Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.87532
Peer-review started: August 14, 2023
First decision: September 14, 2023
Revised: October 21, 2023
Accepted: January 8, 2024
Article in press: January 8, 2024
Published online: March 18, 2024
Processing time: 213 Days and 9.3 Hours
Conditioning regimens employed in autologous stem cell transplantation have been proven useful in various hematological disorders and underlying malig
Core Tip: This literature review study is based on real-world data collected from various published research introducing multiple conditioning regimens for different disorders. Comparisons between regimens of an individual disorder were made using variables such as overall survival, progression free survival, complete remission, and leukemia free survival to conclude a laudable conditioning regimen having trivial adverse effects. The article is designed to discuss the conditioning regimens employed in autologous stem cell transplantation for various diseases. The primary objective of conducting this review is to highlight the various conditioning regimens, and discuss both the positive and the negative consequences along with proposing a treatment that is both efficacious and harmless.
- Citation: Maqbool S, Baloch MF, Khan MAK, Khalid A, Naimat K. Autologous hematopoietic stem cell transplantation conditioning regimens and chimeric antigen receptor T cell therapy in various diseases. World J Transplant 2024; 14(1): 87532
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/87532.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.87532
Over the years, many treatment regimens have been crafted for multifarious diseases, and consequently, endorsement of hematopoietic stem cell (HSC) transplantation (HSCT) was a strategic approach for hematological disorders or under
According to recent research, peripheral blood is 99% of the time used as a donor in autologous stem cell transplants[3]. In contrast, blood cells used in allogeneic stem cell transplantation (Allo-SCT) are taken from potential donors or cord blood units[4]. Today, more than 50000 HSCT procedures are performed annually worldwide. In Europe, are more than one-half of autologous transplants that are performed are autologous[5].
Conditioning regimens are devised in order to eradicate tumor cells and prevent graft rejection. In the 1970s, successful bone marrow transplantation (BMT) using cyclophosphamide (Cy) and total body irradiation (TBI) was reported[6]. Carmustine, etoposide, cytarabine, and melphalan (BEAM) is the most used conditioning regimen for Hodgkin's lym
This article is designed to discuss the conditioning regimens employed in autologous stem cell transplantation (Auto-SCT) for various diseases. The primary objective of conducting this review is to highlight the various conditioning regimens, and discuss both the positive and the negative consequences along with proposing a treatment that is both efficacious and harmless.
The discovery of induced pluripotent stem cells by the reprogramming of human and mouse fibroblasts in 2006 with traits like embryonic stem cells (ESCs) proved to be a landmark in the field of medicine[12]. This discovery ultimately paved the way for modern and significant contributions to drug discovery, cell therapy, basic research, and the widespread use of autologous cell-based therapy[13]. Since the isolation of human ESCs, valuable approaches have been made generally focused on directed differentiation to generate pluripotent hematopoietic stem and progenitor cells to be manipulated in cellular therapy and to treat malignancies[14-16].
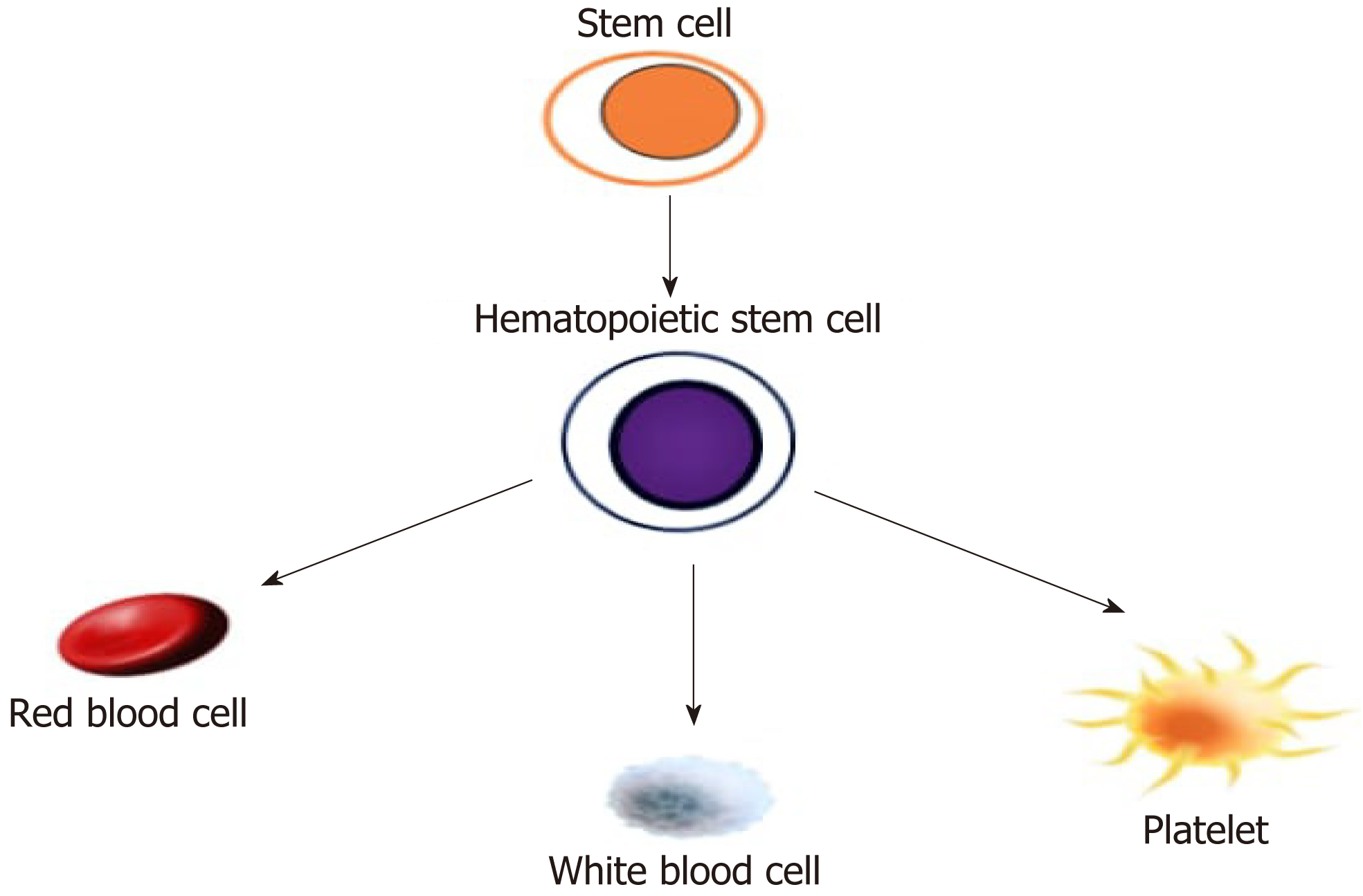
Since the very beginning, the stem cell concept has been crafted into a hierarchical tree-like model where the stem cells are sitting on the root of a branching family tree and the multipotent stem cells originate in an orderly branching fashion from their ancestral root[17]. To summarize, HSCs are immature ESCs that harbor the potential to differentiate into their lineage of cells including red blood cells, white blood cells, and platelets as shown in Figure 1[18].
HSCT is the most widely used cellular immunotherapy, and is an indispensable treatment for many malignant, congenital, and acquired hematological ailments[19]. HSCT is a requisite after chemotherapy or radiotherapy to con
In autologous hematopoietic stem cell transplantation (ASCT), the stem cells are harvested from the recipient's own bone marrow, peripheral blood, or umbilical cord units. This mode of transplantation is effective since it reduces the occ
Allogeneic transplantation uses fresh HSCs, so the collection from the donor as well as the conditioning of the patient occurs at the same time and reduces the risk of cell reduction via thawing or freezing[21]. Patients who undergo Allo-SCT require a longer period of immunosuppression in order to avert the likelihood of transplant rejection.
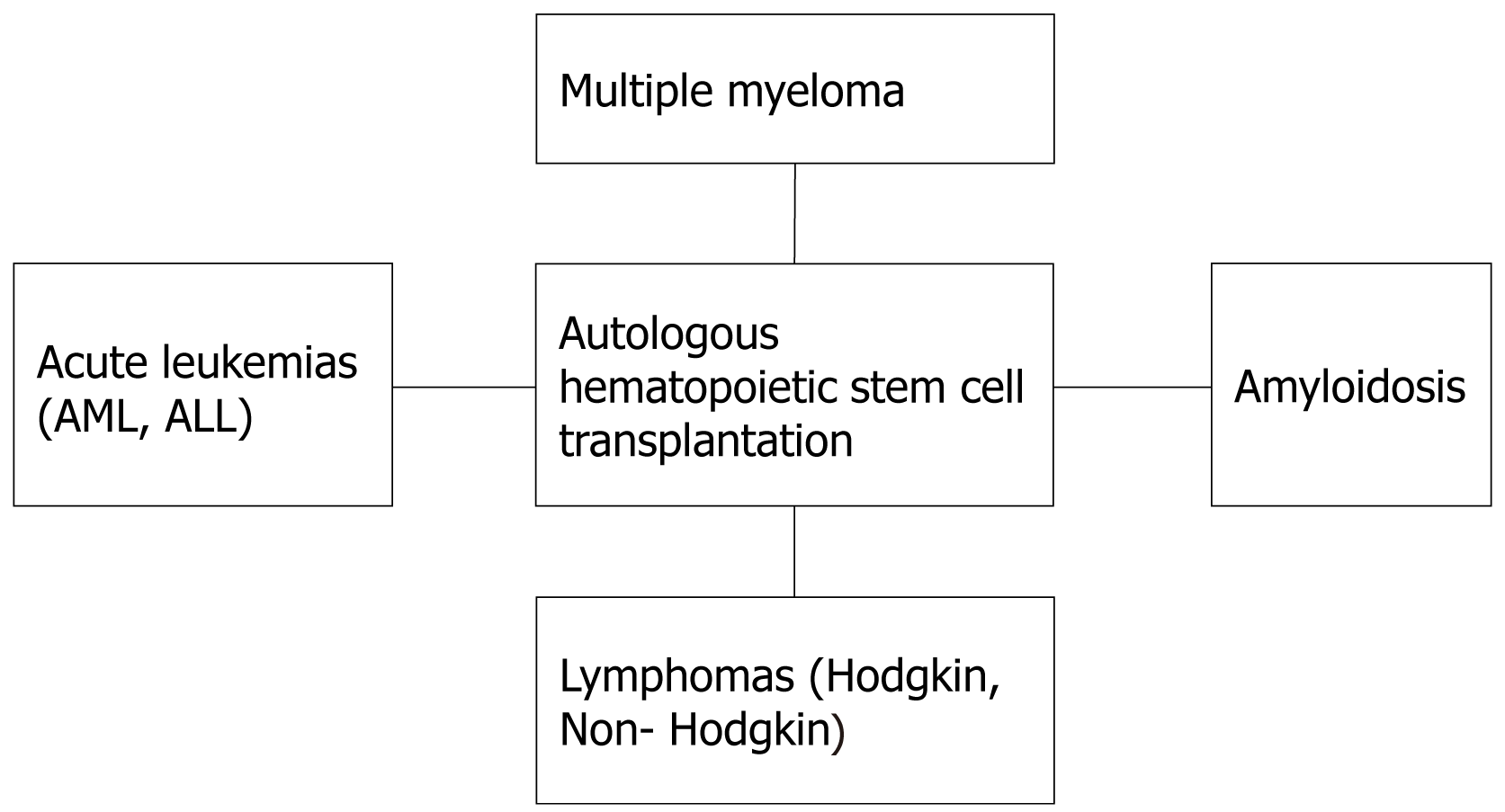
Owing to the great advancements in the field of medicine, Auto-SCT has now been regarded as an established therapeutic approach for many haemato-oncological, immunological, and hereditary conditions with the potential of cure. In 2012, the number of Auto-SCTs performed reached over one million[4]. There are following diseases for which the ASCT is being performed more frequently (Figure 2).
MM is an incurable, malignant B-cell neoplasm characterized by uncontrolled, destructive growth of mutated plasma cells along with the dissemination of multiple tumor cells throughout the bone marrow[22]. With the progress in the field of medical oncology, various drugs of paramount significance have been developed for the treatment of MM (e.g., proteasome inhibitors and immunomodulatory drugs)[23].
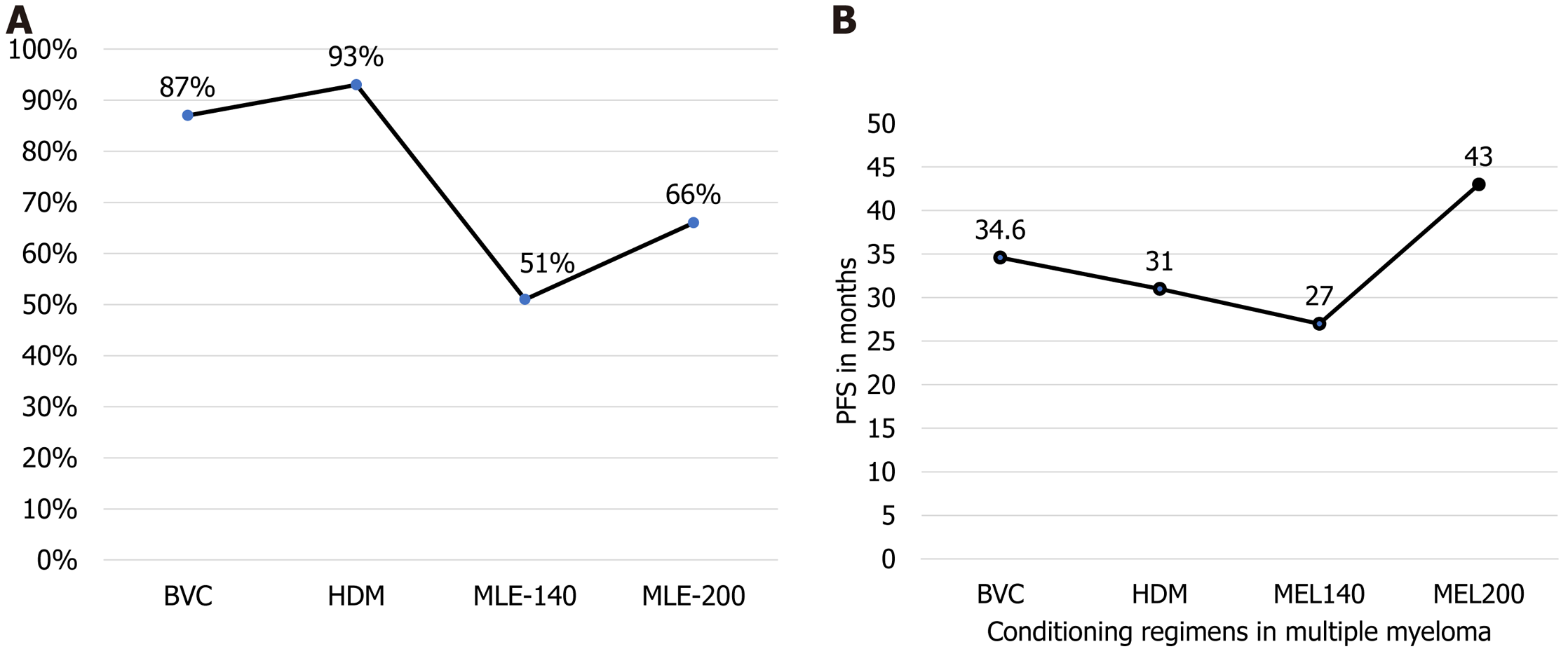
The process of Auto-SCT is carried out in four basic steps: The mobilization, apheresis of mobilized stem cells, utilization of conditioning regimen and, finally, reinfusion[24]. According to a retrospective study by Brioli et al[25] involving 187 patients with MM and a comparison of high dose melphalan (MEL) 200 mg/m2 (MEL 200) and low dose MEL 140 mg (MEL 140) conditioning regimens, the MEL 200 was used in 112 (60%) and MEL 140 in 75 (40%) of the patients. OS was found higher among patients treated with MEL 200 as compared to those who were given MEL 140 (66% vs 51% at 5 years) as mentioned in Figure 3.
A study by Nishihori et al[26] reviewing the effectiveness of various treatment modalities in MM also showed promising benefits by utilization of Bortezomib along with high dose MEL.
During the last decade, genetically engineered chimeric antigen receptor (CAR)-T cell therapy has been developed with the identification of several target antigens like CD19, CD38, CD138, and B-cell maturation antigen (BCMA)[27]. However, CAR-T cells targeting CD19 are the most identified CAR-T cells that are being used in hematological mali
In recent years, the therapeutic and prognostic profile of acute myeloid leukemia (AML) has been improved due to recent advances in chemotherapeutic agents and the rising trend of ASCT to consolidate adult patients with AML[29]. AML is a rare diagnosis. Due to high neoplasm potential, it is associated with a large number of leukemia-associated deaths with a reduced OS rate. The presence of balanced translocation between chromosome 8 and 21 [t(8;21)], inversion of chromosome 16, and translocation between chromosomes 15 and 17 [t(15;17)] has also been implicated in acute promyelocytic leukemia pathogenesis along with some genetic and epigenetic alterations[30]. Although recent advances have been paving an excellent pathway for halting the disease progression and improving OS rate, AML is still posing some serious therapeutic challenges to be overcome.
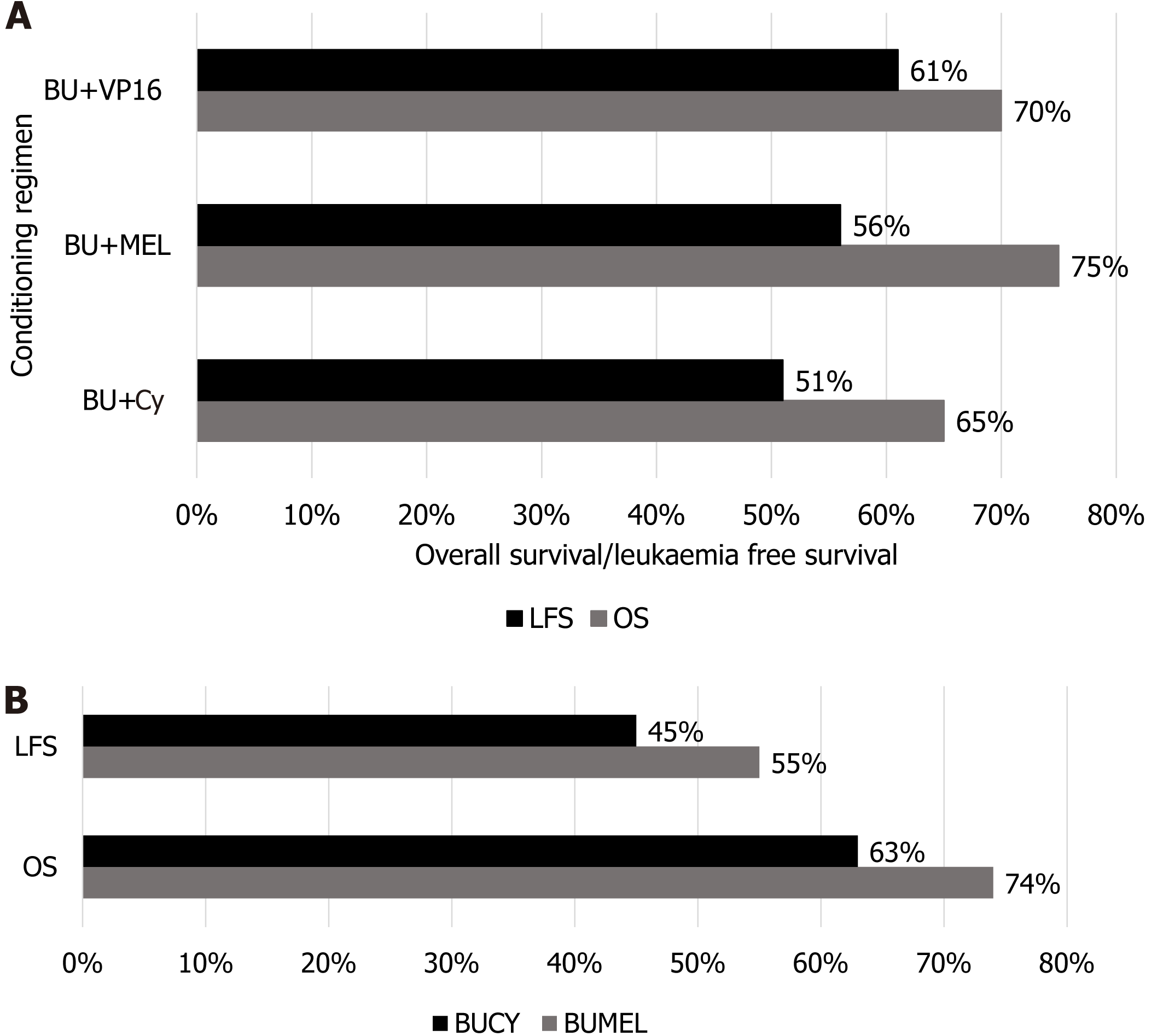
According to a retrospective analytical study involving 952 patients with AML by Nagler et al[31], the median age of patients was 50.5 years with 56% of the population (n = 531) consisting of the male population. The effectiveness of intravenous (IV) busulfan (BU) in ASCT was ascertained in this study and comparison was made with oral BU utilization in patients undergoing ASCT. IV conditioning regimens based mainly on BU (12.8 mg/kg) combined with Cy (120 mg/kg) were administered in about 517 patients, the combination of IV BU (12.8 mg/kg) and MEL (140 mg/kg) was given to 234 patients, a combination of IV BU and etoposide was tried in 82 patients, and the IV BU and idarubicin were administered in 46 patients. Outcomes in terms of 2-year OS, leukemia free survival (LFS), and relapsed incidence were assessed. However, the effectiveness of all combinations was surprisingly higher in patients aged less than 50 as compared to older patients; OS was 67% ± 2%, LFS was 53% ± 2%, and relapse incidence (RI) was 40% ± 2%. Out of all the combinations discussed herein, the combination of IV BU (12.8 mg/kg) with MEL (140 mg/kg) was associated with significantly improved OS as compared to other three combinations, validating the effectiveness of IV BU and MEL as a regimen of choice when compared with other regimens used either IV or oral BUT that was actually showing the greater toxicity profile than IV BU administration with a low incidence of veno-occlusive disease[31].
The conditioning regimen is now considered the real estate of Auto-SCT success because it not only creates the space to transplant the HSCs but also eradicates the disease itself. A study conducted by Gorin et al[32] using the data from a registry of the European Society for Blood and Marrow Transplantation to compare the effectiveness of two standard conditioning regimens, i.e., BU + MEL and BU + Cy, in Auto-SCT for AML patients. The first regimen consisted of BU (12.8 mg/kg) and MEL (140 mg/kg) combined (BUMEL) and the second consisted of BU (12.8 mg/kg) and Cy (120 mg/kg) (BUCY). This study involved 853 patients with available cytogenetics of AML and BUMEL therapy was used in 30% of the patients (n = 257), while 70% of the patients (n = 596) were administered with BUCY therapy and the outcomes were evaluated in terms of RI, LFS, and finally OS. The findings were truly mandating the utilization of the BUMEL regimen against BUCY due to reduced RI (39.5% vs 52.2%; P = 0.003), better LFS (55.4% vs 44.6%; P = 0.005), and finally better OS rate (73.8% vs 63%; P = 0.0007), validating the higher effectiveness of BUMEL regimen in ASCT[32]. When the OS was compared between other conditioning regimens used vs BUMEL in ASCT for patients with AML, the BUMEL regimen was found to be highly effective on all grounds, making it the conditioning regimen of choice with excellent ultimate outcomes as shown in Figure 4.
The construction of a CD-70 CAR-T cell can prove to be a breakthrough in the field of oncology and medicine. CD70 is a type 2 transmembrane glycoprotein and a member of the tumor necrosis factor ligand family that is now increasingly being utilized as a therapeutic target for the treatment of AML; however, there is still very much to discover about this therapeutic approach. The antitumor activity of a CD70-specific monoclonal antibody along with hypomethylating agents for the treatment of patients with AML has been showing promising benefits[33]. Therefore, we can hope that in the future, designing of CAR-T cells will be conducive to the treatment of hematological malignancies with minimal myelotoxicity.
Acute lymphoblastic leukemia (ALL) is a familiar pediatric carcinoma marked by chromosomal translocations and somatic mutations[34].
Lee et al[35] carried out a retrospective study using myeloablative therapy. They inducted 44 patients from March 2009 to January 2014 and the efficacy was assessed by complete remission (CR). These patients underwent HSCT using a once-daily IV conditioning regimen. The regimen included BU (120 mg/m2 for patients > 1 year of age and 80 mg/m2 for patients < 1 year of age), fludarabine 40 mg/m2, and etoposide 20 mg/kg. Results showed that 28 (63.6%), 12 (27.3%), and 1 (2.3%) patients achieved 1st, 2nd, and 3rd CR, respectively, while two (4.5%) patients had no remission at the time of HSCT. The complications reported in this study included elevated AST and/or ALT or total bilirubin[35].
To compare the efficacy of TBI plus etoposide and myeloablative regimen (including fludarabine, thiotepa, and IV BU/treosulfan), Peters et al[36] in 2021 conducted a multi-centre and randomized trial in high-risk ALL patients. Efficacy was measured in terms of treatment related mortality (TRM). They inducted 417 patients and randomly assigned them to two cohorts. Cohort 1 was given TBI and IV etoposide (60 mg/kg) while cohort 2 was administered with fludarabine (30 mg/m2) once daily, thiotepa (5 mg/kg) twice daily, and treosulfan (14 g/m2)/BU once daily. Following the TBI-based regimen and myeloablative regimen, the 2-year TRM was 0.02 [95% confidence interval (95%CI): 0.01 to 0.05] and 0.09 (95%CI: 0.05 to 0.14), respectively, thus showing that TBI plus etoposide regimen had good disease control.
For hematologic malignancies, CAR-T cell therapy has been unfolded as an efficacious therapeutic option. Its mechanism of action involves the patient’s own T-cells that in turn express receptors modified to recognize specific epitopes of tumor-associated antigens on the target cell surface[37]. Numerous trials have been carried out to investigate the efficacy of this therapy. Subklewe et al[38] conducted “the pivotal global ELIANA trail” (NCT02435849) using genetically modified CD19-directed T-cell products, “Tisagenlecleucel”. In another phase 1 trial (NCT01044069), Davila et al[39] pointed out the plausibility of CAR-T cell therapy. In this study, 16 patients were enrolled and given a 19-28z infusion of CAR-T cells after salvage chemotherapy. This blatantly boosted the overall complete response rate to 88%, which is higher than that expected with salvage chemotherapy alone.
To sum up, the introduction of CAR-T cell therapy has provided new directions to the field of oncology and medicine; however, ASCT is widely preferred because of being inexpensive. Moreover, CAR-T cell therapy needs further evolution by health professionals.
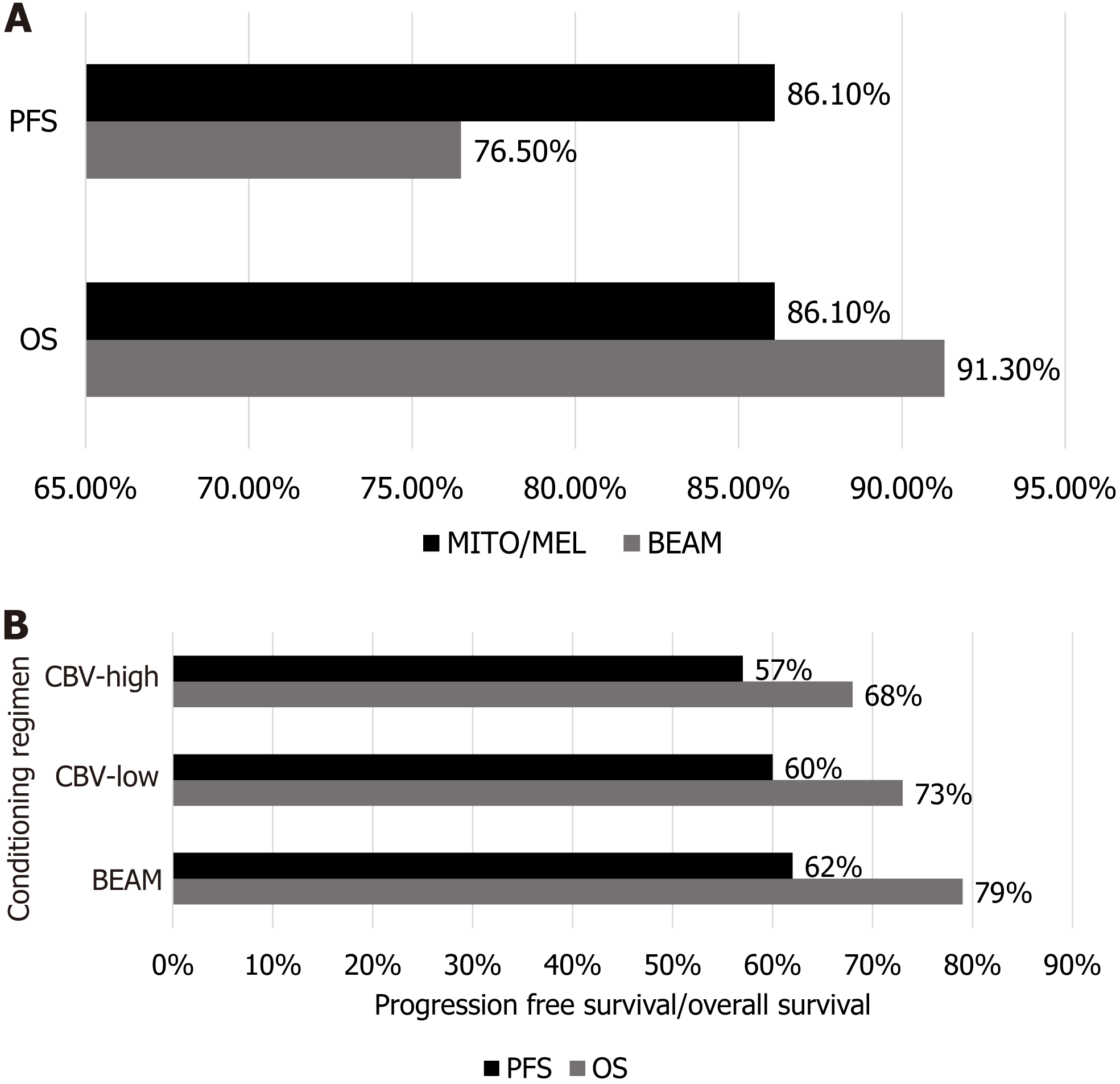
A retrospective, multi-center study by Yeral et al[42] involving 142 patients with HL undergoing ASCT showed the comparison of two conditioning regimens with end points represented by OS and progression free survival (PFS). The two conditioning regimens used were BEAM (carmustine 300 mg/m2 given at day 6, etoposide 200 mg/m2 and cyta
According to a study by Chen et al[43] involving 1012 patients with HL, BEAM and Cy, carmustine, and etoposide (CBV)-low or CBV-high were the most used regimens with a 3-year OS of 79% and PFS of 62% in the BEAM group, OS of 73% and PFS of 60% in the CBV-low, and OS of 68% and PFS of 57% in the CBV-high group. However, the BEAM-based regimen was most effective in HL with better OS and PFS as compared to other regimens as shown in Figure 5.
CAR T-cell therapy of B-cell malignancies has proved to be effective. Ramos et al[44] showed how the same approach of CAR-T cells specific for CD30 (CD30.CAR-Ts) can be used to treat HL.
Non-HLs (NHLs) are a diverse collection of lymphoproliferative tumors with a greater propensity to expand to extr
Between May 19, 2015 and September 15, 2016, Locke et al[47] carried out a single-arm, multicenter phase 1/2 study in which 119 patients were enrolled and 108 were given axicabtagene ciloleucel. Seven patients participated in phase 1, while the remaining 107 were enrolled in phase 2 studies. After receiving IV fludarabine and Cy as conditioning chemo
Between February 25, 2011 and April 3, 2014, Okay et al[48] selected 1503 previously untreated patients for a randomized, open-label, phase 3 study. The forecasts for OS at 5 years, survival without disease, and survival without events were 81.9%, 46.5%, and 41.4%, respectively. All patients displayed neutropenia and thrombocytopenia. All indi
CAR T-cell therapy has emerged as a standard of care for treating a number of disorders in recent years, overcoming any potential drawbacks associated with conventional therapies. Clinical trials of anti-CD19 CAR-T cell therapy for the treatment of refractory or relapsed B-NHL have produced encouraging effective outcomes[50].
Amyloidosis (AL) is a clonal plasma cell dyscrasia characterized by the accumulation of misfolded fibrillar proteins in extracellular tissues, leading to organ failure and eventually death. Though associated with high treatment-related mortality, for nearly 20 years Auto-SCT has been used and demonstrated improved survival and a prolonged treatment-free interval[51].
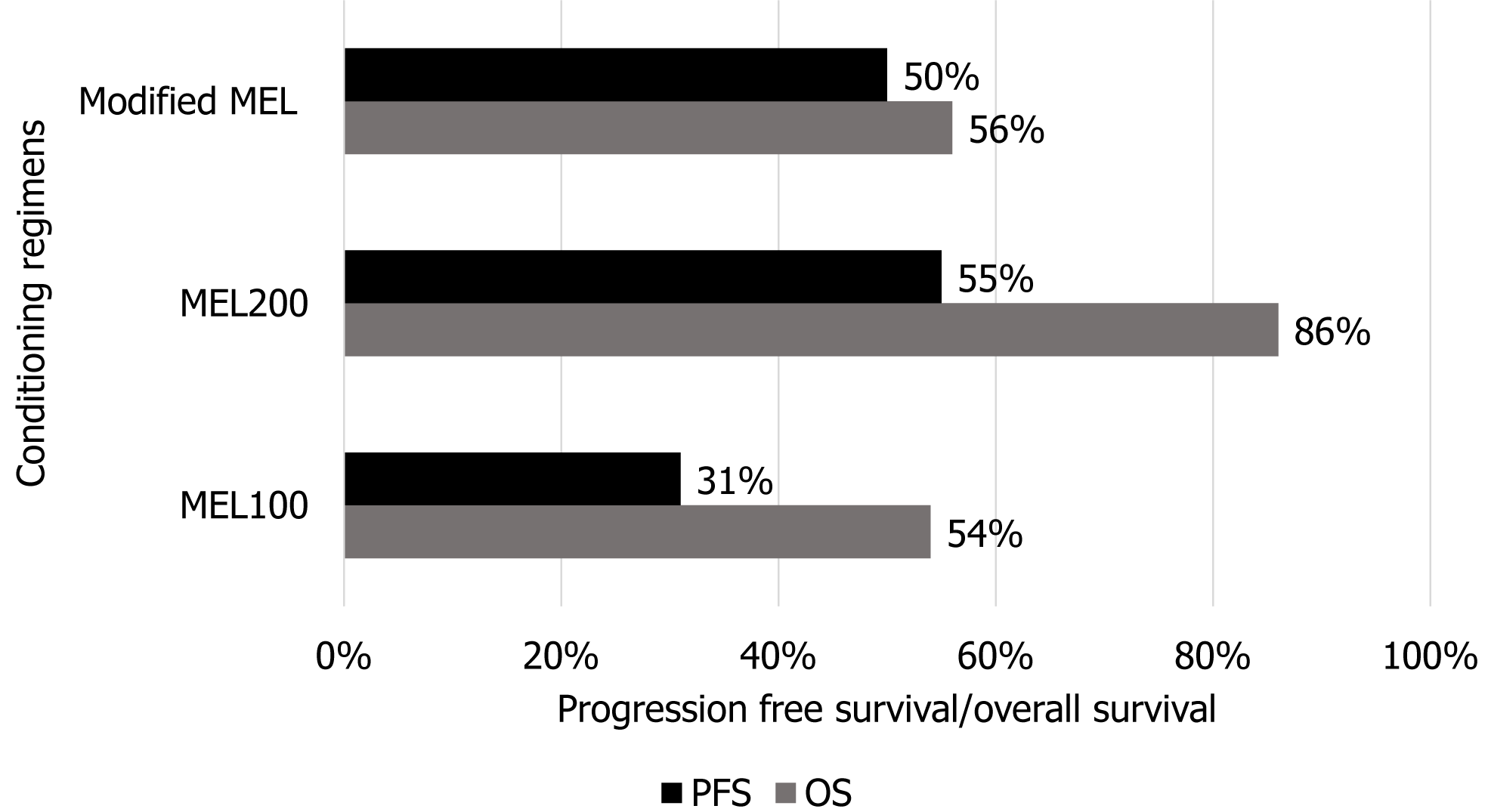
According to a study by Tandon et al[52] involving 457 diagnosed cases of light chain AL undergoing AHSCT, two conditioning regimens, one with full dose MEL (200 mg/m2) and the other with low or reduced intensity MEL (100 mg/kg), were compared. Complete response was observed in high dose Mel group (53% vs 37%, P = 0.003), and the PFS was also validating the effectiveness of high dose Mel regimen when compared with low dose Mel group (55% vs 31%; P < 0.001) as shown in Figure 6.
Similarly, a trial labeled SWOG (S0115) conducted by Sanchorawala et al[53] involved 93 patients diagnosed with ligh-chain amyloidosis (AL), AL with myeloma (AM), and host-based high-risk myeloma (hM), with 59, 9, and 25 patients in each group. The patients were treated with sequential doses of modified MEL (100 mg/m2). The estimated 2- and 5-year OS was 69%, 56%, and 80%, and 56%, 42%, and 55% for AL, AM, and hM, respectively. The estimated 5-year PFS was 50%, 30%, and 50% in AL, ALM, and hM, respectively. Skinner et al[54] evaluated 701 consecutive patients with AL between July 1994 and June 2002. Fifty-six percent (394) of the patients met the eligibility criteria for high dose MEL treatment. Overall median survival was 4.6 years and 56% of the patients remained alive. The estimated 5-year survival rate was 47%.
Strategies for the treatment of hematologic malignancies have evolved as the use of immunotherapy is an attractive approach. Rosenzweig et al[55] provided preclinical data evaluating bone marrow specimens for BCMA and CS1 expression in ten AL patients. All the AL samples expressed high levels of CS1 (76.5% ± 4.7%) but low levels of BCMA (4.9% ± 0.8%). The study reported the unique nature of plasma clonal cells in AL patients because of the scarcity of BCMA expression.
This literature review study is based on real-world data collected from various published research introducing multiple conditioning regimens for different disorders. Comparisons between regimens for an individual disorder were made using variables such as OS, PFS, CR, and LFS to conclude a laudable conditioning regimen having trivial adverse effects. In MM, the most effective regimen was high dose MEL given at a dose of 200 mg/m2/d. However, for ALL, CAR-T cell therapy was preferred in the context of better OS and LFS. With respect to HL, MITO/MEL overtook BEAM in view of PFS and vice versa regarding OS. NHL patients were administered MITO (60 mg/m2) and MEL (180 mg/m2) which showed promising results. Lastly, AL is considered, and the regimen that proved to be competent was MEL 200 (200 mg/m2). This article presents a descriptive picture of diseases and the regimens employed in them along with mentioning the most successful regimen.
| 1. | Bazinet A, Popradi G. A general practitioner's guide to hematopoietic stem-cell transplantation. Curr Oncol. 2019;26:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, Bence-Bruckler I, Birch P, Bredeson C, Chen J, Fergusson D, Halpenny M, Hamelin L, Huebsch L, Hutton B, Laneuville P, Lapierre Y, Lee H, Martin L, McDiarmid S, O'Connor P, Ramsay T, Sabloff M, Walker L, Freedman MS. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 3. | Ali N, Adil SN, Shaikh MU. Autologous Hematopoietic Stem Cell Transplantation-10 Years of Data From a Developing Country. Stem Cells Transl Med. 2015;4:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Balassa K, Danby R, Rocha V. Haematopoietic stem cell transplants: principles and indications. Br J Hosp Med (Lond). 2019;80:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Baldomero H, Aljurf M, Zaidi SZA, Hashmi SK, Ghavamzadeh A, Elhaddad A, Hamladji RM, Ahmed P, Torjemane L, Abboud M, Tbakhi A, Khabori MA, El Quessar A, Bazuaye N, Bekadja MA, Adil S, Fahmy O, Ramzi M, Ibrahim A, Alseraihy A, Ben Abdejalil N, Sarhan M, Huneini MA, Mahmal L, ElSolh H, Hussain F, Nassar A, Al-Hashmi H, Hamidieh AA, Pasquini M, Kodera Y, Kröger N, Mohty M, Jaimovich G, Rolon JM, Paulson K, Greinix H, Weisdorf D, Horowitz M, Nunez J, Gratwohl A, Passweg J, Koh M, Szer J, Niederwieser D, Novitzky N; East-Mediterranean (EMBMT) and African (AfBMT) Blood and Marrow Transplantation Groups and the Worldwide Network for Blood and Marrow Transplantation (WBMT). Narrowing the gap for hematopoietic stem cell transplantation in the East-Mediterranean/African region: comparison with global HSCT indications and trends. Bone Marrow Transplant. 2019;54:402-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Lum SH, Hoenig M, Gennery AR, Slatter MA. Conditioning Regimens for Hematopoietic Cell Transplantation in Primary Immunodeficiency. Curr Allergy Asthma Rep. 2019;19:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Kanda Y, Sakamaki H, Sao H, Okamoto S, Kodera Y, Tanosaki R, Kasai M, Hiraoka A, Takahashi S, Miyawaki S, Kawase T, Morishima Y, Kato S; Japan Marrow Donor Program. Effect of conditioning regimen on the outcome of bone marrow transplantation from an unrelated donor. Biol Blood Marrow Transplant. 2005;11:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Samara Y, Mei M. Autologous Stem Cell Transplantation in Hodgkin Lymphoma-Latest Advances in the Era of Novel Therapies. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Ikehara S, Shi M, Li M. Novel conditioning regimens for bone marrow transplantation. Blood and Lymphatic Cancer: Targets and Therapy 2013; 3: 1-9. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Burt RK, Patel D, Thomas J, Yeager A, Traynor A, Heipe F, Arnold R, Marmont A, Collier D, Glatstein E, Snowden J. The rationale behind autologous autoimmune hematopoietic stem cell transplant conditioning regimens: concerns over the use of total-body irradiation in systemic sclerosis. Bone Marrow Transplant. 2004;34:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, Maloney DG, Storb R. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 543] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 13. | Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 457] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 14. | Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 446] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32:554-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 16. | Wang L, Li L, Menendez P, Cerdan C, Bhatia M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005;105:4598-4603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 597] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 18. | Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20:303-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 736] [Article Influence: 122.7] [Reference Citation Analysis (0)] |
| 19. | Fraint E, Ulloa BA, Feliz Norberto M, Potts KS, Bowman TV. Advances in preclinical hematopoietic stem cell models and possible implications for improving therapeutic transplantation. Stem Cells Transl Med. 2021;10:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Merli P, Algeri M, Del Bufalo F, Locatelli F. Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoblastic Leukemia. Curr Hematol Malig Rep. 2019;14:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (4)] |
| 21. | Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, Falkenburg JH, Farge-Bancel D, Gennery A, Kröger N, Lanza F, Marsh JC, Nagler A, Peters C, Velardi A, Mohty M, Madrigal A. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015;50:1037-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Costa LJ, Zhang MJ, Zhong X, Dispenzieri A, Lonial S, Krishnan A, Freytes C, Vesole D, Gale RP, Anderson K, Wirk B, Savani BN, Waller EK, Schouten H, Lazarus H, Meehan K, Sharma M, Kamble R, Vij R, Kumar S, Nishihori T, Kindwall-Keller T, Saber W, Hari PN. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:1615-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ; Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1422] [Cited by in RCA: 1399] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 24. | Kimambo EH, Li LH, Ma DD, Ling Y, Miao WJ, Li WF, Ji XB. Usulfan, Etoposide and Cyclophosphamide vs High-Dose Melphalan as a Conditioning Regimen for Autologous Hematopoietic Stem Cell Transplantation in Patients with Multiple Myeloma. Ann Hematol Oncol Res. 2022;2:1012. |
| 25. | Brioli A, Mügge LO, Scholl S, Hilgendorf I, Sayer HG, Yomade O, Ernst T, Hochhaus A, von Lilienfeld-Toal M. Full Dose or Reduced Dose Melphalan (MEL) for Autologous Stem Cell Transplantation (ASCT) in Multiple Myeloma (MM): A Single Center Analysis on 187 Consecutive Patients. Blood 2018; 132: 4625. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Nishihori T, Alsina M. Advances in the autologous and allogeneic transplantation strategies for multiple myeloma. Cancer Control. 2011;18:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | van de Donk NWCJ, Usmani SZ, Yong K. CAR T-cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol. 2021;8:e446-e461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 28. | Teoh PJ, Chng WJ. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 2021;11:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 29. | Gorin NC. History and Development of Autologous Stem Cell Transplantation for Acute Myeloid Leukemia. Clin Hematol Int. 2021;3:83-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 458] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 31. | Nagler A, Labopin M, Gorin NC, Ferrara F, Sanz MA, Wu D, Gomez AT, Lapusan S, Irrera G, Guimaraes JE, Sousa AB, Carella AM, Vey N, Arcese W, Shimoni A, Berger R, Rocha V, Mohty M. Intravenous busulfan for autologous stem cell transplantation in adult patients with acute myeloid leukemia: a survey of 952 patients on behalf of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Gorin NC, Labopin M, Czerw T, Pabst T, Blaise D, Dumas PY, Nemet D, Arcese W, Trisolini SM, Wu D, Huynh A, Zuckerman T, Meijer E, Cagirgan S, Cornelissen J, Houhou M, Polge E, Mohty M, Nagler A. Autologous stem cell transplantation for adult acute myelocytic leukemia in first remission-Better outcomes after busulfan and melphalan compared with busulfan and cyclophosphamide: A retrospective study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Cancer. 2017;123:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Sauer T, Parikh K, Sharma S, Omer B, Sedloev D, Chen Q, Angenendt L, Schliemann C, Schmitt M, Müller-Tidow C, Gottschalk S, Rooney CM. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood. 2021;138:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 34. | DeAngelo DJ, Jabbour E, Advani A. Recent Advances in Managing Acute Lymphoblastic Leukemia. Am Soc Clin Oncol Educ Book. 2020;40:330-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 35. | Lee JW, Kang HJ, Kim S, Lee SH, Yu KS, Kim NH, Jang MK, Kim H, Song SH, Park JD, Park KD, Shin HY, Jang IJ, Ahn HS. Favorable outcome of hematopoietic stem cell transplantation using a targeted once-daily intravenous busulfan-fludarabine-etoposide regimen in pediatric and infant acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant. 2015;21:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, Shaw PJ, Staciuk R, Ifversen M, Pichler H, Vettenranta K, Svec P, Aleinikova O, Stein J, Güngör T, Toporski J, Truong TH, Diaz-de-Heredia C, Bierings M, Ariffin H, Essa M, Burkhardt B, Schultz K, Meisel R, Lankester A, Ansari M, Schrappe M; IBFM Study Group, von Stackelberg A; IntReALL Study Group, Balduzzi A; I-BFM SCT Study Group, Corbacioglu S; EBMT Paediatric Diseases Working Party, Bader P. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J Clin Oncol. 2021;39:295-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 37. | Pehlivan KC, Duncan BB, Lee DW. CAR-T Cell Therapy for Acute Lymphoblastic Leukemia: Transforming the Treatment of Relapsed and Refractory Disease. Curr Hematol Malig Rep. 2018;13:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | Subklewe M, von Bergwelt-Baildon M, Humpe A. Chimeric Antigen Receptor T Cells: A Race to Revolutionize Cancer Therapy. Transfus Med Hemother. 2019;46:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 39. | Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2030] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 40. | Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018;68:116-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 41. | Demiroğlu H, Çiftçiler R, Büyükaşık Y, Göker H. Prediction of Stem Cell Mobilization Failure in Patients with Hodgkin and Non-Hodgkin Lymphoma. Turk J Haematol. 2021;38:204-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Yeral M, Aytan P, Gungor B, Boga C, Unal A, Koc Y, Kaynar L, Buyukkurt N, Eser B, Ozdoğu H. A Comparison of the BEAM and MITO/MEL Conditioning Regimens for Autologous Hematopoietic Stem Cell Transplantation in Hodgkin Lymphoma: An Analysis of Efficiency and Treatment-Related Toxicity. Clin Lymphoma Myeloma Leuk. 2020;20:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Chen YB, Lane AA, Logan B, Zhu X, Akpek G, Aljurf M, Artz A, Bredeson CN, Cooke KR, Ho VT, Lazarus HM, Olsson R, Saber W, McCarthy P, Pasquini MC. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 44. | Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu MF, Ivanova A, Wang T, Shea TC, Rooney CM, Dittus C, Park SI, Gee AP, Eldridge PW, McKay KL, Mehta B, Cheng CJ, Buchanan FB, Grilley BJ, Morrison K, Brenner MK, Serody JS, Dotti G, Heslop HE, Savoldo B. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol. 2020;38:3794-3804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 299] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 45. | Singh R, Shaik S, Negi BS, Rajguru JP, Patil PB, Parihar AS, Sharma U. Non-Hodgkin's lymphoma: A review. J Family Med Prim Care. 2020;9:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 46. | Soekojo CY, Kumar SK. Stem-cell transplantation in multiple myeloma: how far have we come? Ther Adv Hematol. 2019;10:2040620719888111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P, Flinn IW, Farooq U, Goy A, McSweeney PA, Munoz J, Siddiqi T, Chavez JC, Herrera AF, Bartlett NL, Wiezorek JS, Navale L, Xue A, Jiang Y, Bot A, Rossi JM, Kim JJ, Go WY, Neelapu SS. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1739] [Article Influence: 217.4] [Reference Citation Analysis (0)] |
| 48. | Okay M, Büyükaşık Y, Demiroğlu H, Malkan ÜY, Çiftçiler R, Aladağ E, Aksu S, Haznedaroğlu İC, Sayınalp N, Özcebe Oİ, Göker H. Mitoxantrone-melphalan conditioning regimen for autologous stem cell transplantation in relapsed/refractory lymphoma. Turk J Med Sci. 2019;49:985-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Hahn L, Lim H, Dusyk T, Sabry W, Elemary M, Stakiw J, Danyluk P, Bosch M. BeEAM conditioning regimen is a safe, efficacious and economical alternative to BEAM chemotherapy. Sci Rep. 2021;11:14071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Yin Z, Zhang Y, Wang X. Advances in chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Biomark Res. 2021;9:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Sharpley FA, Petrie A, Mahmood S, Sachchithanantham S, Lachmann HJ, Gillmore JD, Whelan CJ, Fontana M, Martinez-Naharro A, Quarta C, Hawkins PN, Wechalekar AD. A 24-year experience of autologous stem cell transplantation for light chain amyloidosis patients in the United Kingdom. Br J Haematol. 2019;187:642-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Tandon N, Muchtar E, Sidana S, Dispenzieri A, Lacy MQ, Dingli D, Buadi FK, Hayman SR, Chakraborty R, Hogan WJ, Gonsalves W, Warsame R, Kourelis TV, Leung N, Kapoor P, Kumar SK, Gertz MA. Revisiting conditioning dose in newly diagnosed light chain amyloidosis undergoing frontline autologous stem cell transplant: impact on response and survival. Bone Marrow Transplant. 2017;52:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Sanchorawala V, Hoering A, Seldin DC, Finn KT, Fennessey SA, Sexton R, Mattar B, Safah HF, Holmberg LA, Dean RM, Orlowski RZ, Barlogie B. Modified high-dose melphalan and autologous SCT for AL amyloidosis or high-risk myeloma: analysis of SWOG trial S0115. Bone Marrow Transplant. 2013;48:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL, Anderson JJ, O'Hara C, Finn KT, Libbey CA, Wiesman J, Quillen K, Swan N, Wright DG. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 55. | Rosenzweig M, Urak R, Walter M, Lim L, Sanchez JF, Krishnan A, Forman S, Wang X. Preclinical data support leveraging CS1 chimeric antigen receptor T-cell therapy for systemic light chain amyloidosis. Cytotherapy. 2017;19:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhong Y, China S-Editor: Lin C L-Editor: Wang TQ P-Editor: Zhang YL