transplant Association Eurotransplant Liver Intestine Advisory Committee; Eurotransplant Liver Intestine Advisory Committee. Validation of the donor risk index in orthotopic liver transplantation within the Eurotransplant region. Liver Transpl. 2012;18:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Liu JH