Published online Sep 18, 2023. doi: 10.5500/wjt.v13.i5.276
Peer-review started: July 13, 2023
First decision: August 4, 2023
Revised: August 6, 2023
Accepted: August 25, 2023
Article in press: August 25, 2023
Published online: September 18, 2023
Processing time: 63 Days and 6.8 Hours
Although the availability of related living donors (LDs) provides a better chance for receiving kidney transplantation (KT), the evaluation protocols for LD selection remain a safeguard for the LD’s safety. These protocols are variable from one center to another, resulting in variable rates of decline of the potential LDs (PLDs). The decline of willing PLDs may occur at any stage of evaluation, starting from the initial contact and counseling to the day of operation.
To identify the causes of the decline of PLDs, the predictors of PLD candidacy, and the effect on achieving LDKT.
A retrospective study was performed on the willing PLDs who attended our outpatient clinic for kidney donation to their related potential recipients between October 2015 and December 2022. The variables influencing their candidacy rate and the fate of their potential recipients were studied. Two groups of PLDs were compared: Candidate PLDs after a completed evaluation vs non-candidate PLDs with a complete or incomplete evaluation. A multivariate logistic regression was performed to assess the factors contributing to the achievement of PLD candidacy.
Of 321 willing PLDs, 257 PLDs (80.1%) accessed the evaluation to variable extents for 212 potential recipients, with a mean age (range) of 40.5 ± 10.4 (18-65) years, including 169 females (65.8%). The remaining 64 PLDs (19.9%) did not access the evaluation. Only 58 PLDs (18.1%) succeeded in donating, but 199 PDLs (62.0%) were declined; exclusion occurred in 144 PLDs (56.0%) for immunological causes (37.5%), medical causes (54.9%), combined causes (9.7%), and financial causes (2.1%). Regression and release occurred in 55 PLDs (17.1%). The potential recipients with candidate PLDs were not significantly different from those with non-candidate PLDs, except in age (P = 0.041), rates of completed evaluation, and exclusion of PLDs (P < 0.001). There were no factors that independently influenced the rate of PLD candidacy. Most patients who failed to have KT after the decline of their PLDs remained on hemodialysis for 6 mo to 6 years.
The rate of decline of willing related PLDs was high due to medical or immunological contraindications, release, or regression of PLDs. It reduced the chances of high percentages of potential recipients in LDKT.
Core Tip: The rate of decline of willing related potential living kidney donors (PLDs) was high (82%). The causes of decline included exclusion by the transplant team due to contraindications of donation, release after disqualification of the potential recipients, and regression due to withdrawal of the decision by the PLD. PLD exclusion was the commonest form of decline due to medical or immunological contraindications. The high rate of PLD decline resulted in the loss of chances of kidney transplantation for high percentages of potential recipients who were left on dialysis for variably long periods, who died, or who were lost to an unknown fate.
- Citation: Gadelkareem RA, Abdelgawad AM, Mohammed N, Reda A, Azoz NM, Zarzour MA, Hammouda HM, Khalil M. Reasons and effects of the decline of willing related potential living kidney donors. World J Transplant 2023; 13(5): 276-289
- URL: https://www.wjgnet.com/2220-3230/full/v13/i5/276.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i5.276
Living donor kidney transplantation (LDKT) is the optimal form of renal replacement therapy. It shortens the waiting times and provides better survival rates. Hence, it is recommended as the first choice of treatment for each candidate patient with end-stage renal disease (ESRD), especially with the availability of related potential LDs (PLDs)[1,2]. However, it is not easy to find a willing and suitable LD. In addition, the preparation of a potential donor-recipient pair for KT is a complex sequential process[3,4]. Relative to the variability of assessment protocols, the reported acceptance rate of LDs is variable at 8.0%-18.4%[4,5]. This variability in acceptance of LDs is significant among countries, medical societies, health organizations, and KT units[3,6].
A large proportion of those PLDs may initially be declined for demographic issues, such as unsuitable age and genetic unrelatedness, or excluded later during preparation due to different medical reasons[5,7,8]. Although the exclusion of a willing potential LD may negatively reflect on the potential recipients by reducing their chances of transplantation, it is still a paramount principle not to violate the donor’s safety for the recipient’s benefit[3-5]. In our center, the maintenance of this narrow-margin principle between the donor’s safety and the recipient’s benefit through the assessment process was the motivator for the conduct of the current study. The aim was to assess the reasons for the decline of PLDs and their effects on the fate of potential recipients.
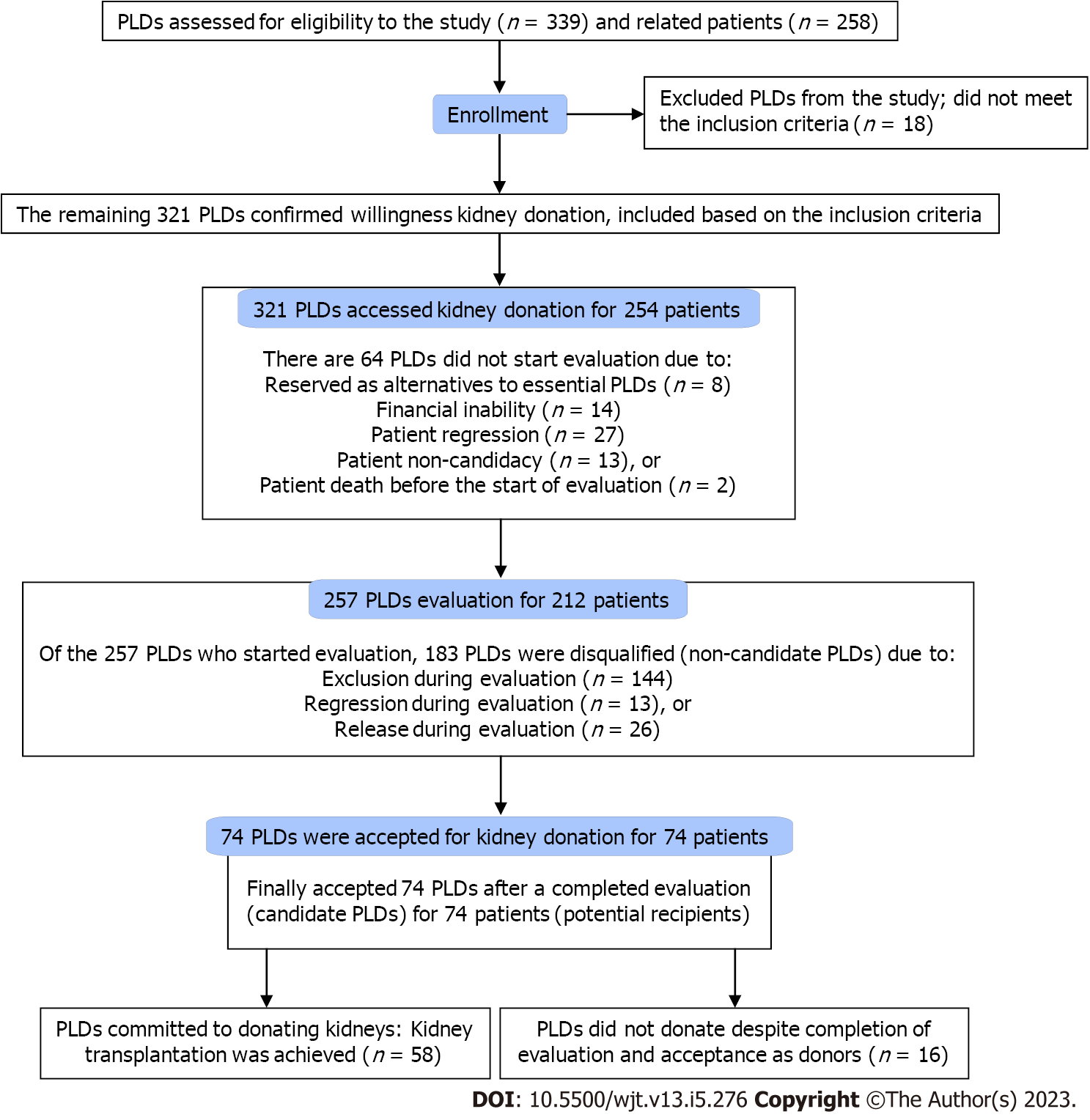
A retrospective study was carried out by reviewing the data of the PLDs of ESRD patients who presented to our center seeking KT from October 2015 to December 2022. The inclusion criteria were a related PLD presenting to our center for donation to a related, intended patient with ESRD. Exclusion criteria included an initial failure to confirm the willingness to donate a kidney (Figure 1).
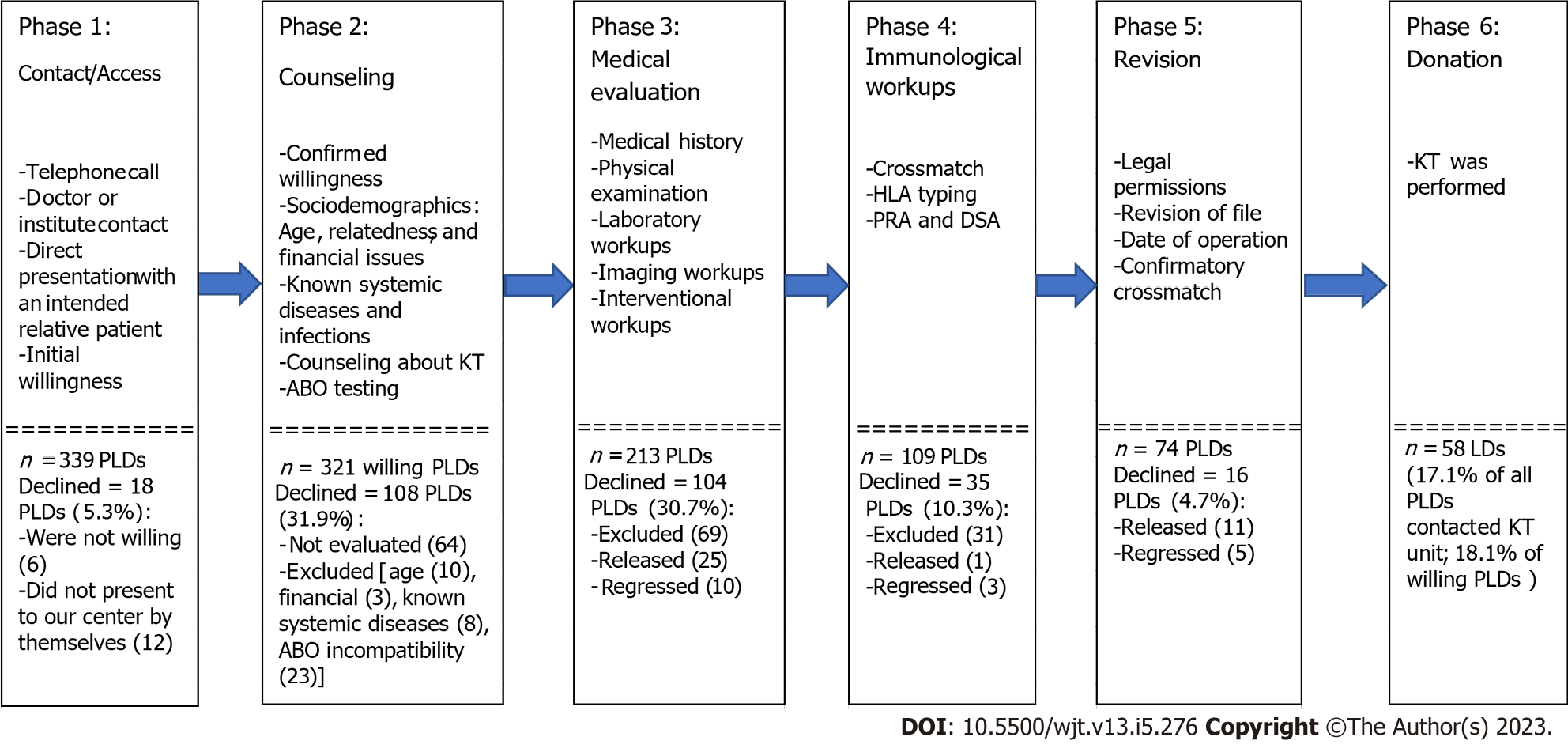
In our policy, the process of PLD evaluation is differentiated into six phases, from the initial contact to the achievement of a donation (Figure 2). Owing to the unavailability of a national waitlisting program and the nature of related living kidney donation (LKD), PLDs directly present with their intended recipients at the KT center. The initial two phases consist of contact with the KT center, confirmation of willingness to donate a kidney to an intended related potential recipient, counseling about KT, sociodemographic evaluation (age, familial relationships, and financial issues), and blood group matching. Initial history taking is usually performed at the first contact or counseling session, excluding previously known systemic diseases, financial issues, and factors violating the integrity of volunteer donation.
The third and fourth phases are multidisciplinary steps, including medical and immunological evaluations. The medical evaluation consists of detailed medical history, physical examinations, laboratory workups, and imaging workups. Kidney function was evaluated using the Technetium 99-diethylenetriamine pentaacetate renography for measurement of the total and split glomerular filtration rates in all PLDs. However, the anatomical features were evaluated by abdominal ultrasonography and contrast-enhanced computed tomography with renal angiography. In addition, psychosocial assessment was a routine workup to evaluate the mental status of the PLDs, motives for donation, cognitive capacity, expectations after donation, and exclude any psychogenic drive for self-harm. Furthermore, evaluation and exclusion of drug addiction was performed. The immunological workups include crossmatching, human leukocytic antigen (HLA) typing, and panel reactive antibody tests.
The fifth phase includes medicolegal permissions, determination of the date of surgery, and revision of the important tests. The sixth phase is the donation achievement.
The primary outcome of this study was the rate of PLD candidacy. It was defined as the percentage of PLDs with complete preparation for LKD and acceptance for donation, either when the transplantation was performed or it was cancelled due to causes related to the patient. Because the relevant characteristics of the intended patients were significant for the identification of the causes of decline of PLDs and their fates, these characteristics of those intended patients and their distribution per the outcomes of evaluation of their PLDs were studied. Each patient’s file was examined for relevant demographic and clinical characteristics and related PLDs. The studied characteristics included the number, age, relatedness form and degree, decline form (exclusion, release, or regression), and causes of the decline of PLDs. In addition, the fate of PLDs and patients with declined PLDs was studied. Here, the relatedness was presented relative to the genetic relatedness degrees (ABO-relatedness).
According to the primary outcome, the PLDs were differentiated into two groups. The first group was the candidate donors, including the finally accepted donors with a completed evaluation and preparation for donation. The second group was the non-candidate donors, including the remaining donors who were disqualified as PLDs, either with or without initial acceptance or a completed evaluation. The characteristics of both groups were compared with each other. The secondary outcomes were the rate of PLD decline in each phase of evaluation and the fate of patients with declined PLDs.
Throughout the process of LKD, the different statuses of the PLD were distinguished from each other as well-defined clinical events. They were defined to describe the events of PLD evaluation, from access to the achievement of LKD (Table 1). Based on these definitions, the outcomes of the study were estimated.
| Term | Definition |
| PLD | An individual who confirmed his willingness to donate a kidney to an intended patient at the initial counseling settings and was ready to start the evaluation for kidney donation, regardless of the commencement of the evaluation |
| Related PLD | PLD who had a relative intended patient with end-stage renal disease up to the 4th degree of genetic relatedness. Regardless of their genetic relatedness, the wife or husband of a recipient was considered a related PLD |
| Excluded PLD | PLD who was disqualified as a kidney donor and excluded from the process of kidney donation by KT team due to causes that disqualify candidacy to donate a kidney, such as medical, immunological, or financial causes |
| Regressed PLD | PLD who withdrew his decision of kidney donation at any stage after an initial confirmation of the donation decision and before the operation |
| Released PLD1 | PLD who was still willing and completed or was still continuing the evaluation, but the related intended patient was withdrawn from KT preparation due to any cause |
| Candidate PLD | PLD who completed all the steps of evaluation and was finally accepted by the KT team for kidney donation, regardless of the later regression or release from donation |
| Accepted PLD | PLD who completed the evaluation without exclusion from kidney donation and was accepted for donation without release or regression from his willingness |
| LD | PLD becomes a LD when he succeeds in donating a kidney to his/her intended patient, which also means KT was achieved |
| Relatedness degrees and forms2 | First degree: father, mother, son, daughter, wife, and husband. Second degree: brother, sister, grandfather, grandmother, grandson, and granddaughter. Third degree: nephew, uncle and aunt. Fourth degree: cousins |
Official documents from the Civil Registry Office were requested to document the degree of genetic relatedness between the PLD and the intended recipient. Routinely, the birth certificates and national identity numbers for all PLDs and their intended recipients were the basic documents. In our center, the first and second degrees of genetic relatedness of PLDs were routinely allowed, based on these routine documents. If there was difficulty finding a PLD, the third and fourth degrees were allowed after investigations, and they were mostly processed similarly to the unrelated PLDs. In the latter instances, further documentation was warranted, such as a family genealogy tree from the Civil Registry Office and consent registered in the Real Estate Publicity Department and Documentation Office.
The transparency of the donation as an unpaid act was verified via multiple processes to identify and exclude any financial agreements in these cases: (1) Direct confrontation of the PLD and intended recipient with this issue during counseling and warning them that KT would not be done if there was any violation to the moral donation principle; (2) The KT ethical committee, which is composed of three medical professors who do not belong to the KT team, has the authority to investigate and revise the process of preparation, including the soundness of donation principles; (3) Each patient with a PLD of more than the second degree genetic relatedness had to introduce the proofs (official papers or documents) of the relatedness to his PLD from the Civil Registry Office; (4) As mentioned above, each PLD accepted for donation of his kidney had to sign a consent that the donation was for free without any financial or non-financial rewards from the intended recipient or from other relatives. This consent was documented by the Real Estate Publicity Department and Documentation Office; and (5) The Egyptian Supreme Committee of Organ Transplantation revises all these files and documents to prove the family tree and degree of relatedness between each candidate PLD and the intended recipient, with a special attention to exclude any financial agreements.
This study was conducted as part of a research project on the outcomes of LDKT performed in our center. The institutional review board number is 17200148/2017.
Statistical analysis was performed with EasyMedStat software (version 3.21.4; www.easymedstat.com). The continuous data were presented as mean ± standard deviation and range. The categorical data were presented as frequency and percentage for each category. Two groups of PLDs were compared: candidate PLDs after a completed evaluation vs non-candidate PLDs with a complete or incomplete evaluation. Normality and hetereoskedasticity of continuous data were assessed with the White test (or Shapiro-Wilk in multivariate analysis) and Levene’s test, respectively. Continuous outcomes were compared with the unpaired Student t-test, Welch t-test, or Mann-Whitney U test according to the data distribution. Categorical outcomes were compared with the chi-squared or Fisher’s exact test. A multivariate logistic regression was performed to assess the factors contributing to the achievement of PLD candidacy. The data were checked for multicollinearity with the Belsley-Kuh-Welsch technique. A P value < 0.05 was considered statistically significant.
A total of 302 patients were referred to our center for related LDKT during the time frame of this study. The mean age (range) was 32.0 ± 11.7 (12-66) years. Of them, 44 patients (14.6%) did not have PLDs at presentation. The remaining 258 patients (85.4%) had 1-3 PLDs, constituting a total of 339 related PLDs. Eighteen PLDs (5.3%) were considered unwilling to donate a kidney as they could not confirm their willingness at the initial contact and presentation and were excluded from the study. However, the remaining 321 PLDs (94.7%) confirmed their initial willingness to donate to 254 relative patients and were included in the study (Figures 1 and 2). The mean (range) age of all the willing PLDs was 39.7 ± 10.4 (18-65) years, and they included 116 males (36.1%) and 205 females (63.9%).
Despite the confirmed willingness, 64 of 321 PLDs (19.9%) did not start the evaluation. The causes included financial inability in 14 PLDs (21.9%), serving as reserve donors in 8 PLDs (12.5%), patient non-candidacy to KT in 13 PLDs (20.3%), patient regression from KT in 27 PLDs (42.2%), and patient death in 2 PLDs (3.1%).
The remaining 257 PLDs (80.1%) were relatives of 212 patients (83.5%) (Tables 2-5). At the different levels of evaluation and preparation for the final acceptance as LDs (Figure 2), these 257 PLDs variably passed these levels of evaluation with two main outcomes. The first outcome was the completion of the evaluation with candidacy in 74 PLDs (28.8%). The second outcome was the failure to achieve the candidacy outcome in 183 PLDs (71.8%) (Table 5). The causes of the latter outcome were PLD exclusion in 144 PLDs (72.4%), regression from the donation decision in 18 PLDs (9%), and release after the disqualification of their potential recipients in 37 PLDs (18.6%) (Tables 3 and 4). In PLDs with candidacy for donation, 16 PLDs (21.6%) did not commit to donation. Accordingly, only 58 LDs (78.4%) succeeded in committing to donating a kidney to their related recipients, representing 18.1% of the total 321 PLDs (Figures 1 and 2; Table 4). Single and multiple PLDs were found to be related to 165 patients (77.8%) and 47 patients (22.2%), respectively. The respective percentages of achieved donations (26.7% vs 29.8%) were not significantly different (P = 0.674).
| Characteristics | Total patients, n = 212 | Patients with candidate PLDs, n = 74 | Patients with non-candidate PLDs, n = 138 | P value |
| mean ± SD (range)/number (%) | ||||
| Mean age in yr | 31.2 ± 10.6 (13-66) | 29.1 ± 9.6 (13-57) | 32.9 ± 12.0 (14-66) | 0.041 |
| Sex, n = 212 | ||||
| Males | 173 (81.6%) | 67 (90.5%) | 106 (76.8%) | 0.087 |
| Females | 39 (18.4%) | 7 (9.5%) | 32 (23.2%) | |
| Status of dialysis at presentation, n = 212 | ||||
| Preemptive | 19 (9.0%) | 5 (6.8%) | 14 (10.1%) | 0.462 |
| On regular hemodialysis | 193 (91.0%) | 69 (93.2%) | 124 (89.9%) | |
| Primary kidney disease, n = 212 | ||||
| Unknown | 167 (78.8%) | 56 (75.7%) | 111 (80.4%) | 0.088 |
| Systemic disease | 14 (6.6%) | 3 (2.7%) | 11 (8.0%) | |
| Glomerulonephritis | 6 (2.8%) | 3 (4.0%) | 3 (2.2%) | |
| Hereditary renal disease | 5 (3.8%) | 2 (2.7%) | 3 (2.2%) | |
| Obstructive uropathy | 11 (5.2%) | 8 (10.8%) | 3 (2.2%) | |
| Urolithiasis | 9 (4.2%) | 2 (2.7%) | 7 (5.1%) | |
| Categories of primary kidney disease, n = 212 | ||||
| Unknown | 167 (78.8%) | 56 (75.7%) | 111 (80.4%) | 0.154 |
| Systemic disease | 14 (6.6%) | 3 (4.0%) | 11 (8.0%) | |
| Local, renal/urinary | 31 (14.6%) | 15 (20.3%) | 16 (11.6%) | |
| Patients per number of PLDs, n = 212 | ||||
| Patients with one PLD | 165 (77.8%) | 53 (71.6%) | 112 (81.2%) | 0.265 |
| Patients with two PLDs | 39 (18.4%) | 17 (23.0%) | 22 (15.9%) | |
| Patients with three PLDs | 8 (3.8%) | 4 (5.4%) | 4 (2.9%) | |
| Characteristics | Total patients, n = 212 | Patients with candidate PLDs, n = 74 | Patients with non-candidate PLDs, n = 138 | P value |
| mean ± SD (range)/number (%) | ||||
| Patients per extent of evaluation of their PLDs1, n = 212 | ||||
| Completed 1 | 71 (33.5%) | 54 (73%) | 17 (12.3%) | < 0.001 |
| Completed 1/incomplete 1 | 15 (7.1%) | 7 (9.5%) | 8 (5.8%) | |
| Completed 1/incomplete 2 | 2 (0.9%) | 2 (2.7%) | 0 (0%) | |
| Completed 1/not evaluated 1 | 7 (3.3%) | 6 (8.1%) | 1 (0.7%) | |
| Completed 2 | 3 (1.4%) | 3 (4.1%) | 0 (0%) | |
| Completed 2/incomplete 1 | 1 (0.5%) | 0 (0%) | 1 (0.7%) | |
| Completed 3 | 2 (0.9%) | 2 (2.7%) | 0 (0%) | |
| Incomplete 1 | 94 (44.3%) | 0 (0%) | 94 (68.1%) | |
| Incomplete 1/not evaluated 1 | 1 (0.5%) | 0 (0%) | 1 (0.7%) | |
| Incomplete 1/not evaluated 2 | 1 (0.5%) | 0 (0%) | 1 (0.7%) | |
| Incomplete 2 | 13 (6.1%) | 0 (0%) | 13 (9.4%) | |
| Incomplete 3 | 2 (0.9%) | 0 (0%) | 2 (1.5%) | |
| Patients per acceptance of their PLDs1, n = 212 | ||||
| Accepted 1 | 44 (20.6%) | 44 (59.5%) | 0 (0%) | < 0.001 |
| Accepted 1/excluded 1 | 8 (3.8%) | 8 (10.8%) | 0 (0%) | |
| Accepted 1/excluded 2 | 2 (0.9%) | 2 (2.7%) | 0 (0%) | |
| Accepted 1/not evaluated 1 | 4 (1.9%) | 4 (5.4%) | 0 (0%) | |
| Excluded 1 | 81 (38.2%) | 0 (0%) | 81 (0%) | |
| Excluded 2 | 14 (6.6%) | 0 (0%) | 14 (10.1%) | |
| Excluded 3 | 2 (0.9%) | 0 (0%) | 2 (1.5%) | |
| Excluded 1/released 1 | 5 (2.4%) | 1 (1.4%) | 4 (2.9%) | |
| Excluded 1/regressed 1 | 3 (1.4%) | 1 (1.4%) | 2 (1.5%) | |
| Excluded 1/not evaluated 1 | 2 (0.9%) | 0 (0%) | 2 (1.5%) | |
| Excluded 1/not evaluated 2 | 1 (0.5%) | 0 (0%) | 1 (0.7%) | |
| Excluded 2/released 1 | 2 (0.9%) | 1 (1.4%) | 1 (0.7%) | |
| Excluded 2/regressed 1 | 1 (0.5%) | 1 (1.4%) | 0 (0%) | |
| Released 1 | 28 (13.2%) | 7 (9.5%) | 21 (15.2%) | |
| Released 1/not evaluated 1 | 2 (0.9%) | 2 (2.7%) | 0 (0%) | |
| Regressed 1 | 12 (5.7%) | 3 (4.1%) | 9 (6.5%) | |
| Regressed 2 | 1 (0.5%) | 0 (0%) | 1 (0.7%) | |
| Characteristics | Total patients, n = 212 | Patients with candidate PLDs, n = 74 | Patients with non-candidate PLDs, n = 138 | P value |
| mean ± SD (range)/number (%) | ||||
| Patients per number of excluded PLDs, n = 121 | ||||
| Patients with one excluded PLD | 100 (82.6%) | 10 (71.4%) | 90 (84.1%) | 0.764 |
| Patients with two excluded PLDs | 19 (15.7%) | 4 (28.6%) | 15 (14.0%) | |
| Patients with three excluded PLDs | 2 (1.7%) | 0 (0%) | 2 (1.9%) | |
| Patients per causes of exclusion of their PLDs, n = 1211 | ||||
| Combined immunological and medical causes | 14 (11.6%) | 3 (21.4%) | 11 (10.3%) | 0.680 |
| HLA-incompatibility | 24 (19.8%) | 3 (21.4%) | 21 (19.6%) | |
| ABO-incompatibility | 20 (16.5%) | 1 (7.1%) | 19 (17.8%) | |
| ABO and HLA-incompatibility | 2 (1.7%) | 0 (0%) | 2 (1.9%) | |
| Age | 8 (6.6%) | 0 (0%) | 8 (7.5%) | |
| Diabetes mellitus | 4 (3.3%) | 0 (0%) | 4 (3.7%) | |
| HCV positive | 5 (4.1%) | 2 (14.3%) | 3 (2.8%) | |
| Hypertension | 11 (9.1%) | 4 (28.6%) | 7 (6.5%) | |
| Leprosy | 1 (0.8%) | 0 (0%) | 1 (0.9%) | |
| Low GFR | 4 (3.3%) | 1 (7.1%) | 3 (2.8%) | |
| High potential recurrence of primary kidney disease | 6 (5.0%) | 0 (0%) | 6 (5.6%) | |
| Proteinuria | 12 (9.9%) | 0 (0%) | 12 (11.2%) | |
| Psoriasis | 1 (0.8%) | 0 (0%) | 1 (0.9%) | |
| Rheumatoid arthritis | 1 (0.8%) | 0 (0%) | 1 (0.9%) | |
| Urolithiasis | 5 (4.1%) | 0 (0%) | 5 (4.7%) | |
| Financial causes | 3 (2.5%) | 0 (0%) | 3 (2.8%) | |
| Patients per main category of causes of exclusion of their PLDs, n = 121 | ||||
| Combined immunological and medical causes | 14 (11.6%) | 3 (21.4%) | 11 (10.3%) | 0.866 |
| Immunologic mismatches | 46 (38%) | 4 (28.6%) | 42 (39.3%) | |
| Medical causes | 58 (47.9%) | 7 (50.0%) | 51 (47.7%) | |
| Financial causes | 3 (2.5%) | 0 (0%) | 3 (2.8%) | |
| Patients per timing of PLDs regression, n = 18 | ||||
| During evaluation | 13 (72.2%) | 0 (0%) | 13 (100.0%) | NA |
| After evaluation | 5 (27.8%) | 5 (100.0%) | 0 (0%) | |
| Patients per cause of release of PLDs, n = 37 | ||||
| Patient death | 4 (10.8%) | 3 (27.3%) | 1 (3.9%) | 0.186 |
| Patient regression | 22 (59.5%) | 6 (54.5%) | 16 (61.5%) | |
| Patient non-candidacy | 11 (29.7%) | 2 (18.2%) | 9 (34.6%) | |
| Patients per timing of release of PLDs, n = 37 | ||||
| During evaluation | 26 (70.3%) | 0 (0%) | 26 (100.0%) | NA |
| After evaluation | 11 (29.7%) | 11 (100.0%) | 0 (0%) | |
| Fate of patients with evaluated PLDs, n = 212 | ||||
| Transplantation in our center | 58 (27.4%) | 58 (78.4%) | 0 (0%) | NA |
| Transplantation in another center | 14 (6.6%) | 1 (1.4%) | 13 (9.4%) | 0.024 |
| On hemodialysis | 122 (57.6%) | 12 (16.2%) | 110 (79.7%) | < 0.001 |
| Death | 9 (4.2%) | 3 (4.1%) | 6 (4.4%) | 0.920 |
| Unknown | 9 (4.2%) | 0 (0%) | 9 (6.5%) | 0.024 |
| Characteristics | Total PLDs, n = 257 | Candidate PLDs, n = 74 | Non-candidate PLDs, n = 183 | P value |
| mean ± SD (range)/number (%) | ||||
| Mean age in yr | 40.5 ± 10.4 (18-65) | 41.0 ± 10.5 (21-60) | 40.4 ± 10.5 (18-65) | 0.498 |
| Sex | ||||
| Female | 169 (65.8%) | 49 (66.2%) | 120 (65.6%) | > 0.999 |
| Male | 88 (34.2%) | 25 (33.8%) | 63 (34.4%) | |
| Form of relatedness1 | ||||
| Aunt | 4 (1.6%) | 4 (5.4%) | 0 (0%) | 0.286 |
| Brother | 51 (19.8%) | 14 (18.9%) | 37 (20.2%) | |
| Cousin | 4 (1.6%) | 0 (0%) | 4 (2.2%) | |
| Daughter | 4 (1.6%) | 1 (1.4%) | 3 (1.6%) | |
| Father | 23 (8.9%) | 7 (9.5%) | 16 (8.7%) | |
| Husband | 6 (2.3%) | 2 (2.7%) | 4 (2.2%) | |
| Mother | 76 (29.6%) | 23 (31.1%) | 53 (29%) | |
| Nephew | 1 (0.4%) | 0 (0%) | 1 (0.6%) | |
| Sister | 53 (20.6%) | 13 (17.6%) | 40 (21.9%) | |
| Son | 4 (1.6%) | 1 (1.4%) | 3 (1.6%) | |
| Uncle | 1 (0.4%) | 1 (1.4%) | 0 (0%) | |
| Wife | 30 (11.7%) | 8 (10.8%) | 22 (12.0%) | |
| Degree of relatedness | ||||
| First | 143 (55.6%) | 42 (56.8%) | 101 (55.2%) | 0.020 |
| Second | 104 (40.5%) | 27 (36.5%) | 77 (42.1%) | |
| Third | 6 (2.3%) | 5 (6.8%) | 1 (0.6%) | |
| Fourth | 4 (1.6%) | 0 (0%) | 4 (2.2%) | |
| Extent of evaluation | ||||
| Complete | 109 (42.4%) | 74 (100.0%) | 35 (19.1%) | NA |
| Incomplete | 148 (57.6%) | 0 (0%) | 148 (80.9%) | |
| Fate of PLDs | ||||
| Donated | 58 (22.6%) | 58 (78.4%) | 0 (0%) | NA |
| Excluded | 144 (56.0%) | 0 (0%) | 144 (78.7%) | NA |
| Regressed2 | 18 (7.0%) | 5 (6.8%) | 13 (31.6%) | |
| During evaluation | 13 (72.2%) | 0 (0%) | 13 (100.0%) | NA |
| After evaluation | 5 (27.8%) | 5 (100%) | 0 (0%) | |
| Released | 37 (14.4%) | 11 (14.9%) | 26 (68.4%) | |
| Causes of donor release | ||||
| Patient death | 4 (10.8%) | 3 (27.3%) | 1 (3.9%) | 0.186 |
| Patient regression | 22 (59.5%) | 6 (54.5%) | 16 (61.5%) | |
| Patient non-candidacy | 11 (29.7%) | 2 (18.2%) | 9 (34.6%) | |
| Timing of PLDs release | ||||
| During evaluation | 26 (70.3%) | 0 (0%) | 26 (100.0%) | NA |
| After evaluation | 11 (29.7%) | 11 (100.0%) | 0 (0%) | |
| Time spent in PLDs evaluation in mo | 2.2 ± 1.5 (0.5-6.0) | 4.0 ± 0.9 (1-6) | 1.5 ± 1.2 (0.5-5.0) | < 0.001 |
In the 144 excluded PLDs, the causes of exclusion were immunological incompatibilities in 54 PLDs (37.5%), medical abnormalities in 79 PLDs (54.9%), and financial inability in 3 PLDs (2.1%). Combined immunological and medical causes occurred in 14 PLDs (9.7%). In addition, the exclusion was distributed per intended patient (Tables 3 and 4). Exclusion occurred before or after completion of the evaluation in 109 PLDs (75.7%) and 35 PLDs (24.3%), respectively.
A comparison was performed between patients with candidate PLDs and patients with non-candidate PLDs. Their characteristics and PLD-related distributions were not significantly different, except in the mean age, which was lower in patients with candidate PLDs (P = 0.041). During a variable follow-up period, patients with declined PLDs mostly remained on dialysis for 6 mo to 6 years (Tables 2-4).
Also, a comparison was performed between the candidate and non-candidate PLDs. Their characteristics were not significantly different, except in the degree of relatedness to their potential recipients (P = 0.020) and the time spent in the evaluation of PLDs. The latter was lower in patients with non-candidate PLDs (P < 0.001) (Table 5).
A multivariate logistic regression analysis of factors influencing the candidacy of PLDs was carried out. It revealed that the on-dialysis potential recipient (P = 0.451), younger age PLDs (P = 0.925), male PLDs (P = 0.940), second or higher degrees of relatedness to the recipient vs the first degree (P = 0.834), and multiplicity of PLDs (P = 0.123) were not significantly associated with the rate of candidacy of PLDs for LKD after completion of preparation (Table 6).
| Variables | Modality | Odds ratio | P value |
| Dialysis status | Preemptive vs on dialysis | 0.66 (0.23-1.94) | 0.451 |
| Number of potential donors | Single vs multiple | 1.69 (0.87-3.28) | 0.123 |
| Donor age | Increasing age | 1.0 (0.97-1.03) | 0.925 |
| Donor sex | Men vs women | 1.02 (0.55-1.92) | 0.940 |
| Relatedness degree | First vs more than first | 1.07 (0.55-2.1) | 0.834 |
The state of the decline of PLDs is related to the availability of PLDs, the balance between the donor’s safety and the recipient’s benefit, and the achievement of LDKT as the optimal outcome of all these issues[5,6,8]. The decline of PLDs is the backbone of the failure to maintain the availability of PLDs for the majority of patients[6]. Its burden extends between and reflects on the medical and psychosocial integrities of the PLDs and their intended recipients. However, the current literature is still insufficient to resolve this problem because the rates of acceptance of LDs are still variably low[5]. Hence, the current situation mandates further study of the two main aspects of the process of evaluating PLDs. First, the root causes of the failure of high proportions of those PLDs to achieve the task of donating a kidney represent a major topic, despite the limited current literature. Many studies addressed the identification of these causes to help reduce their effects on the process of LKD[5,6,8]. Second, the fate of the intended recipients who had their PLDs lost is the other aspect that may be more critical in the process because those potential recipients may have their all chances for KT permanently lost also[5,6,9,10].
The availability of a related LD provides a better chance for receiving KT, and it represents the only source of grafts in many countries and programs[2,9]. This fact may raise caution to try to decrease the rate of decline of PLDs in those programs to afford the needs of the increasing pool of potential recipients over time. However, the evaluation protocols for suitable LD selection remain a safeguard against violations of donor safety, implementing a complex evaluation process[2,9,10]. According to this process, KT centers usually evaluate LD acceptance and exclusion processes in the context of the principle of non-violation of the donor’s safety[3,11]. Our center implements only the LDKT strategy; hence, we evaluated the rate of candidacy and acceptance of LDs, the causes of declined PLDs, and the fate of potential recipients with declined PLDs.
Although the current rate of successful candidacy of PLDs is similar to other reported values from other centers, it is relatively hard to expect the exact percentage of PLDs who ultimately succeed in committing to LKD. This uncertainty in the acceptance rates can be attributed to the variability in the assessment protocols and stages from one center to another and from one country to another[5,8]. The stages of evaluation in our center may be different from those in other centers, due to socioeconomic factors and different policies of donation[5,9]. We considered HLA-typing at the late stage of evaluation due to sociodemographic reasons. Immunological tests for HLA typing and crossmatching are costly. However, other routine and multidisciplinary laboratory evaluations can be individualized into separate steps to catch any abnormalities in medical and laboratory workups with relatively low costs. In addition, related PLDs have higher chances of being HLA-matched with their intended recipients. On the other hand, this latter characteristic may be the reason why the mean age of the potential recipients was significantly lower than that of the PLDs. First-degree and second-degree relatedness between the PLDs and their potential recipients provided high proportions of parents and older sisters or brothers as PLDs for their relatives.
The efficiency of the evaluation of PLDs should consider the needs of the PLD, the intended recipient, and the qualifications of the healthcare system. The timing of the evaluation of multiple PLDs is an important issue[12]. In the current study, only the early stages of evaluation, including counseling and blood group (ABO) compatibility testing, can be carried out simultaneously due to financial causes. In addition, high proportions of PLD decline occur in the early stages of evaluation[5]. Similarly, the current results showed that more than two-thirds of PLDs were declined during the early evaluation phases.
The causes of the decline of PLDs are various and can be classified into PLD-related and patient-related causes[13]. In the current study, the major PLD-related causes included medical, immunological, and sociodemographic factors or combinations of them. ABO and HLA incompatibilities were responsible for high percentages of excluded PLDs. Trials to expand the pool of LDs may need novel strategies, such as accepting LDs with abnormalities that are not accepted in the standard criteria for LDs. The variability of the causes of decline mandates the variability of these strategies. Hence, this practice should be implemented under strict control in LDKT programs because it may have impacts on preserving full safety issues[3,4,11]. On the same principle, such policies have not been permitted in our center protocols to avoid the violation of donor safety.
About half of the evaluated PLDs in the current study were excluded due to immunological causes, including both ABO and HLA incompatibilities. These immunological barriers can be managed by strategies such as incompatible LDKT and paired or exchange LKD (PKD)[14]. The former strategy is certainly not acceptable due to the relatively inferior outcomes compared to the matched ABO-compatible or HLA-compatible patients and the potential higher costs for desensitization[15]. However, PKD or kidney-sharing programs seem to be more effective for the KT programs that are based on the LDKT strategy due to their low costs and high efficiency. They are currently recommended to reduce the decline rate of PLDs, which may increase the acceptance rate of PLDs to more than 50% and provide better chances of finding high-quality donors for those who already have matched PLDs. They overcome considerable proportions of ABO-incompatible and HLA-incompatible PLDs. Unfortunately, these programs have not been established in our country so far, allowing a high rate of PLD loss. However, it has gradually become the focus of some interested researchers in our KT community[16,17].
The PLDs could be disqualified from donation for different medical contraindications. These reasons vary from one program to another program[3,5]. In the current study, medical contraindications were found in more than 60% of the causes of the decline of PLDs, similar to previously reported experiences[5]. They included several clinical forms, such as systemic diseases, infections, urolithiasis, and primary kidney diseases with hereditary or familial patterns. These reasons may benefit from the relaxation of the standard criteria for donation, such as accepting those PLDs with mild hypertension, obesity, proteinuria, sporadic urolithiasis, or microscopic hematuria[3,18].
Another right for PLD is autonomy, which provides the full capacity to preserve the ability to withdraw at any stage of preparation up to the date of surgery[19]. This right may contribute to the decline of PLDs due to withdrawal or regression from the decision to donate. In our study, 18 PLDs (7%) regressed from donation after confirmed initial willingness due to fear of health concerns, and 5 PLDs withdrew their decision after completion of the evaluation. Their potential recipients failed to find other suitable donors and remained on hemodialysis. Although it is disappointing to the potential recipients, PLD regression usually occurs in a small percentage of PLDs. However, it warrants the help of the team to support the PLDs in their decision and communicate with the potential recipient to deliver the decision[19]. Connaughton et al[5] reported that 15.5% of PLDs withdrew from donation during evaluation. A study by Liu et al[20] reported self-ranking health conditions, insufficient support in decision-making, value clarity, and conflicts in the decisions as factors of withdrawal.
For a PLD, withdrawal or being withdrawn indicates more time for finding another PLD. In our study, some patients found suitable donors after many trials among relatives. There is no doubt that this process was time-consuming, and the patients waited on dialysis for years. Also, some of them died while they were waiting for a donor. Moreover, the majority of the potential recipients are now still waiting on regular dialysis or have been transplanted in other places that mostly adopt the unrelated LDKT. The latter policy may predispose to such an unfavorable act of paid LKD. Hence, we preserved this policy for limited indications, such as cases of hereditary or familial primary renal diseases, including polycystic kidney disease. However, the in-depth discussion of the point of paid LKD is beyond the scope of this study.
Patient-related causes of the decline of PLDs included patient withdrawal from KT, non-candidacy, and death during preparation. We defined those PLDs as released because the intended recipients were disqualified. In an interview study by Pronk et al[21], patients expressed moral causes for regression from accepting related PLDs, such as reluctance to accept a kidney from close relatives and fear of being considered selfish.
The duration of PLD evaluation is variable and may be lengthy for repeating or confirmatory workups. Understanding the reasons that may prolong the evaluation may help reduce unnecessary delays[12]. The duration of evaluation in this study varied from 2 wk to 6 mo, with an average of 2.2 mo. This variation was due to the consideration of all PLDs with incomplete or complete evaluations.
Most of the potential recipients with declined PLDs remained on hemodialysis for variable periods ranging from 6 mo to 6 years. This means that the chances of achieving LDKT were reduced for those potential recipients when their PLDs were declined. Only 14 patients (6.6%) with declined PLDs succeeded in having LDKT in other centers, representing low chances of having LDKT similar to the results of previous studies[5,8,13]. In addition, patients with multiple PLDs did not significantly have higher chances of achieving KT. This might be attributed to the healthcare system implemented in our country, where most patients sought medical consultations in private clinics, and their PLDs had initial evaluations with their private physicians before the presentation to our center. PLDs with known systemic diseases and ABO typing can easily be excluded. In turn, this may be an explanation for the presentation of a single PLD in 77.8% of patients.
The outcomes of the current study should attract attention to the formulation of efficient plans to reduce the rates of decline of PLDs and promote LKD through the introduction of strategies such as PKD to our national program. Also, national initiatives for the education of the public, patients, and general practitioners about the advantages of LDKT and LKD may help reduce the decline of PLDs caused by the reluctance and low medical literacy of those individuals.
To the best of our knowledge, the current study is the first from Egypt that specifically addressed the topic of the decline of PLDs among related PLDs and recipients. This is a very important step in the development of an integrated national KT program, which has only been dependent on LKD until now. The current study may encourage other centers to conduct similar studies to provide better evidence of the problem and formulate a plan to overcome the causes of the decline of PLDs. In addition, the aim, rationale, and outcomes of this study were parallel to many studies from different countries[3,5,6,8,13], which may strengthen the effect of its outcomes on the improvement of our practice and healthcare system management.
The limitations of this study included the retrospective nature of the methods. The retrospective nature may be the reason that the regression of PLDs and their recipients was not reported in detail. It is unknown whether these events were due to improper counseling, sociodemographic characteristics, or the low integrity of the healthcare system. In addition, a relatively short follow-up period limited the evaluation of the long-term effect of the decline of PLDs on the fate of some intended recipients. Moreover, it was a single-center experience, which warrants further national or multicenter studies for the generalizability of these results. However, most of the available literature comes from retrospective single-center studies[3,5,8,13].
The willing, related PLDs have a mean age higher than their potential recipients due to relatedness; most of them were parents or older relatives. Also, their potential recipients had primary kidney diseases that typically affect young people. The rate of decline of the willing, related PLDs was high, reaching about 82%. The causes could be classified as PLD-related or potential recipient-related, depending on the side of the cause. In addition, they could be differentiated into exclusion due to contraindications, release after disqualification of the potential recipients, and regression due to withdrawal of the decision by the PLDs, based on the autonomy of decision-making. PLD exclusion was the commonest form during or after the completion of the evaluation due to medical or immunological contraindications. These high percentages of PLD decline resulted in the loss of the chance to obtain LDKT for a high percentage of potential recipients who were left on dialysis for variably long periods, who died, or who were lost to an unknown fate. In our country, this study represents an initial scientific step in the evidence-based evaluation of the situation of LD selection and its deficits. The high rate of decline of PLDs reported here may draw attention to implementing more research on this topic.
The evaluation protocols for living kidney donor (LD) selection are usually strict but remain a safeguard against violations of LD safety. Hence, the decline of willing potential living donors (PLDs) may occur at any stage of evaluation due to different causes, resulting in variable rates of decline of PLDs.
The rate of decline of willing related LDs seems to be a modifiable variable for improving LD kidney transplantation (LDKT).
To identify the causes of the decline of PLDs, the predictors of PLD candidacy, and the effect on achieving LDKT.
A retrospective study was performed on willing PLDs who attended our outpatient clinic for kidney donation to their related potential recipients between October 2015 and December 2022. Two groups of PLDs were compared: Candidate PLDs after a completed evaluation vs non-candidate PLDs with a complete or incomplete evaluation. A multivariate logistic regression was performed to assess the factors contributing to the achievement of PLD candidacy.
Of 321 willing PLDs, 257 (80.1%) accessed the evaluation to variable extents for 212 potential recipients, with a mean age (range) of 40.5 ± 10.4 (18-65) years. The remaining 64 PLDs (19.9%) did not access the evaluation due to serving as alternatives to essential PLDs, financial causes, and patient-related factors. Only 58 PLDs (18.1%) achieved donation, but 199 PDLs (62.0%) were declined. Exclusion occurred in 144 PLDs (56%) for immunological causes (37.5%), medical causes (54.9%), combined causes (9.7%), and financial causes (2.1%), but regression and release occurred in 55 PLDs (17.1%). The number of potential recipients with candidate PLDs was not significantly different from that with non-candidate PLDs, except in age (P = 0.041), rates of completed evaluation, and exclusion of PLDs (P < 0.001). In the multivariate analysis, there were no independent factors that influenced the rate of PLD candidacy. Most patients who failed to have KT after the decline of their PLDs remained on hemodialysis for 6 mo to 6 years.
Despite the availability of willing related PLDs for most potential recipients, their rate of decline was high. The causes were various, including medical or immunological contraindications, release, and regression of PLDs. Hence, the chances of LDKT were reduced or lost in a high percentage of potential recipients.
Trials to reduce the rate of decline of PLDs should not be at the expense of LD safety. However, revision and identification of the causes of PLD decline may help increase the chances of patients for KT, especially with the application of strategies that overcome the immunological barriers of LDKT and low medical literacy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Cabezuelo AS, Spain; Sarier M, Turkey; Ghazanfar A, United Kingdom S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 565] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Simforoosh N, Shemshaki H, Nadjafi-Semnani M, Sotoudeh M. Living related and living unrelated kidney transplantations: A systematic review and meta-analysis. World J Transplant. 2017;7:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Kim IK, Tan JC, Lapasia J, Elihu A, Busque S, Melcher ML. Incidental kidney stones: a single center experience with kidney donor selection. Clin Transplant. 2012;26:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Gambaro G, Zaza G, Citterio F, Naticchia A, Ferraro PM. Living kidney donation from people at risk of nephrolithiasis, with a focus on the genetic forms. Urolithiasis. 2019;47:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Connaughton DM, Harmon G, Cooney A, Williams Y, O’Regan J, O’Neill D, Cunningham P, Counihan A, O’Kelly P, McHale S, Denton M, O’Seaghdha CM, Magee C, Conlon P, Little D, Keogan M, de Freitas DG. The Irish living kidney donor program - why potential donors do not proceed to live kidney donation? Clin Transplant. 2016;30:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Arunachalam C, Garrues M, Biggins F, Woywodt A, Ahmed A. Assessment of living kidney donors and adherence to national live donor guidelines in the UK. Nephrol Dial Transplant. 2013;28:1952-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Skrunes R, Svarstad E, Reisæter AV, Vikse BE. Familial clustering of ESRD in the Norwegian population. Clin J Am Soc Nephrol. 2014;9:1692-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Lapasia JB, Kong SY, Busque S, Scandling JD, Chertow GM, Tan JC. Living donor evaluation and exclusion: the Stanford experience. Clin Transplant. 2011;25:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Rashad H, Fahmy A, Eissa A, Elsherbiny A. Committee XII: Renal Transplantation. In: Mourad S, Shalaby M, El Halaby, Morsy A, Elgamasy AN, Hamouda H. Egyptian Urological Guidelines Book. [cited 1 April 2023]. Available from: http://eug-eg.net/. |
| 10. | Gadelkareem RA, Abdelgawad AM, Reda A, Azoz NM, Zarzour MA, Mohammed N, Hammouda HM, Khalil M. Preemptive living donor kidney transplantation: Access, fate, and review of the status in Egypt. World J Nephrol. 2023;12:40-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (5)] |
| 11. | Romagnoli J, Salerno MP, Mamode N, Calia R, Spagnoletti G, Bianchi V, Maresca M, Piccirillo N, Putzulu R, Piselli P, Cola E, Zini G, Citterio F. Expanding the Living Donor Pool "Second Act": Laparoscopic Donor Nephrectomy and ABO-Incompatible Kidney Transplantation Improve Donor Recruitment. Transplant Proc. 2015;47:2126-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Habbous S, Arnold J, Begen MA, Boudville N, Cooper M, Dipchand C, Dixon SN, Feldman LS, Goździk D, Karpinski M, Klarenbach S, Knoll GA, Lam NN, Lentine KL, Lok C, McArthur E, McKenzie S, Miller M, Monroy-Cuadros M, Nguan C, Prasad GVR, Przech S, Sarma S, Segev DL, Storsley L, Garg AX; Donor Nephrectomy Outcomes Research (DONOR) Network. Duration of Living Kidney Transplant Donor Evaluations: Findings From 2 Multicenter Cohort Studies. Am J Kidney Dis. 2018;72:483-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | AlBugami MM, AlOtaibe FE, Boqari D, AlAbadi AM, Hamawi K, Bel’eed-Akkari K. Why Potential Living Kidney Donors Do Not Proceed for Donation: A Single-Center Experience. Transplant Proc. 2019;51:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kher V, Jha PK. Paired kidney exchange transplantation - pushing the boundaries. Transpl Int. 2020;33:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | de Weerd AE, Betjes MGH. ABO-Incompatible Kidney Transplant Outcomes: A Meta-Analysis. Clin J Am Soc Nephrol. 2018;13:1234-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Elrggal ME, Tawfik M, Gawad MA, Sheasha HA. Kidney paired donation program, a national solution against commercial transplantation? J Egypt Soc Nephrol Transplant. 2018;18:6-10. [DOI] [Full Text] |
| 17. | Hassaballa MA. Kidney Paired Donation. Exp Clin Transplant. 2022;20:59-61. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Young A, Storsley L, Garg AX, Treleaven D, Nguan CY, Cuerden MS, Karpinski M. Health outcomes for living kidney donors with isolated medical abnormalities: a systematic review. Am J Transplant. 2008;8:1878-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Grossi AA, Sever MS, Hellemans R, Mariat C, Crespo M, Watschinger B, Peruzzi L, Demir E, Velioglu A, Gandolfini I, Oniscu GC, Hilbrands L, Mjoen G. The 3-Step Model of informed consent for living kidney donation: a proposal on behalf of the DESCaRTES Working Group of the European Renal Association. Nephrol Dial Transplant. 2023;38:1613-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Liu KL, Wang HH, Hsieh CY, Huang XY, Lin CT, Lin KJ, Chiang YJ, Chien CH. Kidney Donation Withdrawal and Related Factors Among the Potential Donors of Living Kidney Transplant. Transplant Proc. 2020;52:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Pronk MC, Slaats D, Zuidema WC, Hilhorst MT, Dor FJMF, Betjes M, Weimar W, van de Wetering J, Massey EK. "What if this is my chance to save my life?" A semistructured interview study on the motives and experiences of end-stage renal disease patients who engaged in public solicitation of a living kidney donor. Transpl Int. 2018;31:318-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |