Published online Jun 18, 2023. doi: 10.5500/wjt.v13.i4.107
Peer-review started: March 22, 2023
First decision: April 11, 2023
Revised: May 16, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: June 18, 2023
Processing time: 86 Days and 0.6 Hours
Pancreatic islet transplantation is a minimally invasive procedure aiming to reverse the effects of insulin deficiency in patients with type 1 diabetes (T1D) by transplanting pancreatic beta cells. Overall, pancreatic islet transplantation has improved to a great extent, and cellular replacement will likely become the mainstay treatment. We review pancreatic islet transplantation as a treatment for T1D and the immunological challenges faced. Published data demonstrated that the time for islet cell transfusion varied between 2 and 10 h. Approximately 54% of the patients gained insulin independence at the end of the first year, while only 20% remained insulin-free at the end of the second year. Eventually, most transplanted patients return to using some form of exogenous insulin within a few years after the transplantation, which imposed the need to improve immunological factors before transplantation. We also discuss the immunosuppressive regimens, apoptotic donor lymphocytes, anti-TIM-1 antibodies, mixed chimerism-based tolerance induction, induction of antigen-specific tolerance utilizing ethylene carbodiimide-fixed splenocytes, pretransplant infusions of donor apoptotic cells, B cell depletion, preconditioning of isolated islets, inducing local immunotolerance, cell encapsulation and immunoisolation, using of biomaterials, immunomodulatory cells, etc.
Core Tip: Type 1 diabetes (T1D) is associated with loss of beta-cell mass and insulin secretion. Regardless of its nature, autoimmune or idiopathic, the loss of own insulin secretion is a hallmark dysfunction in T1D mellitus; thus, therapeutic options are aimed at either replacing the missing insulin or restoring physiological insulin secretion to achieve normoglycemia and postponing micro- and macrovascular complications. Nevertheless, the need to completely replace the depleted pancreatic secretion also leads to the emergence of new therapeutic horizons, including pancreas and islet cell transplantation. However, this approach also meets several immunological challenges-cellular and antibody-mediated rejection and loss of function. To improve the outcomes, several approaches are performed: Immunosuppression, apoptotic donor lymphocytes, anti-TIM-1 antibodies, mixed chimerism-based tolerance induction, induction of antigen-specific tolerance utilizing ethylene carbodiimide-fixed splenocytes, infusion of donor apoptotic cells before transplantation, combined with anti-CD40L antibodies and rapamycin, preconditioning of isolated islets, inducing local immunotolerance, cell encapsulation and immunoisolation, using of biomaterials, immunomodulatory cells, etc. mesenchymal stem cells, as an adjunct therapy to islet transplantation, can promote long-term graft survival, possibly by reducing inflammation and enhancing immune tolerance.
- Citation: Kabakchieva P, Assyov Y, Gerasoudis S, Vasilev G, Peshevska-Sekulovska M, Sekulovski M, Lazova S, Miteva DG, Gulinac M, Tomov L, Velikova T. Islet transplantation-immunological challenges and current perspectives. World J Transplant 2023; 13(4): 107-121
- URL: https://www.wjgnet.com/2220-3230/full/v13/i4/107.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i4.107
Pancreatic islet transplantation is a minimally invasive procedure aiming to reverse the effects of insulin deficiency by transplanting pancreatic beta cells[1]. Pancreatic islet transplantation can be done with autologous and allogeneic islets. While autologous islet transplantation has the advantage of being derived from the same patient, eliminating the risk of immune rejection, its widespread utilization is limited due to several drawbacks, including the need for pancreatectomy, which may have associated surgical risks, and the limited availability of functional islets from a single organ in patients with advanced disease. On the other hand, allogeneic islets are taken from different individuals of the same species, usually for treating type 1 diabetes (T1D), with followed immunological response complications[2].
Typical for T1D is the continuing pancreatic beta cell destruction, which could be autoimmune (Type 1A) or non-autoimmune (Type 1B), resulting in decreased or absent insulin production. As a result, it increases in incidence yearly and is associated with severe hypoglycemia, ketoacidosis, and vascular complications[3]. Although exogenous insulin analogs are considered the primary treatment option for managing T1D in response to hyperglycemia, they cannot accurately resemble the timing and dosing of physiological insulin secretion. Moreover, exogenous insulin therapy is associated with an increased risk of severe side effects such as hypoglycemia, weight gain, lipodystrophy, etc.[4]. Therefore, there is an ongoing effort to improve the treatment options[5]. Among them, pancreatic islet transplantation is promising to become the mainstay in the treatment process[6].
As a minimally invasive procedure, islet transplantation is ideal for high-risk surgical patients burdened with cardiovascular disease[7]. It does not follow the significant complications of vascularized pancreas transplantation, and with minimal intra-operational complications, such as bleeding and portal vein thrombosis, the mortality is negligible. On the negative side, multiple donors for a single patient are needed, while the alternative whole pancreatic transplantation treatment needs 1 and rarely 2 pancreases. This makes it a rather wasteful procedure[8]. An adequate islet number must be transplanted for patients to become insulin-independent. A single transplantation is often insufficient; several sequential transplantations are needed for satisfactory glycaemic and insulin results[9]. Early attempts had been made as early as 1893. Still, the milestone that grabbed the scientific community's attention was the ground-breaking Edmonton protocol, with its non-corticosteroid immunosuppressive treatment[9] and the other studies regarding the benefits of islet transplantation on glucose metabolism improvement[10,11]. Studies have shown that 5-year insulin independence has increased manifold[12-14].
Overall, pancreatic islet transplantation has improved to a great extent, and cellular replacement will likely become the mainstay treatment. Our goal was to review pancreatic islet transplantation as a treatment for T1D and the immunological challenges faced. To prepare this narrative review, we search the main databases, Medline, PubMed, and Scopus, in conformity with the principles of writing a narrative review[15].
The main procedural steps are pre-transplant assessment, pancreas procurement, islet isolation, tissue culture, transplantation, and post-transplant evaluation[8].
In pre-transplant assessment, eligible patients are chosen. Strong indications include recurrent severe hypoglycemic shocks, impaired awareness of hypoglycemia, undetectable C-peptide, age between 18-65, and a diagnosis of more than five years[16]. Additionally, previous kidney transplantation has been shown to impact the outcomes of islet transplantation positively. Studies have reported that patients who have undergone a kidney transplant before islet transplantation have higher graft survival rates, improved glycemic control, and reduced insulin requirements compared to those without a prior kidney transplant. This may be attributed to the immunosuppressive regimen used for kidney transplantation, which may enhance the success of islet transplantation by preventing the rejection of the transplanted islets[17]. Exclusion criteria include poorly controlled hypertension, heart disease, macroalbuminuria, glomerular filtration rate < 80 mL/min/1.73 m2 and potential contraindications for immunosuppression. Current indications do not include the pediatric population[18]. In the transplantation of allogeneic pancreatic beta cells, ABO and human leucocyte antigen histocompatibility have to be assessed. The number of islet donors is generally limited, but new xenografts with islets from other species, typically porcine islets, and stem cell technologies could tackle this critical problem[19].
In the stage of pancreas procurement, the pancreas is removed from donors and preserved in the University of Wisconsin solution for up to 24 h. Important in this stage is the capsule to be kept intact. The pancreas is delivered to the islet isolation center when procurement is ready[20]. The islet isolation process involves the preparation of the pancreas, which is carefully cleaned of surrounding tissues and dissected to expose the islets of Langerhans. The pancreas is then cannulated and perfused with a collagenase enzyme solution for 10 min, which distends the pancreas to facilitate the separation of the islets from the surrounding stroma. Next, the distended pancreas is cut and set into the Ricordi Chamber, an automated device designed to facilitate the islet isolation process. The chamber employs a series of automatic steps to separate the islets from the exocrine tissue, including filtration and density gradient centrifugation. Finally, the isolated islets are processed using a COBE 2991 cell processor, which further separates the islets from any residual exocrine tissue, and the purified islets are then cultured for transplantation[21,22].
In the hands of the proper expert, the tissue culture stage of islet isolation represents a critical step in preparing isolated islets for transplantation. This stage allows the islets to recover from the stress induced by the previous steps of the isolation procedure, during which they may have been subjected to mechanical and enzymatic stress. The tissue culture stage typically involves the placement of the purified islets into a nutrient-rich media in a controlled environment, where they are allowed to recover for several hours to several days. During this time, the islets are carefully monitored for signs of viability and function, including assessment of insulin secretion and glucose-stimulated insulin release. This stage also allows for flexibility in scheduling the subsequent transplant procedure, as the islets can be stored under optimal conditions until the transplant recipient is ready to receive them. The success of the tissue culture stage is highly dependent on the expertise of the individual performing the procedure, as optimal conditions must be maintained to ensure the viability and function of the isolated islets[23].
Before transplantation begins, the final transplantation islet site has to be decided. The liver is considered preferable for transplantation, although different places are being tested for better islet survival and function[24]. Upon islet infusion, an "instant blood-mediated inflammatory reaction" is described with platelet consumption, activation of coagulation, and the complement system[25].
The post-transplant period following islet transplantation is characterized by a prolonged period of recovery during which insulin independence may not be immediately achieved. The transplanted islets may take months to years to fully integrate into the recipient's body[6] and establish a functional vascular supply. During this period, the transplanted islets are subject to immunological attacks from the recipient's immune system, which can compromise their function and survival. A combination of induction, maintenance, and antirejection immunosuppressive drugs are typically used to prevent rejection of the transplanted islets. However, a notable irony is that many of these immunosuppressive drugs have diabetogenic properties, which can exacerbate preexisting metabolic abnormalities in transplant recipients. As such, these drugs must be carefully balanced against the need to maintain optimal islet function and prevent rejection[26].
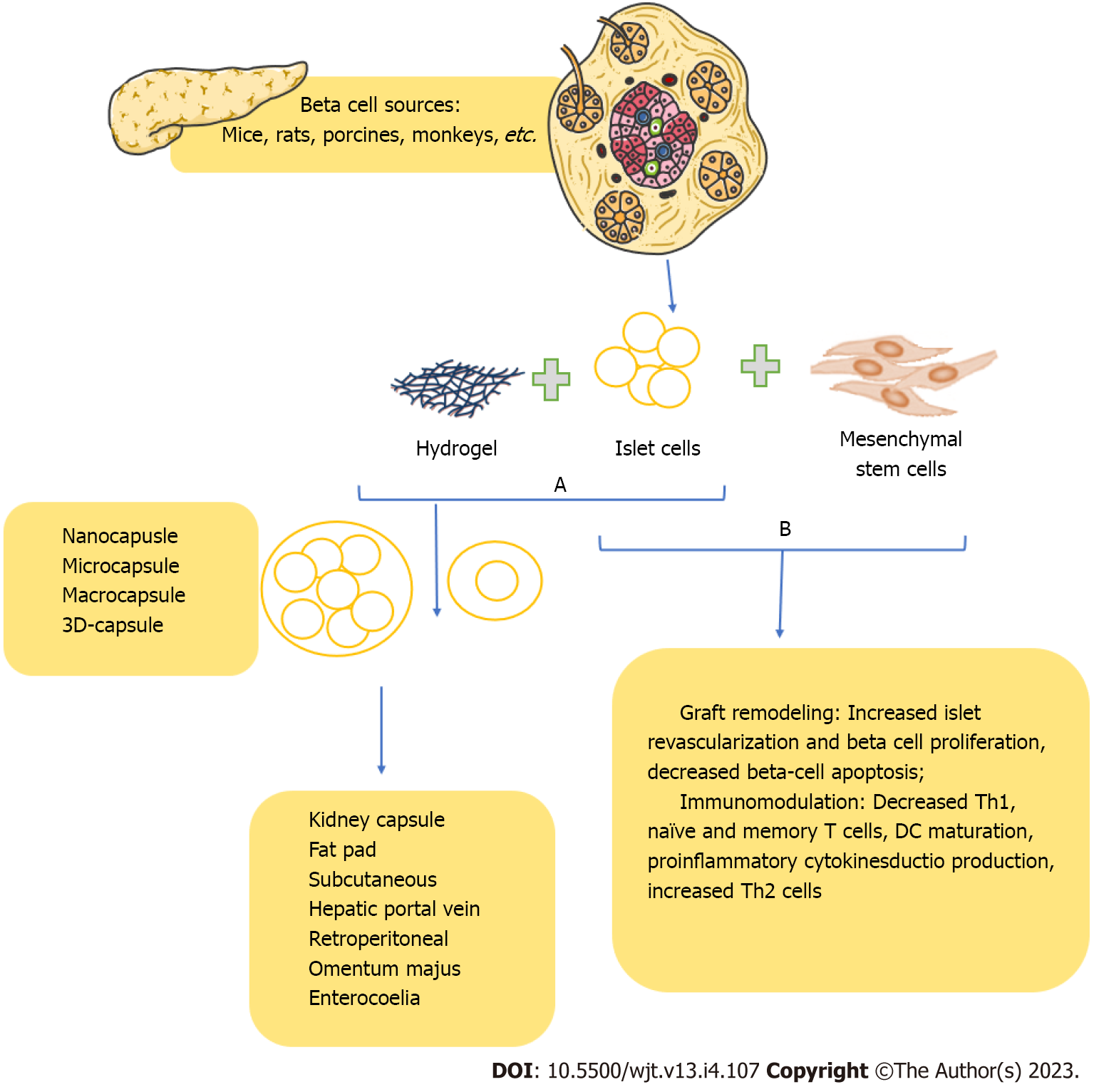
Recent advances in islet transplantation have focused on immunoisolation, which involves the encapsulation of transplanted islets in a protective membrane to prevent their recognition and subs
For example, alginate hydrogels are commonly used due to their biocompatibility, ease of fabrication, and ability to protect transplanted islets from the immune system. While early studies in small animal models have shown promising results, with sustained islet function and reduced immunosuppressive drug requirements, translation to larger animals and humans has been less successful, with limited long-term success and significant technical challenges in maintaining membrane integrity and permeability. Using traditional immunosuppressive regimens remains a crucial component of current islet transplantation protocols, albeit with the recognized risks of diabetogenicity and other adverse effects[11].
Type 1 diabetes mellitus (T1DM) is a metabolic disease distinct by hyperglycemia, insulin deficiency, and a lifelong need for exogenous insulin replacement treatment[3,29]. T1DM is an autoimmune disease that develops in genetically predisposed individuals under the influence of environmental factors, which triggers autoimmunity to pancreatic beta cells. Although it is defined as "diabetes of young age", T1DM can also affect adults[30]. In general, T1DM is divided into two subtypes, 1A and 1B[31]. While T1ADM is associated with autoantibodies against islet cells [glutamic acid decarboxylase (anti-GAD65), tyrosine phosphatases islet antigen 2 (IA-2), IA-2β insulin, or zinc transporter 8[32], also observed in patients with T2D[33], T1BDM, in turn, is a relatively small subtype that is not mediated by the immune system and has an unclear genesis.
T1DM is related to other autoimmune conditions such as celiac disease[34,35], Hashimoto thyroiditis, Addison's disease, pernicious anemia, etc.[36]. Moreover, patients with diabetes may have a comp
Regardless of the subtype, the loss of insulin secretion is a hallmark dysfunction in T1DM, and therapeutic options aim to replace the missing insulin or restore physiological insulin secretion to achieve normoglycemia and prevent micro- and macrovascular complications. Within the last few years, we have seen a rapid evolution in the therapy of T1DM[39]. First, tangible progress marked the discovery of insulin in 1921-22 by Banding and Macleod, saving from certain death children with diabetes. The subsequent development of new analog insulins with a better therapeutic and safety profile results in better control of hyperglycemia and a reduced risk of hypoglycemia, respectively. The introduction of insulin pumps with continuous subcutaneous insulin administration[40] and the implementation of modern technologies in diabetes control with continuous glucose monitoring systems combined with glucose prediction algorithms enabling the development of artificial pancreas delivery systems[41] marks extraordinary progress in managing T1DM.
Nevertheless, the need to completely replace the depleted pancreatic secretion also leads to the emergence of new therapeutic horizons, including pancreas and islet cell transplantation. They allow not only to achieve independence from exogenous insulin administration and the need to monitor blood sugar but also successfully to afford counterregulatory hormone secretion and pancreatic exocrine function[42].
T1DM was thought to be a T cell-mediated autoimmune illness for many decades. This belief persists, but multiple recent discoveries hint at a role for beta cells beyond being a non-provoking victim of an autoimmune onslaught[38].
The interaction between genetic vulnerability and probable triggers is likely to begin at a young age, gradually leading to the loss of tolerance to self and, eventually, the development of clinical symptoms. The result is determined by genetic predisposition, decreased removal of the apoptotic cell remains, altered immune regulation, and environmental triggers (i.e., viral infections). In addition, autoreactivity may exist under physiological settings, and illness may arise if the integrity of the complicated regulatory process is compromised[43].
The beta cells are destroyed by islet-infiltrating cells (i.e., CD8+ cytotoxic lymphocytes and macrophages), resulting in insulitis. In addition, macrophages release cytokines that are harmful to beta cells. Secondary considerations are autoantibodies, which serve as the foundation for clinical diagnosis[43].
Initially, B lymphocytes are known to play a secondary role in T1DM that even occurs in severe congenital B-lymphocyte immunodeficiency[44]. Xiu et al[45] considerably delayed disease development in NOD mice by depleting B-lymphocytes using an anti-CD20 antibody. They concluded that this was not due to T effector cell reduction or T regulatory (Tregs) induction but rather to a decrease in the development of autoreactive T cells[45].
However, autoreactive T cells are part of the typical T cell repertoire. In T1D, beta cells live in an inflammatory environment and participate in their destruction. Additionally, metabolic activity is what causes beta cell malfunction and destruction. Insulitis is characterized by inflammation, associated with substantial metabolic, epigenetic, and autoantigenic alterations that expose beta cells to the immune system[46]. In line with this, immunotherapy may be insufficient to treat T1D, although beta cell therapy may help reduce beta cell immunogenicity and islet autoimmunity[47].
It was demonstrated recently that innate immunity components might play a role in T1D pathogenesis, such as pattern recognition receptors and proinflammatory cytokines[47]. Nevertheless, the accompanying inflammation of the islets leads to damaged beta cells and loss of insulin production.
Animal studies (i.e., non-obese diabetic mice) and human studies in T1D revealed defects in thymic selection, expansion of effector T cells, impaired homeostasis and FoxP3+ Tregs[48]. However, even if we accept the immune system's role in the development of T1D, science cannot assume that the disease is entirely a result of dysfunctional immunity, i.e., autoreactive T cells. Recent research focuses on the participation of the peripheral immune system in the targeted tissue and the role of beta cells in the autoimmune process[49,50].
Indeed, when we accept this conception, it was demonstrated that T1D is usually characterized by less beta-cell mass, functional capacity and inability to control glycemia. Usually, beta cells undergo metabolic stress, inflammatory environment and other factors that increase the expression of specific adhesion molecules and other receptors, making them prone to immune attacks[46].
Islet transplantation has been considered a potential cure for T1D by replacing the damaged beta cells. However, its effectiveness is dependent on the underlying cause of the disease. For example, if T1D results from a pancreatic dysfunction leading to the loss of beta cells, then islet transplantation may be a viable option. However, if T1D is viewed as an autoimmune disorder, the presence of autoreactive T and B cells can lead to the disease's recurrence and limit the transplantation's efficacy[47]. In such cases, alternative approaches such as immunomodulatory therapies, co-transplantation with immune cells, or encapsulation of islets can be explored to improve the success rate of islet transplantation.
Patients with T1D or pancreatogenic (type 3c) diabetes (also known as insulin-deficient) may benefit from islet isolation from a deceased donor followed by transplantation of allogeneic islets in the liver. This can help alleviate hypoglycemia while stabilizing glycemic lability, and maintaining glycemic control, ultimately improving quality of life and frequently eliminating the need for insulin therapy. Replacement of islet function by transplantation addresses the underlying pathophysiology of long-standing T1D with sub-total annihilation of islet alpha-cells and the associated loss of the alpha-cell response to hypoglycemia[19]. This allows for the avoidance of hypoglycemia and stabilization of glycemic lability, which would otherwise contribute to impaired awareness of hypoglycemic states. Patients with T1D uncontrolled hyperglycemia, demonstrated by the recurring episodes of diabetes-associated ketoacidosis or quickly progressing severe complications related to the disease, might also benefit from islet transplantation[51,52].
Patients with T1D complicated by an allergy or resistance to insulin that is administered subcutaneously are a rare but essential indication for this treatment[53]. Finally, alloislets (from a viable allograft pancreatectomy) re-transplantation has been successfully executed in a patient with T1D who was initially given the pancreas transplant for hypoglycemia unawareness. Similarly, a T1D patient received simultaneous pancreas/kidney transplantation complicated by pancreas graft arterial anastomosis bleeding[54]. Notably, the degree of glycemic control achieved within the first five days after surgery determines the chances of accomplishing long-term insulin independence[55].
We analyzed the literature data published on islet transplantation focusing on the clinical outcomes[55-60]. Our results have been summarized in Table 1. The total number of included patients was 372. We established that the time for islet cell transfusion varied between 2 and 10 h. Approximately 54% of the patients gained insulin independence at the end of the first year, while only 20% remained insulin-free at the end of the second year. Most patients have received islet cells in the liver, and only 38 patients have IC harvested in the spleen. Another interesting fact we discovered was the high percentage of opioid-free patients after this intervention.
| Ref. | Patients (n) | Time of infusion of islets (h) | HbA1c, 1-yr, median (%) | HbA1c, 2-yr, median (%) | Insulin independence 1-yr, median (%) | Insulin independence 2-yr, median (%) | IEQ harvested/g pancreas, median (range) | IEQ transplanted/g pancreas, median (range) | Opioid and pain-relieving | Organ placement |
| Sutherland et al[56] | 173 | 2-7 | NR | NR | 32 | 24 | < 1000 IE/kg | (> 5000, 2500-5000 and < 2500 IE/kg) | NR | 173 liver |
| Ahmad et al[57] | 45 | 7-10 | NR | NR | 40 | NR | NR | 297889 ± 49480 | 72% | 45 liver |
| Rodriguez Rilo et al[58] | 22 | 9 | NR | NR | 41 | NR | 245457 (range 20850 to 607466-175234) | 350428 (range 31500 to 1164000-299321 | 82% | 22 liver |
| Webb et al[59] | 46 | NR | 7 | 6.7 | 12 | 5 | 1876 (249-12271) | I130029 (24332-958078) | NR | 42 liver; 2 spleen; 2 both |
| Garcea et al[60] | 50 | NR | Approximately 6 | Approximately 6 | 24 | 10 | NR | NR | 60% | 85 liver |
| Johnston et al[55] | 36 | 8-9 | NR | 6.8 | 50 | 33 | 358959 (45000–672000) | 4308 (769–9942) | 30% | 36 spleen |
Unfortunately, the Collaborative Islet Transplant Registry reported 71% insulin independence in the first year and 24% in the third from the islet transplant centers[61]. Eventually, most transplanted patients need exogenous insulin within a few years after the transplantation[62].
Some additional factors can also improve the outcomes after islet transplantation. For example, experiments in mice and rats with Vitamin D show promising results on glycemia and tumor necrosis factor-α (TNF-α) production in islet transplantation[63]. In addition, analogs of vitamin D3 are shown to prevent the autoimmune destruction of transplanted islets in non-obese mice[64]. This is a promising direction for research on humans due to the well-known anti-inflammatory effects of vitamin D3 in vivo[65,66].
At this point, the main complication after allogeneic islet transplantation is the chronic rejection conducted by activated T cells. This is also the main barrier to accomplishing long-term engraftment. One of the ways to maintain immune tolerance to the allograft is to administer immunosuppression[67].
However, this could be toxic for the islet grafts, leading to worsening long-term function of the islets, increased risk of infections, development of cardiovascular and renal diseases, de novo diabetes, neurotoxicity and malignancies[68].
The ultimate goal of islet transplantation is to achieve donor-specific immune tolerance. A recently proposed method for tolerance induction using apoptotic donor lymphocytes (ADLs) in animal models (i.e., non-human primates)[69]. ADLs employ clonal depletion, anergy, expansion of Treg cells, regulatory B cells (Bregs), etc. Usually, these mechanisms act together to induce and maintain tolerance. However, this approach also meets several challenges.
Initially, the immune rejection after transplantation starts with innate immune cells infiltration into the islet grafts (i.e., macrophages), followed by donor-specific lymphocyte response, consisting of T cells (CD4+ and CD8+) and B cells. In line with this, the protocol comprised of T cell depletion and anti-TNF agents may enhance short-term graft survival[67]. However, this protocol has a significant drawback-it cannot modulate antibody-mediated rejection[70,71].
Targeting Bregs (i.e., low-affinity antibodies against TIM-1, essential for Breg development) results in considerably longer islet cell survival (about 30% of mice attained engraftment over 3 mo)[72]. Surprisingly, anti-TIM-1 treatment of B cell-depleted recipients significantly increased interferon-γ and prevented the typically seen rise in Th2 cytokines[72].
Furthermore, in a mouse islet transplant model, a combination of anti-CD45RB and anti-TIM-1 antibodies synergized in establishing tolerance in all recipients. Depending on the presence of interleukin (IL)-10-producing B cells in the recipient, the combined antibody therapy significantly increased the regulatory lymphocytes[73]. Furthermore, the study implied that B cells expressing CD19 and TIM-1 are part of tolerance development and maintenance. These results might clarify why B cell reduction decreased the effectiveness of dual antibody therapy.
Cross-reactive memory T and B cells could substantially impede immunological tolerance in animals and humans after transplantation. However, tolerance development in non-human models or humans would be more complex than in rat models, owing to cross-reactive memory immune cells. Yet, a few hopeful treatments exist, such as mixed chimerism through hematopoietic cell transplantation[74,75] or ADL exposure[76], which have led us to anticipate that immune tolerance can eventually be attained in people.
Oura et al[77] published the results of a non-human islet transplantation model where a nonmyeloablative condition regimen induced the mixed chimerism-based tolerance. The latter consisted of total body irradiation, and administration of horse anti-thymocyte globulin, monoclonal antibodies (i.e., anti-CD154, anti-CD8, etc.), or cyclosporine (the so-called calcineurin inhibitor-free regimen)[77]. As a result, temporary chimerism did not prompt tolerance to increase the islet graft survival. Eventually, the islet stopped functioning shortly after chimerism disappeared[77]. Oura et al[77] also found that islet recipients had greater levels of inflammatory cytokines (i.e., TNF-α and IL-17) in blood circulation than kidney recipients[77]. This study implies that excessive levels of inflammatory mediators following islet transplantation may impede islet graft tolerance induction. Since isolated islet grafts could induce a significant systemic inflammatory response, this should be the focus of future research to improve tolerance development and graft survival.
Induction of immune tolerance utilizing ethylene carbodiimide (ECDI)-fixed splenocytes in combination with particular antigens or peptides is a method used in transplantation models, including islet transplantation. Kheradmand et al[78] demonstrated various mechanisms (i.e., anergy, clonal depletion, employment of Tregs, etc.) via donor ECDI-fixed splenocytes administration. These splenocytes possess direct and indirect allospecificities that target allogeneic host responses. These mechanisms act synergistically to cause tolerance after transplantation[78]. In addition, Tregs and myeloid-derived cells that exert immunosuppression are activated and increased in number in the case of ECDI-fixed splenocytes infusion[79].
Allotransplantation in sensitized patients with pre-formed donor-specific memory lymphocytes and antibodies increases the risk of allograft rejection. Dangi et al[80] showed that administration of donor apoptotic cells, anti-CD40L antibodies, and rapamycin before transplantation resulted in a considerable extension of islet graft in allosensitized patients (median survival time, 35 d)[80]. Sato and Marubashi[69] confirmed that invading B lymphocytes play an essential part in the chronic rejection of the islet graft by stimulating local T cells. Therefore, ECDI-fixed splenocytes from the donor infused into sensitized recipients efficiently reduced alloreactive B cells. However, the latter could be switched by contemporary B cell invasion into the graft. As a result, in B cell-depleted patients, a method to regulate concurrent B cell invasion is required[69].
Moreover, islet grafts might be more resistant to immunological tolerance induction. Compared to kidney grafts, the considerably increased immunogenicity of islet grafts may impede tolerance induction in islet transplantation[77]. Islet grafts have relatively strong cytokine secretion activity because pancreatic islets are endocrine cells. Furthermore, cell stressors during the isolation process cause islet inflammation, increasing the immunogenicity of the islet graft before transplantation.
These conclusions imply that the stress during the separation method activates the proinflammatory gene program. Islet isolation entails many steps, including pancreatic distention, digesting with collagenase, and purification. Therefore, the islets should be injured throughout each phase by hypoxia and heated ischemia, production of activated proteolytic enzymes by acinar cells, and oxidative and mechanical stress[69].
According to estimates, around half of the transplanted islets are irreparably destroyed around the transplantation period (from hours to days). In addition, more than a quarter of islet grafts are known to be lost shortly after the portal vein infusion[81]. Therefore, the initial inflammatory response is crucial in instant transplanted islet loss due to immediate blood-mediated inflammatory reaction (IBMIR). During IBMIR, coagulation pathways are activated, proinflammatory cytokines are produced, and innate immune cells infiltrate the graft[82], all contributing to the islet's acute cell-mediated damage. Additionally, IBMIR is distinguished by coagulation and complement systems activation, fast activation and binding of platelets and leukocyte recruitment and infiltration[83].
Preconditioning isolated islets with sublethal genotoxic stress may be a potential technique for lowering islet immunogenicity and extending islet transplant life. It is reasonable to believe that preconditioning therapy for reducing graft immunogenicity will synergistically impact tolerance induction therapy, including the ADL regimen[69].
Applying the cellular treatment is a novel approach to induce local immunotolerance and avoid islet rejection. In addition, the administration of stem cell-derived beta cells during islet transplantation improves graft performance while reducing the negative consequences of systemic immunosuppression. Recent advances in T1D cell replacement treatments (i.e., non-encapsulation and local immunomodulatory techniques) are addressed in this concise review[84]. They include alteration of islet/cell, use of biomaterials that provide immunomodulation, and immunomodulatory cell co-transplantation.
Co-transplantation of pancreatic islets with mesenchymal stem cells (MSCs) is one such approach that has attracted attention. Studies have shown that using MSCs as an adjunct therapy to islet transplantation can promote long-term graft survival, possibly by reducing inflammation and enhancing immune tolerance[85]. For instance, co-transplantation of adipose tissue-derived MSCs and pancreatic islets improved glycemic control and regulation of the Th17/Treg function streptozotocin-induced diabetic mice model[86]. Encapsulation, on the other hand, is another technique that has been extensively studied for its potential to protect transplanted islets from immune rejection while allowing for efficient nutrient and oxygen exchange. In addition, Vegas et al[87] demonstrated that beta cells derived from human stem cells, when implanted into mice with preserved immune competence, resulted in long-term glycemic control[87]. Thus, further investigation into these novel strategies for T1D cell replacement therapies may provide new insights and solutions to the ongoing challenges in this field.
Therefore, methods for immunoisolation or beta cell encapsulation are one approach to improving graft performance. Still, it has its own set of obstacles, which causes a loss in cell viability over time (Figure 1B). Although altering human islets in clinical applications is implausible, creating universal cells from pluripotent stem cells that can elude immune identification offers enormous promise in diabetic cell treatments. However, despite these breakthroughs, critical problems like the persistence of genomic and epigenetic modifications and cell phenotypes stability remain unanswered. Additionally, although these cells are hypoimmunogenic, their safety should be carefully maintained because cells that elude the immune system are intrinsically dangerous.
Similarly, undifferentiated stem cells can potentially develop into teratomas in vivo because it is well-known that both embryonic and induced pluripotent stem cells can differentiate into all three germ layers. Therefore, they can form teratomas if not fully differentiated[88]. Theoretically, the presence of a few remaining undifferentiated pluripotent stem cells can cause undesirable teratomas after transplantation. Although "suicide genes" could be incorporated into stem cells for increased safety[89], it is still uncertain how these cells would behave in people over time, necessitating additional research.
Biomaterials combined with immunomodulation give multiple instruments for locally modulating immune responses and are an intriguing way to assist cell transplantation. This technique has apparent advantages, including safety as "nonliving" materials. Furthermore, biomaterials are generally simple to mass-produce. In contrast, cell modification or immunomodulatory cell preparation is sometimes difficult, in addition to the necessity of good manufacturing processes that must fulfill clinical requirements. Yet, given the restricted ligands and the eventual exhaustion of coated reagents, the long-term durability of biomaterials and delivery techniques remains challenging. Hence, there is a need for new approaches for the retention or restocking of the supplied reagents in the future[84].
Interestingly, immunomodulatory cells operate as "living" medicine repositories and, if engrafted, may boost functional stability by producing cytokines continuously or expressing surface markers to affect the immune system. Improvements in these immunoregulatory cells' acquisition, retention, stability, potency and localization are required to increase their effectiveness and safety. As we create T1D therapies and cures, a functioning resolution will likely need a multi-modal methodology involving several immuno-modalities and tissue engineering methods. The strategy for the 3D-engineered biomaterial tissue construct coupled with both invisible to the immune response cells and accessory cells that exert could be employed to provide long-term effective and safe cell treatments for T1D. Examining the disease's heterogeneity and customizing therapy procedures is critical to reaching the best possible outcomes[84].
Additionally, because transplanted islets are isolated from deceased donors who are not human leukocyte antigen (HLA)-matched to recipients, the use of multiple donors and the potential need to discontinue immunosuppression in the case of a clinically failed islet-alone graft increases the risk of HLA sensitization in islet transplant recipients. Most transplant patients currently have an unexplained slow loss of islet graft function may be partly caused by allograft rejection. However, discovering anti-HLA antibodies during graft deterioration remains uncommon[90].
Future pathways for improving the outcomes of islet transplantation include obtaining alternative sources of insulin-secreting cells, attempts to improve the immune protection and revascularization of the transplanted tissue, and methods for enhancing viability[91].
Islets obtained from human embryonic stem cells (hESC) are in early-phase clinical trials[92]. hESC islets should theoretically not require immunosuppression or HLA silencing, which would allow the treatment of children. However, alternative strategies, such as xenogeneic sources of islets and human-induced pluripotent stem cells[93], are also being researched.
Several therapeutical approaches to improve islet survivability are currently in the preclinical phase of research. These include cellular therapies such as MSCs[94], regulatory T-cells[95], as well as modulators of the liver niche with anti-inflammatory agents[96] and growth factors[97]. MSCs appear promising as their anti-inflammatory and immunomodulatory properties have been used in humans for other conditions and could, in theory, enable them to reduce the immunosuppression dose[98]. In addition, improving vascularity through gene therapy[99] of the transplant has also been a sought-after strategy for future development.
Last but not least, various scaffolding methods, as well as alternative implant sites, are undergoing research to enhance the viability of the grafts. For example, dexamethasone-loaded microplate-enriched collagen-coated polydimethylsiloxane scaffolds have improved transplant outcomes and survival[100]. While the liver currently remains the localization of choice for islet transplantation, several other sites are being investigated, such as intramuscular[101], gastric submucosa[102], thymus, testes and the eyes[103].
T1DM is an immune-associated metabolic disease characterized by hyperglycemia, absolute insulin deficiency, and a lifelong need for exogenous insulin replacement treatment. The implementation of modern technologies in diabetes control with continuous glucose monitoring systems combined with glucose prediction algorithms enables the development of artificial pancreas delivery systems. Nevertheless, the need to completely replace the depleted pancreatic secretion also leads to the emergence of new therapeutic horizons, including pancreas and islet cell transplantation. They allow not only to achieve independence from exogenous insulin administration and the need to monitor blood sugar but also successfully to afford counterregulatory hormone secretion and pancreatic exocrine function. At this point, the main complication after allogeneic islet transplantation is the chronic rejection conducted by activated T cells and autobodies-mediated rejection, the main barrier to accomplishing long-term engraftment. To improve the outcomes, several approaches are performed: Immunosuppression, ADLs, anti-TIM-1 antibodies, mixed chimerism-based tolerance induction, induction of antigen-specific tolerance utilizing ECDI-fixed splenocytes, infusion of donor apoptotic cells before transplantation, therapy with anti-CD40L antibodies and rapamycin, preconditioning of isolated islets, inducing local immunotolerance, cell encapsulation and immunoisolation, using of biomaterials, immunomodulatory cells, etc.
| 1. | Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 525] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 2. | Khan K, Desai CS. Islet Transplantation in Children. Curr Gastroenterol Rep. 2019;21:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1462] [Cited by in RCA: 1742] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 4. | Ku HT, Zhang N, Kubo A, O'Connor R, Mao M, Keller G, Bromberg JS. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells. 2004;22:1205-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Akil AA, Yassin E, Al-Maraghi A, Aliyev E, Al-Malki K, Fakhro KA. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med. 2021;19:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV; T1D Exchange Clinic Network. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 1025] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 7. | Maffi P, Secchi A. Islet Transplantation Alone Versus Solitary Pancreas Transplantation: an Outcome-Driven Choice? Curr Diab Rep. 2019;19:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Johnson PR, Jones KE. Pancreatic islet transplantation. Semin Pediatr Surg. 2012;21:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AM. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 648] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 10. | Digon BJ 3rd. History of islet transplantation. Curr Diab Rep. 2009;9:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Farney AC, Sutherland DE, Opara EC. Evolution of Islet Transplantation for the Last 30 Years. Pancreas. 2016;45:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1206] [Article Influence: 57.4] [Reference Citation Analysis (1)] |
| 13. | Qi M, Kinzer K, Danielson KK, Martellotto J, Barbaro B, Wang Y, Bui JT, Gaba RC, Knuttinen G, Garcia-Roca R, Tzvetanov I, Heitman A, Davis M, McGarrigle JJ, Benedetti E, Oberholzer J. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta Diabetol. 2014;51:833-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Lablanche S, Borot S, Wojtusciszyn A, Bayle F, Tétaz R, Badet L, Thivolet C, Morelon E, Frimat L, Penfornis A, Kessler L, Brault C, Colin C, Tauveron I, Bosco D, Berney T, Benhamou PY; GRAGIL Network. Five-Year Metabolic, Functional, and Safety Results of Patients With Type 1 Diabetes Transplanted With Allogenic Islets Within the Swiss-French GRAGIL Network. Diabetes Care. 2015;38:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 522] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 16. | Othonos N, Choudhary P. Who Should Be Considered for Islet Transplantation Alone? Curr Diab Rep. 2017;17:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Chetboun M, Jannin A, Kerr-Conte J, Pattou F, Vantyghem MC. 1921-2021: From insulin discovery to islet transplantation in type 1 diabetes. Ann Endocrinol (Paris). 2021;82:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | McCall M, Shapiro AM. Islet cell transplantation. Semin Pediatr Surg. 2014;23:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Rickels MR, Robertson RP. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr Rev. 2019;40:631-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 20. | Tsujimura T, Kuroda Y, Kin T, Avila JG, Rajotte RV, Korbutt GS, Ryan EA, Shapiro AM, Lakey JR. Human islet transplantation from pancreases with prolonged cold ischemia using additional preservation by the two-layer (UW solution/perfluorochemical) cold-storage method. Transplantation. 2002;74:1687-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 847] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 22. | Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets. 2018;10:80-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Vantyghem MC, de Koning EJP, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394:1274-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Cayabyab F, Nih LR, Yoshihara E. Advances in Pancreatic Islet Transplantation Sites for the Treatment of Diabetes. Front Endocrinol (Lausanne). 2021;12:732431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 275] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Stratta RJ, Farney AC, Rogers J, Orlando G. Immunosuppression for pancreas transplantation with an emphasis on antibody induction strategies: review and perspective. Expert Rev Clin Immunol. 2014;10:117-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Ghasemi A, Akbari E, Imani R. An Overview of Engineered Hydrogel-Based Biomaterials for Improved β-Cell Survival and Insulin Secretion. Front Bioeng Biotechnol. 2021;9:662084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Zhang Q, Gonelle-Gispert C, Li Y, Geng Z, Gerber-Lemaire S, Wang Y, Buhler L. Islet Encapsulation: New Developments for the Treatment of Type 1 Diabetes. Front Immunol. 2022;13:869984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Leslie RD, Kolb H, Schloot NC, Buzzetti R, Mauricio D, De Leiva A, Yderstraede K, Sarti C, Thivolet C, Hadden D, Hunter S, Schernthaner G, Scherbaum W, Williams R, Pozzilli P. Diabetes classification: grey zones, sound and smoke: Action LADA 1. Diabetes Metab Res Rev. 2008;24:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40:10-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 381] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 32. | Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32:468-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Zaharieva ET, Velikova TV, Tsakova AD, Kamenov ZA. Prevalence of Positive Diabetes-Associated Autoantibodies among Type 2 Diabetes and Related Metabolic and Inflammatory Differences in a Sample of the Bulgarian Population. J Diabetes Res. 2017;2017:9016148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Bao F, Yu L, Babu S, Wang T, Hoffenberg EJ, Rewers M, Eisenbarth GS. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Hoffenberg EJ, Bao F, Eisenbarth GS, Uhlhorn C, Haas JE, Sokol RJ, Rewers M. Transglutaminase antibodies in children with a genetic risk for celiac disease. J Pediatr. 2000;137:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Triolo TM, Armstrong TK, McFann K, Yu L, Rewers MJ, Klingensmith GJ, Eisenbarth GS, Barker JM. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care. 2011;34:1211-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 37. | Vasilev G, Kabakchieva P, Miteva D, Batselova H, Velikova T. Effectiveness and safety of COVID-19 vaccines in patients with diabetes as a factor for vaccine hesitancy. World J Diabetes. 2022;13:738-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 38. | Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 798] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 39. | Hirsch IB, Juneja R, Beals JM, Antalis CJ, Wright EE. The Evolution of Insulin and How it Informs Therapy and Treatment Choices. Endocr Rev. 2020;41:733-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 40. | Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 41. | Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Toffanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ 3rd, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B; International Artificial Pancreas Study Group. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61:2230-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 42. | Fridell JA, Stratta RJ, Gruessner AC. Pancreas Transplantation: Current Challenges, Considerations, and Controversies. J Clin Endocrinol Metab. 2023;108:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 43. | Zóka A, Műzes G, Somogyi A, Varga T, Szémán B, Al-Aissa Z, Hadarits O, Firneisz G. Altered immune regulation in type 1 diabetes. Clin Dev Immunol. 2013;2013:254874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzij JG, Roep BO. Development of type 1 diabetes despite severe hereditary B-cell deficiency. N Engl J Med. 2001;345:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 46. | Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021;17:150-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (1)] |
| 47. | Pino SC, Kruger AJ, Bortell R. The role of innate immune pathways in type 1 diabetes pathogenesis. Curr Opin Endocrinol Diabetes Obes. 2010;17:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Clark M, Kroger CJ, Tisch RM. Type 1 Diabetes: A Chronic Anti-Self-Inflammatory Response. Front Immunol. 2017;8:1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 49. | Culina S, Lalanne AI, Afonso G, Cerosaletti K, Pinto S, Sebastiani G, Kuranda K, Nigi L, Eugster A, Østerbye T, Maugein A, McLaren JE, Ladell K, Larger E, Beressi JP, Lissina A, Appay V, Davidson HW, Buus S, Price DA, Kuhn M, Bonifacio E, Battaglia M, Caillat-Zucman S, Dotta F, Scharfmann R, Kyewski B, Mallone R; ImMaDiab Study Group. Islet-reactive CD8(+) T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 50. | Bottazzo GF. Lawrence lecture. Death of a beta cell: homicide or suicide? Diabet Med. 1986;3:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Rickels MR, Stock PG, de Koning EJP, Piemonti L, Pratschke J, Alejandro R, Bellin MD, Berney T, Choudhary P, Johnson PR, Kandaswamy R, Kay TWH, Keymeulen B, Kudva YC, Latres E, Langer RM, Lehmann R, Ludwig B, Markmann JF, Marinac M, Odorico JS, Pattou F, Senior PA, Shaw JAM, Vantyghem MC, White S. Defining outcomes for β-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transpl Int. 2018;31:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 52. | Rickels MR, Stock PG, de Koning EJP, Piemonti L, Pratschke J, Alejandro R, Bellin MD, Berney T, Choudhary P, Johnson PR, Kandaswamy R, Kay TWH, Keymeulen B, Kudva YC, Latres E, Langer RM, Lehmann R, Ludwig B, Markmann JF, Marinac M, Odorico JS, Pattou F, Senior PA, Shaw JAM, Vantyghem MC, White S. Defining Outcomes for β-cell Replacement Therapy in the Treatment of Diabetes: A Consensus Report on the Igls Criteria From the IPITA/EPITA Opinion Leaders Workshop. Transplantation. 2018;102:1479-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Gillard P, Ling Z, Lannoo M, Maes B, Maleux G, Pipeleers D, Keymeulen B, Mathieu C. Beta-cell transplantation restores metabolic control and quality of life in a patient with subcutaneous insulin resistance. Diabetes Care. 2004;27:2243-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Nijhoff MF, Dubbeld J, van Erkel AR, van der Boog PJM, Rabelink TJ, Engelse MA, de Koning EJP. Islet alloautotransplantation: Allogeneic pancreas transplantation followed by transplant pancreatectomy and islet transplantation. Am J Transplant. 2018;18:1016-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Johnston PC, Lin YK, Walsh RM, Bottino R, Stevens TK, Trucco M, Bena J, Faiman C, Hatipoglu BA. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab. 2015;100:1765-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Sutherland DER, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, Chinnakotla S, Vickers SM, Bland B, Balamurugan AN, Freeman ML, Pruett TL. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409-424. |
| 57. | Ahmad SA, Lowy AM, Wray CJ, D'Alessio D, Choe KA, James LE, Gelrud A, Matthews JB, Rilo HL. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Rodriguez Rilo HL, Ahmad SA, D'Alessio D, Iwanaga Y, Kim J, Choe KA, Moulton JS, Martin J, Pennington LJ, Soldano DA, Biliter J, Martin SP, Ulrich CD, Somogyi L, Welge J, Matthews JB, Lowy AM. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Webb MA, Illouz SC, Pollard CA, Gregory R, Mayberry JF, Tordoff SG, Bone M, Cordle CJ, Berry DP, Nicholson ML, Musto PP, Dennison AR. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas. 2008;37:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Garcea G, Weaver J, Phillips J, Pollard CA, Ilouz SC, Webb MA, Berry DP, Dennison AR. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: a series of 85 consecutive patients. Pancreas. 2009;38:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Alejandro R, Barton FB, Hering BJ, Wease S; Collaborative Islet Transplant Registry Investigators. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1480] [Cited by in RCA: 1442] [Article Influence: 72.1] [Reference Citation Analysis (1)] |
| 63. | Gurol AO, Okten-Kursun A, Kasapoglu P, Suzergoz F, Kucuksezer UC, Cevik A, Tutuncu Y, Yentur SP, Gurol SD, Kucuk M, Yilmaz MT. The synergistic effect of ω3 and Vit D3 on glycemia and TNF-α in islet transplantation. Cell Mol Biol (Noisy-le-grand). 2016;62:90-98. [PubMed] |
| 64. | Casteels K, Waer M, Laureys J, Valckx D, Depovere J, Bouillon R, Mathieu C. Prevention of autoimmune destruction of syngeneic islet grafts in spontaneously diabetic nonobese diabetic mice by a combination of a vitamin D3 analog and cyclosporine. Transplantation. 1998;65:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Infante M, Ricordi C, Sanchez J, Clare-Salzler MJ, Padilla N, Fuenmayor V, Chavez C, Alvarez A, Baidal D, Alejandro R, Caprio M, Fabbri A. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 66. | Szymczak-Pajor I, Śliwińska A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 67. | Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12:1576-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 68. | Vallabhajosyula P, Hirakata A, Shimizu A, Okumi M, Tchipashvili V, Hong H, Yamada K, Sachs DH. Assessing the effect of immunosuppression on engraftment of pancreatic islets. Transplantation. 2013;96:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Sato N, Marubashi S. Induction of Immune Tolerance in Islet Transplantation Using Apoptotic Donor Leukocytes. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Johnson PRV. Islet Transplantation in the UK. CellR4 Repair Replace Regen Reprogram. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | UK Guidelines on Pancreas and Islet Transplantation. British Transplantation Society. Available from: https://bts.org.uk/wp-content/uploads/2019/09/FINAL-Pancreas-guidelines-FINAL-version-following-consultation.-Sept-2019.pdf. |
| 72. | Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645-3656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 73. | Lee KM, Kim JI, Stott R, Soohoo J, O'Connor MR, Yeh H, Zhao G, Eliades P, Fox C, Cheng N, Deng S, Markmann JF. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, Winn HJ, Colvin RB, Sachs DH, Cosimi AB. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 839] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 76. | Singh A, Ramachandran S, Graham ML, Daneshmandi S, Heller D, Suarez-Pinzon WL, Balamurugan AN, Ansite JD, Wilhelm JJ, Yang A, Zhang Y, Palani NP, Abrahante JE, Burlak C, Miller SD, Luo X, Hering BJ. Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes. Nat Commun. 2019;10:3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 77. | Oura T, Ko DS, Boskovic S, O'Neil JJ, Chipashvili V, Koulmanda M, Hotta K, Kawai K, Nadazdin O, Smith RN, Cosimi AB, Kawai T. Kidney Versus Islet Allograft Survival After Induction of Mixed Chimerism With Combined Donor Bone Marrow Transplantation. Cell Transplant. 2016;25:1331-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, Houlihan JL, Miller SD, Zhang ZJ, Luo X. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Bryant J, Lerret NM, Wang JJ, Kang HK, Tasch J, Zhang Z, Luo X. Preemptive donor apoptotic cell infusions induce IFN-γ-producing myeloid-derived suppressor cells for cardiac allograft protection. J Immunol. 2014;192:6092-6101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Dangi A, Yu S, Lee FT, Burnette M, Knechtle S, Kwun J, Luo X. Donor apoptotic cell-based therapy for effective inhibition of donor-specific memory T and B cells to promote long-term allograft survival in allosensitized recipients. Am J Transplant. 2020;20:2728-2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Eriksson O, Eich T, Sundin A, Tibell A, Tufveson G, Andersson H, Felldin M, Foss A, Kyllönen L, Langstrom B, Nilsson B, Korsgren O, Lundgren T. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9:2816-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 82. | Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;16:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 83. | Hu M, Hawthorne WJ, Yi S, O'Connell PJ. Cellular Immune Responses in Islet Xenograft Rejection. Front Immunol. 2022;13:893985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 84. | Wang X, Brown NK, Wang B, Shariati K, Wang K, Fuchs S, Melero-Martin JM, Ma M. Local Immunomodulatory Strategies to Prevent Allo-Rejection in Transplantation of Insulin-Producing Cells. Adv Sci (Weinh). 2021;8:e2003708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 85. | Scuteri A, Donzelli E, Rodriguez-Menendez V, Ravasi M, Monfrini M, Bonandrini B, Figliuzzi M, Remuzzi A, Tredici G. A double mechanism for the mesenchymal stem cells' positive effect on pancreatic islets. PLoS One. 2014;9:e84309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Rahavi H, Hashemi SM, Soleimani M, Mohammadi J, Tajik N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. 2015;2015:878535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 537] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (1)] |
| 88. | Prokhorova TA, Harkness LM, Frandsen U, Ditzel N, Schrøder HD, Burns JS, Kassem M. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009;18:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 89. | Harding J, Vintersten-Nagy K, Shutova M, Yang H, Tang J, Massumi M, Izadifar M, Izadifar Z, Zhang P, Li C, Nagy A. Induction of long-term allogeneic cell acceptance and formation of immune privileged tissue in immunocompetent hosts. 2019 Preprint. Available from: BioRxiv:716571. [DOI] [Full Text] |
| 90. | Rickels MR, Kearns J, Markmann E, Palanjian M, Markmann JF, Naji A, Kamoun M. HLA sensitization in islet transplantation. Clin Transpl. 2006;413-420. [PubMed] |
| 91. | Paget MB, Murray HE, Bailey CJ, Downing R. From insulin injections to islet transplantation: An overview of the journey. Diabetes Obes Metab. 2022;24 Suppl 1:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Siehler J, Blöchinger AK, Meier M, Lickert H. Engineering islets from stem cells for advanced therapies of diabetes. Nat Rev Drug Discov. 2021;20:920-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 93. | Coe TM, Markmann JF, Rickert CG. Current status of porcine islet xenotransplantation. Curr Opin Organ Transplant. 2020;25:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 94. | Hubber EL, Rackham CL, Jones PM. Protecting islet functional viability using mesenchymal stromal cells. Stem Cells Transl Med. 2021;10:674-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Harden PN, Game DS, Sawitzki B, Van der Net JB, Hester J, Bushell A, Issa F, Brook MO, Alzhrani A, Schlickeiser S, Scotta C, Petchey W, Streitz M, Blancho G, Tang Q, Markmann J, Lechler RI, Roberts ISD, Friend PJ, Hilton R, Geissler EK, Wood KJ, Lombardi G. Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Am J Transplant. 2021;21:1603-1611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 96. | Kuppan P, Kelly S, Polishevska K, Hojanepesov O, Seeberger K, Korbutt GS, Pepper AR. Co-localized immune protection using dexamethasone-eluting micelles in a murine islet allograft model. Am J Transplant. 2020;20:714-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Alwahsh SM, Qutachi O, Starkey Lewis PJ, Bond A, Noble J, Burgoyne P, Morton N, Carter R, Mann J, Ferreira-Gonzalez S, Alvarez-Paino M, Forbes SJ, Shakesheff KM, Forbes S. Fibroblast growth factor 7 releasing particles enhance islet engraftment and improve metabolic control following islet transplantation in mice with diabetes. Am J Transplant. 2021;21:2950-2963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Vandermeulen M, Erpicum P, Weekers L, Briquet A, Lechanteur C, Detry O, Beguin Y, Jouret F. Mesenchymal Stromal Cells in Solid Organ Transplantation. Transplantation. 2020;104:923-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 99. | Staels W, Verdonck Y, Heremans Y, Leuckx G, De Groef S, Heirman C, de Koning E, Gysemans C, Thielemans K, Baeyens L, Heimberg H, De Leu N. Vegf-A mRNA transfection as a novel approach to improve mouse and human islet graft revascularisation. Diabetologia. 2018;61:1804-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Primavera R, Razavi M, Kevadiya BD, Wang J, Vykunta A, Di Mascolo D, Decuzzi P, Thakor AS. Enhancing islet transplantation using a biocompatible collagen-PDMS bioscaffold enriched with dexamethasone-microplates. Biofabrication. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Bertuzzi F, Colussi G, Lauterio A, De Carlis L. Intramuscular islet allotransplantation in type 1 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2018;22:1731-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 102. | Yin Z, Li J, Zheng Y, Wang S, Wang X. Gastric submucosal alleviated pro-inflammation cytokines mediated initial dysfunction of islets allografts. Transpl Immunol. 2021;65:101292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Addison P, Fatakhova K, Rodriguez Rilo HL. Considerations for an Alternative Site of Islet Cell Transplantation. J Diabetes Sci Technol. 2020;14:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nagaya M, Japan; Scuteri A, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR