Published online Jun 18, 2022. doi: 10.5500/wjt.v12.i6.131
Peer-review started: January 7, 2022
First decision: February 21, 2022
Revised: February 25, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 18, 2022
Processing time: 158 Days and 21.1 Hours
Patients with a history of primary brain tumors can be eligible for organ donation under extended criteria. The risk assessment of tumor transmission via organ transplant in primary brain tumors is primarily based on the assessment of tumor histotype and grade. Previous surgeries, chemo-/radiotherapy, and ventriculo-peritoneal shunt placement can lead to a disruption of the blood-brain barrier, concurring to an increase in the transmission risk.
To investigate the role of tumor transmission risk factors in donors with oligoden
We searched PubMed and EMBASE databases for studies reporting extraneural spreading of oligoden
Data on a total of 157 patients were retrieved. The time from the initial diagnosis to metastatic spread ranged from 0 to 325 mo in patients with oligodendrogliomas and 0 to 267 mo in those with astrocytomas. Respectively, 19% and 39% of patients with oligodendroglioma and astrocytoma did not receive any adjuvant therapy. The most frequent metastatic sites were bone, bone marrow, and lymph nodes. The lungs and the liver were the most commonly involved visceral sites. There was no significant correlation between the occurrence of multiple metastases and the administration of adjuvant chemo-/radiotherapy. Patients who developed intracranial recurrences/metastases had a significantly longer extraneural metastasis-free time compared to those who developed extraneural metastases in the absence of any intra- central nervous system spread.
A long follow-up time does not exclude the presence of extraneural metastases. Therefore, targeted imaging of bones and cervical lymph nodes may improve safety in the management of these donors.
Core Tip: Recognized risk factors of tumor transmission from donors with a history of primary brain tumors are previous surgery, chemotherapy,and radiotherapy. We performed a systematic review of the literature on oligodendroglioma and astrocytomas with extraneural metastases, aiming to clarify the role of tumor transmission risk factors. We searched PubMed and EMBASE databases for studies reporting extraneural spreading of these gliomas. Performed treatments do not seem to impact on the timing of metastatic spread, and a long follow-up time does not exclude extraneural spread. Targeted imaging of bones and cervical lymph nodes may improve safety in the management of these donors.
- Citation: Ammendola S, Barresi V, Bariani E, Girolami I, D’Errico A, Brunelli M, Cardillo M, Lombardini L, Carraro A, Boggi U, Cain O, Neil D, Eccher A. Risk factors of extraneural spreading in astrocytomas and oligodendrogliomas in donors with gliomas: A systematic review. World J Transplant 2022; 12(6): 131-141
- URL: https://www.wjgnet.com/2220-3230/full/v12/i6/131.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i6.131
The transplant community has been struggling with the chronic shortage of donor’s organs for transplantation. In order to increase the donor pool, criteria for donation have been expanded[1,2], accepting as donors individuals with a history of malignancies of low metastatic potential. However, transplantation from these donors carries a risk of cancer transmission that should be carefully assessed for each tumor type[1-4].
Organs from donors with a history of a primary brain tumor (PBT) may be considered eligible for transplantation under extended criteria since these tumors have a low propensity to metastasize outside the central nervous system (CNS). These patients represent a relevant subgroup of donors that can increase the number of transplants performed, reducing times on the waiting list[5]. According to the 7th edition of the guidelines on quality and safety of organ transplantation, the risk of transmission for patients with a history of PBT is mainly influenced by the tumor histotype and grade[6]. The risk of tumor transmission in donors with a history of CNS tumors is graded as minimal, low to intermediate, and high or unacceptable; in detail, donors with World Health Organization (WHO) grade I and II PBTs are considered at minimal risk of tumor transmission, while grade III tumors are now considered at low to intermediate risk in the absence of any recognized risk factors, such as previous surgical resections, ventriculo-peritoneal (VP) or ventriculo-atrial shunt placement, and/or chemotherapy/radiotherapy that increase the risk from intermediate to high[6]. These procedures disrupt the blood-brain barrier, increasing the risk of hematogenous and lymphovascular spread of these tumors[7]. Extra-CNS metastases from PBTs do however occur, with a reported prevalence of up to 4.3%[7], and metastases mainly occur in patients with a history of high-grade gliomas and, in particular, of glioblastoma[8-10]. Ventriculo-atrial and VP shunts have also been reported as risk factors for tumor spread[11].
However, the studies on PBT transmission after solid organ transplantation often include limited data on the tumor histological features and the patients’ clinical management[9-13]. In the United Network for Organ Sharing registry, among 642 patients who received organs from a donor with a PBT, three died due to the transmission of a glioblastoma[8,13]. However, no cases of transmission were reported among 96 recipients in the Australian and New Zealand Organ Donation Registry[14], 89 recipients from the Czech Republic registry[15], and 448 recipients from the United Kingdom registry[16]. More recently, Lee et al[17] reported that none of 87 transplant recipients had tumor transmission from 28 donors with PBTs.
To date, there are no reports of transmission of oligodendroglioma to organ transplant recipients, while donor-to-recipient transmission of grade III/IV astrocytic tumors have been previously reported[6]. Though the metastatic potential of these tumors in the context of transplantation needs to be clarified and kept up-to-date. Oligodendrogliomas are CNS diffuse gliomas mainly occurring in adulthood, with a peak incidence in the fourth and fifth decade and a slight male predominance (1.3:1), preferentially arising in the cerebral hemispheres and mostly in the frontal lobe[18]. According to the WHO, oligodendroglioma is defined by the co-occurrence of isocitrate dehydrogenase 1/2 (IDH1/2) mutation and chromosome 1p/19q whole arm codeletion and classified into grade II and grade III (anaplastic oligodendrogliomas) based on the presence of histologic features of anaplasia, such as microvascular proliferation and/or brisk mitotic activity[18].
Tumors of astrocytic lineage, contrary to oligodendrogliomas, have a four-tiered grading system that encompasses a wide spectrum of clinical entities, from grade I tumors characterized by a benign clinical course to grade IV tumors carrying a dismal prognosis[18]. About 5% of all PBTs with extra-CNS metastatic spread are reported to be oligodendrogliomas, while astrocytomas account for about 10% of extraneural metastatic PBTs[19]. However, data on extraneural metastatic spread mostly come from case reports or small case series, and there is no systematic appraisal of the risk factors or patterns of metastatic spread.
In this study, we performed a systematic review of the literature on oligodendrogliomas and astrocytomas with extra-CNS metastases with the aim of identifying clinical or pathological factors that can be helpful to predict the tumor transmission risk and guide decision making in organ transplantation from donors with these tumors.
This literature review was performed in accordance with the PRISMA. A literature search without language restrictions was carried out in the electronic databases MEDLINE-PubMed and EMBASE until December 2020. The search terms were: “oligodendroglioma”, “anaplastic oligodendroglioma”, “astrocytoma”, “anaplastic astrocytoma” “oligodendroglial tumours”, “diffuse glioma” “extracranial metastasis” “oligodendroglioma metastatic to”, “astrocytoma metastatic to”, “extraneural metastases” “primary brain tumours”, “metastatic oligodendroglioma”, “metastatic astrocytoma”. Screening of article titles and abstracts was independently performed by three investigators using Rayyan QCRI reference manager web application[20]. Some references for Journal articles also were searched from (RCA), an artificial intelligence technology-based open citation analysis database (https://www.refer-encecitationanalysis.com, Baishideng Publishing Group Inc., Pleasanton, CA, United States).
The full texts of the articles fulfilling the initial screening criteria were retrieved and reviewed (Supplementary Table 1); disagreement was resolved via consensus. Inclusion criteria were: Case reports, case series, and literature reviews reporting on patients with a history of oligodendroglioma or astrocytoma that subsequently metastasized outside the CNS. Articles with limited data were included if they at least reported the histologic diagnosis of primary and metastatic tumors (Table 1; Supplementary Table 1). We included articles mentioning different tumor histotypes only if findings of each case were further detailed. We excluded articles reporting metastatic disease not histologically confirmed and those concerning only animal models or cell cultures. Articles reporting extracranial metastases from primary glioblastomas were also excluded. Finally, from the included articles we extracted data on: Author and publication year, country, type of paper, sex and age of the patients at metastatic spread, tumor histotype and grade, synchronous or metachronous malignancies, intracranial recurrence, intra-axial spreading, tumor progression, time between the diagnosis and the onset of metastases, sites and number of metastases, tumor progression of the primary neoplasm preceding extracranial extra-CNS spread, prior surgeries, prior radiotherapy and/or chemotherapy, ventriculo-atrial or VP shunt placement, IDH1/2 mutation and 1p/19q codeletion in both the primary and metastatic tumors.
| Clinical features | Oligodendroglioma (%) | Astrocytoma (%) |
| Patients | 90 (100) | 67 (100) |
| Sex | ||
| Male | 52 (58) | 39 (58) |
| Female | 32 (35) | 27 (40) |
| Undisclosed | 6 (7) | 1 (2) |
| Age in yr | 1.5-74.0 (mean: 44.5; median: 46) | 0-82.0 (mean: 31.0, median: 26) |
| Location | ||
| Frontal lobe | 34 (38) | 7 (11) |
| Parietal lobe | 8 (9) | 2 (3) |
| Temporal lobe | 5 (6) | 11 (16) |
| Spine | 1 (1) | 6 (9) |
| NA | 22 (24) | 2 (3) |
| Other sites | 20 (22) | 39 (58) |
| Surgery | ||
| Yes | 79 (88) | 48 (71) |
| No | 2 (2) | 16 (24) |
| Multiple surgeries | ||
| Yes | 44 (49) | 24 (36) |
| No | 35 (39) | 41 (61) |
| Radiotherapy | ||
| Yes | 60 (67) | 49 (73) |
| No | 14 (15) | 15 (22) |
| Chemotherapy | ||
| Yes | 33 (37) | 16 (23) |
| No | 37 (41) | 48 (72) |
| VA/VP shunt | ||
| Yes | 3 (3) | 20 (30) |
| No | 26 (29) | 34 (50) |
| Metastatic sites | ||
| Bone | 48 (53) | 30 (44) |
| Bone marrow | 30 (33) | 6 (8) |
| Lymph nodes | 27 (30) | 24 (30) |
| Cervical | 16 (17) | 14 (17) |
| Retroperitoneal | 3 (3) | 2 (3) |
| Axillary | 2 (2) | - |
| Other | 6 (7) | 7 (10) |
| Lung | 10 (11) | 11 (17) |
| Liver | 8 (9) | 8 (11) |
| Scalp | 8 (9) | 8 (11) |
| Pleura | 5 (6) | 6 (8) |
| Parotid gland | 5 (6) | 3 (4) |
| Breast | 3 (3) | - |
| Chest wall | 3 (3) | 1 (1) |
| Peritoneum | 3 (3) | 10 (14) |
| Kidney | - | 3 (4) |
| Retroperitoneum | 2 (2) | 1 (1) |
| Soft tissues | 1 (1) | 11 (15) |
| Pericardium | 1 (1) | - |
| Pancreas | 1 (1) | 1 (1) |
| Spleen | 1 (1) | - |
| Thymus/mediastinum | 1 (1) | 1 (1) |
| Adrenal gland | 1 (1) | - |
| Muscles | 3 (3) | 2 (3) |
| Intra-CNS metastases/recurrence | ||
| Yes | 43 (48) | 37 (55) |
| No | 19 (21) | 26 (39) |
| Non-conclusive | 1 (1) | 3 (6) |
| Time from the diagnosis to metastatic spread | 0-324 (mean: 53.7; median: 36) | 0-276 (mean: 31.0; median: 13) |
Statistical analysis was performed using open-source software R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) with RStudio 1.4.1106 environment (RStudio Inc, Boston, Massachusetts, United States). The statistical correlation between chemotherapy or radiotherapy and the presence of multiple extra-CNS metastases was analyzed using χ2 and Fischer exact test. Kaplan-Meyer method was used to investigate the correlation between metastasis-free time and metastatic sites, presence/absence of intracranial recurrence, and the occurrence of multiple metastases. A P-value less than 0.05 was considered statistically significant. No institutional review board approval was needed, as no ethical issue is raised by literature reviews.
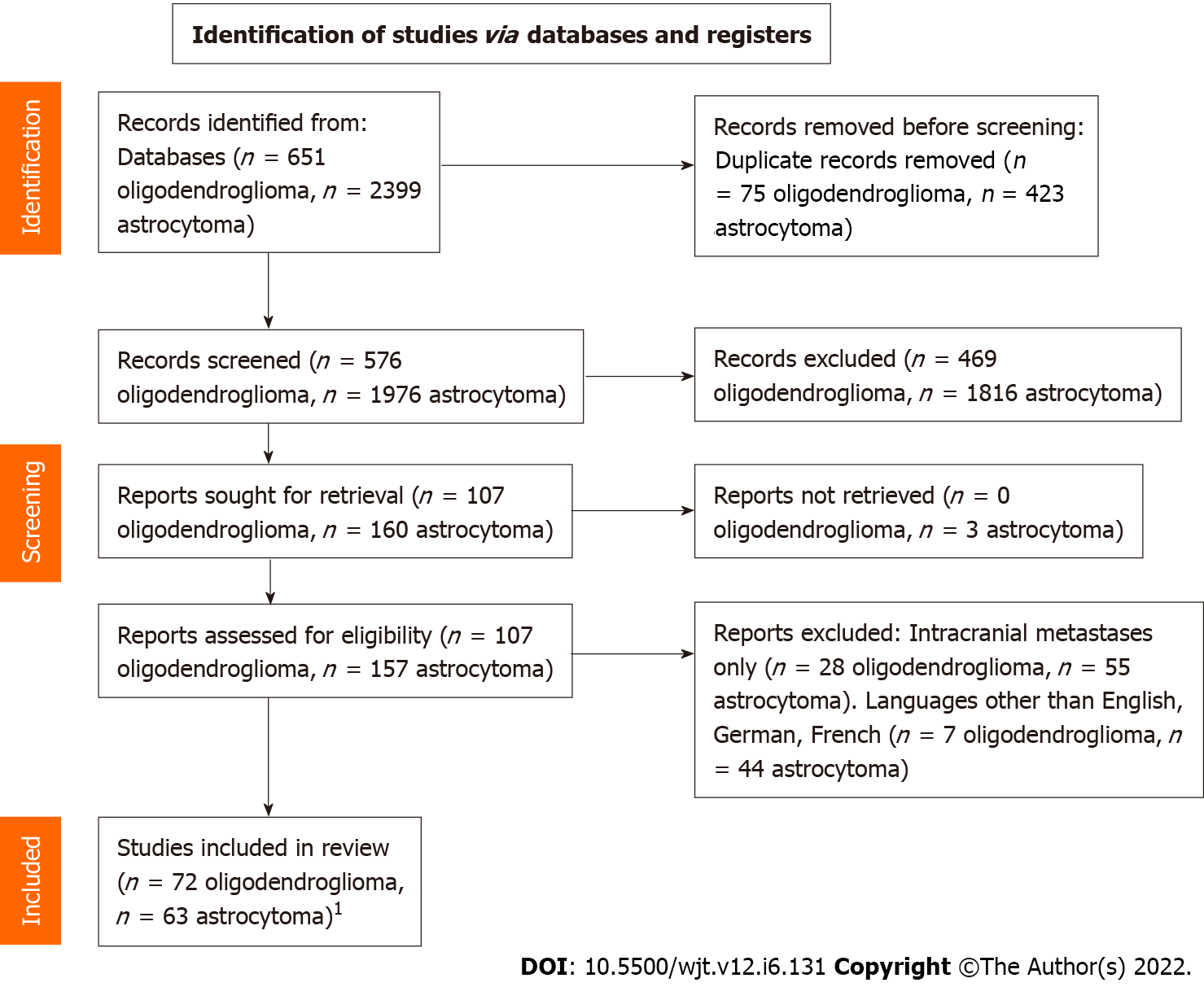
The results are summarized in Table 1 and detailed in Supplementary Table 1. A total of 2675 articles were identified after duplicate removal. After an initial screening on titles and abstracts, we considered 267 articles as potentially relevant to our study. We excluded 3 articles with unavailable full text and 83 reporting only intracranial or spinal drop metastases; 51 articles were excluded due to language restrictions. A PRISMA flow diagram of the literature screening and article exclusion is shown in Figure 1.
The 130 articles included were case series, case reports, and literature review articles reporting data on a total of 90 patients (52 males, 32 females, and 6 with undisclosed sex) with extra-CNS metastases from oligodendroglial tumors and 67 patients with extra-CNS metastatic astrocytoma (39 males, 27 females, and 1 with undisclosed sex) (Table 1; Supplementary Table 1). Age at metastatic spread ranged between 1.5 years to 74.0 years (mean: 44.7; median: 46) in patients with oligodendrogliomas and between 8 mo and 84.0 years (mean: 31.3; median: 26) in patients with astrocytoma.
Among patients with metastatic oligodendrogliomas, 11 (12%) progressed from grade II to III in the intracranial relapse or in the metastasis, and 1 anaplastic oligodendroglioma recurred as a secondary glioblastoma; 2 cases diagnosed as oligoastrocytomas at the initial diagnosis were reported as oligodendrogliomas at recurrence. Twenty-one astrocytic tumors also displayed tumor progression, and 15 patients received a diagnosis of secondary glioblastoma at the time of recurrence or at microscopic evaluation of the metastasis. Time from the initial diagnosis to metastatic spread of oligodendrogliomas ranged from 0 to 325 mo (mean: 54; median: 36) and from 0 to 276 mo for astrocytic tumors (mean: 31; median: 13) (Table 1). One patient with oligodendroglioma and 10 patients with astrocytic tumors were found with extraneural metastatic disease at the time of the first diagnosis.
Two patients with oligodendroglioma and 8 patients with astrocytic tumors did not undergo any surgical resection before metastatic spread. In 7 cases a diagnostic stereotactic biopsy was performed without open craniotomy; the remaining cases received an autoptic diagnosis. Sixty-three (70%) patients with oligodendroglioma and 51 (76%) patients with astrocytoma received radiation therapy, chemotherapy, or both before metastases occurred, while 12 patients with oligodendroglioma and 8 with astrocytoma did not receive any adjuvant therapy. Twenty patients with astrocytoma underwent VP shunt placement, while among patients with oligodendroglioma, only three required VP shunt placement. Forty-three patients with oligodendroglioma (48%) and 37 patients with astrocytomas (55%) had at least one intracranial recurrence and/or intra-CNS metastatic disease before extra-CNS metastases.
Among oligodendrogliomas, metastases were mainly localized at the bone (n = 48), bone marrow (n = 30), and lymph nodes (n = 27), with cervical stations being the most affected (n = 16). Metastases to the scalp were present in 8 cases. The most common visceral metastatic sites were the lung (n = 10), liver (n = 8), and pleural cavity (n = 5). Kidneys were always spared (Table 1). The most common extra-CNS metastatic sites of astrocytoma were instead bone (n = 30) and lymph nodes (n = 24), and in more than half of the cases the cervical nodal stations were affected (n = 14). The scalp was involved in 8 cases and the soft tissues in 11 cases. Visceral metastases were localized to the lungs (n = 11), liver (n = 8), and kidney (n = 3) (Table 1).
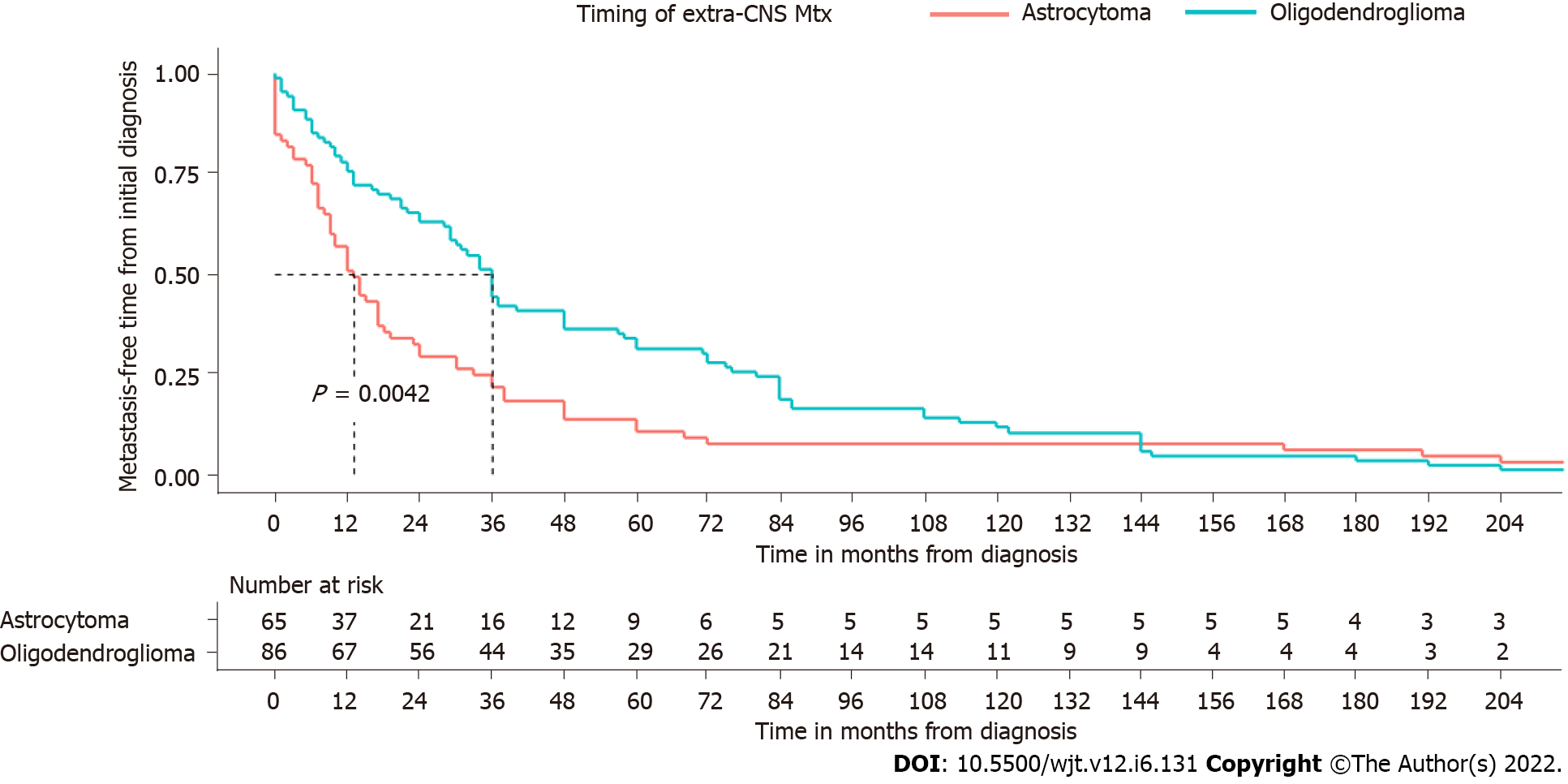
There was a significantly shorter metastasis-free time in patients with astrocytoma than in those with oligodendrogliomas (P = 0.0042), and median time from the diagnosis of the primary tumor to metastatic spread was 36 mo [95%confidence interval (CI): 29-48] in patients with oligodendroglioma and 13 mo in patients with astrocytic tumors (95%CI: 15-41) (Figure 2). There was no significant correlation between timing of metastatic spread and metastatic sites (bone and lymph nodes vs visceral metastases) for both oligodendrogliomas (P = 0.98) and astrocytomas (P = 0.93).
Considering: (1) Surgical procedures; (2) Radiotherapy/chemotherapy; and (3) VP shunt as risk factors for extracranial metastatic spread, in the astrocytoma cohort, 7 patients had extra-CNS metastases without any recognized risk factor, 6 patients displayed only one risk factor, 29 of them had two risk factors, and only 3 patients received all the above-mentioned treatments. All patients with metastatic oligodendroglioma had instead at least one risk factor for extracranial metastatic spread.
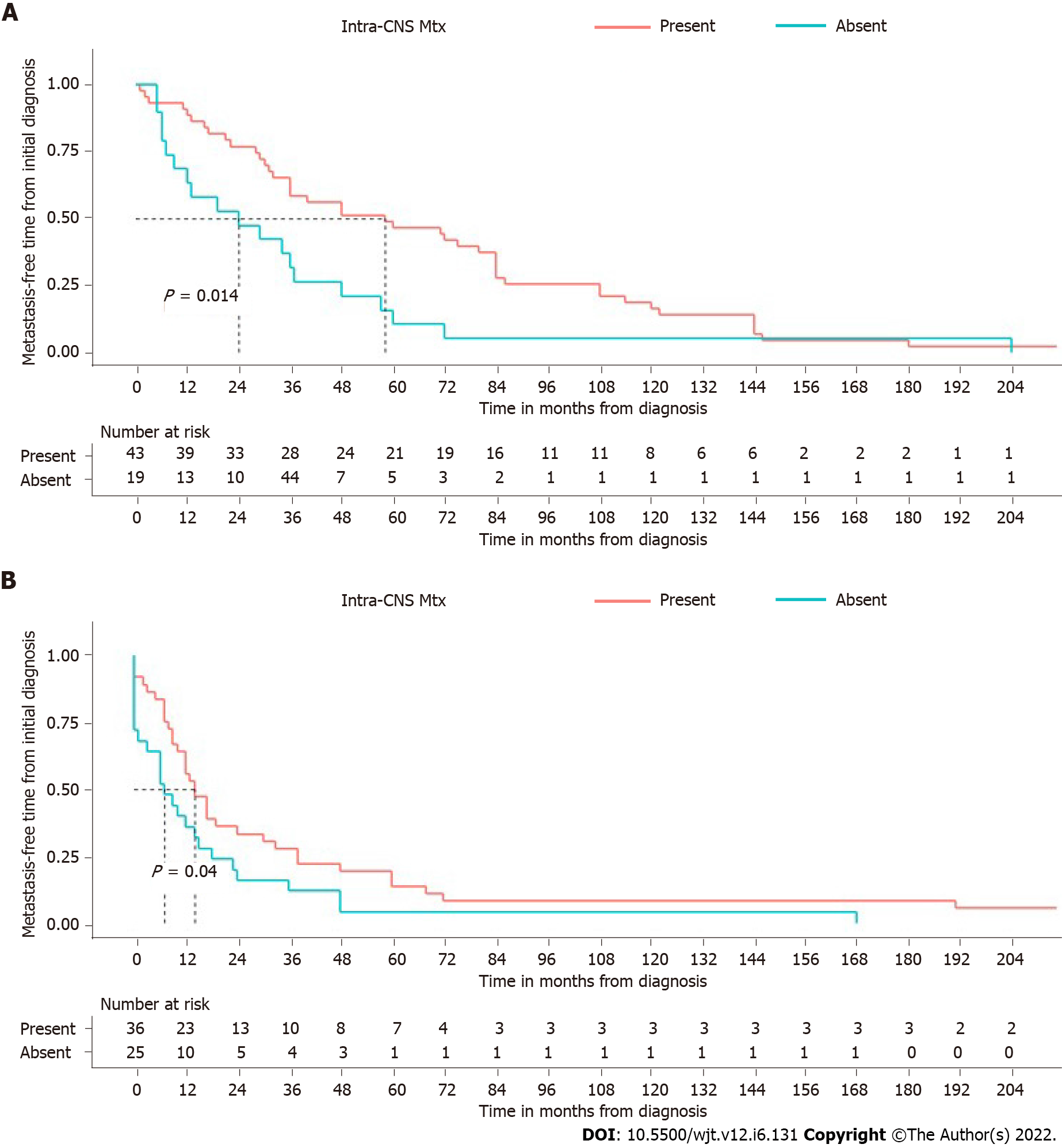
Patients with intracranial recurrence or intra-CNS dissemination of oligodendroglioma had a significantly longer extra-CNS free-time interval (median: 59.8 mo; 95%CI: 36-84) than those who had no local recurrences (median: 24.0 mo; 95%CI: 9-37) (P = 0.014) (Figure 3). The same correlation was present when considering patients with astrocytomas. There is indeed a significant correlation between the presence of intracranial metastases and a longer time before extra-CNS metastatic spread (P = 0.04) (Figure 3).
In this study, we reviewed the literature on oligodendrogliomas and astrocytomas with extra-CNS metastases. Based on the present review, extra-CNS metastasis of these tumor entities may occur, independently from the grade of the primary neoplasm. Indeed, the reported cases of extra-CNS metastases were roughly similar in lower and higher grade oligodendrogliomas. This distinction appears to be less sharp taking into account extraneural metastases from astrocytomas since in many articles the tumor grade is not specified, while terms such as “low grade”, “aggressive” or “malignant” are used as substitutes of the grading system. Indeed, it should be noted that the criteria for tumor grading changed substantially over the past decades. As an example, the tumor reported by James and Pagel[21] in 1951 as oligodendroglioma showed areas of necrosis and moderately conspicuous mitotic activity, which are nowadays considered diagnostic criteria of a higher grade oligodendroglioma. These limitations are partly shared by many transplantation registry data, whose reports cover a wide timespan and in the past were often incomplete, not providing data on donors’ tumor histotypes or the interval between performed treatments and donation[22,23]. According to the Disease Transmission Advisory Committee, recurrence-free survival can be used as a surrogate for transmission risk and donors, with a history of neoplasm diagnosed 5 or more years earlier and with a probability of cure of > 99% are considered at low risk for tumor transmission, while neoplasms with a probability of cure between 90% and 99% are considered at intermediate risk of transmission[24].
According to this literature review, while the extraneural spread of PBT appears to be an earlier event in astrocytic tumors, in oligodendrogliomas it can occur after more than 10 years from the primary diagnosis in a non-negligible number of patients. Indeed, the interval between diagnosis and metastatic spread varied widely among patients, and many of them underwent multiple treatments that have possibly interfered with the natural history of the tumor[25]. Therefore, the possibility of metastatic spread even after many years should be carefully considered when selecting eligible donors for organ transplantation. In light of these findings, taking into account that diffuse gliomas preferentially metastasize to the bone and cervical lymph nodes, we suggest that protocols for potential donors with a present or past history of oligodendroglioma should include ultrasound imaging of the head and neck and/or computerized tomographic scan of the skeleton. A minority of patients also had metastases in transplantable organs such as lungs, liver, and pancreas, while metastases to kidney and heart were not reported in oligodendrogliomas, suggesting that these organs are relatively spared from metastatic spread. This is in accordance with two studies on donors with glioblastoma that described a better outcome in recipients of kidneys than in those with lung or liver grafts and worse outcomes in patients with liver metastases compared to those with other extracranial metastatic sites[9,26].
Of note, patients with intracranial tumor relapse had a significantly longer interval between the initial diagnosis and the metastatic spread. Additionally, we found that patients who had multiple surgeries for intra-CNS relapses or metastases developed extra-CNS disease after a longer time interval than those who had a single surgery. We may speculate that patients with intracranial relapses or metastases have tumors with a lower biological aggressiveness and that acquire “visceral” metastatic potential only in a later stage.
The present review has several limitations. First, we did not include in the literature search articles reporting extracranial metastases from primary glioblastomas, currently classified as grade IV tumors according to the WHO[18]. Moreover, the selected literature covers a wide timespan, and inevitably the changes in the classification of tumor entities and in grading systems represent a limitation to every systematic review on this topic. It should be noted, indeed, that most of the articles included in this review were published before the 2016 update of the WHO classification of CNS tumors and do not always include data on 1p19q codeletion and IDH1/2 mutations[18].
In conclusion, despite the relatively low propensity to metastasize outside the CNS of oligoden
Under extended criteria, patients with a history of primary brain tumor can be eligible for organ donation. Tumor histotype and tumor grade are considered the main risk factors of tumor transmission, and previous surgeries, chemo-/radiotherapy, and ventriculo-peritoneal shunt placement concur to increase the transmission risk.
Most of the literature on the extraneural metastatic spread of diffuse gliomas is based on case reports and case series, and there is a lack of systematic appraisal of patterns of metastatic spread- and on factors concurring to increase the risk of extraneural spreading.
We aimed to collect and analyze the existing literature on extraneural spreading of oligodendroglial and astrocytic tumors in order to identify clinical or pathological factors that could help clinicians to assess the risk of tumor transmission from donors with a history of these gliomas and guide decision making in organ transplantation.
We performed a systematic review of the literature in accordance with the PRISMA guidelines. A literature search without language restrictions was performed in the electronic databases MEDLINE-PubMed and EMBASE, searching for articles, case reports, and case series reporting data on extra-central nervous system metastases of oligodendrogliomas and astrocytomas.
Elapsed time from the initial diagnosis to metastatic spread ranged from 0 to 325 mo and from 0 to 276 mo for oligodendrogliomas and astrocytic tumors, respectively. The most common metastatic sites were bone and lymph nodes for both tumors, while the most common visceral sites were the lungs and the liver in patients with oligodendrogliomas and lungs, liver, and kidneys in patients with astrocytomas. Among patients with astrocytomas, 7 did not undergo surgery, chemo-/radiotherapy or ventriculo-peritoneal shunt placement before the onset of metastases.
A long interval between the tumor diagnosis and the donor’s death does not exclude the possibility of extraneural spreading of these tumors. Bone and lymph nodes are the most common metastatic sites; the lungs and the liver are instead the preferential visceral sites of metastatic spread. Follow-up imaging of the skeleton and cervical lymph nodes could be useful to identify metastatic disease in donors with a history of these gliomas.
The diagnostic advances made recently in tumor classification and targeted follow-up protocols could improve the knowledge on the factors involved in extraneural spreading of gliomas, with repercussions on the tumor transmission risk assessment of potential donors.
| 1. | Kauffman HM, Bennett LE, McBride MA, Ellison MD. The expanded donor. Transplant Rev. 1997;11:165-190. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | López-Navidad A, Caballero F. Extended criteria for organ acceptance. Strategies for achieving organ safety and for increasing organ pool. Clin Transplant. 2003;17:308-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (9)] |
| 3. | Eccher A, Girolami I, Motter JD, Marletta S, Gambaro G, Momo REN, Nacchia F, Donato P, Boschiero L, Boggi U, Lombardini L, Cardillo M, D'Errico A, Neil D, Segev DL, Zaza G. Donor-transmitted cancer in kidney transplant recipients: a systematic review. J Nephrol. 2020;33:1321-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Eccher A, Girolami I, Marletta S, Brunelli M, Carraro A, Montin U, Boggi U, Mescoli C, Novelli L, Malvi D, Lombardini L, Cardillo M, Neil D, D'Errico A. Donor-Transmitted Cancers in Transplanted Livers: Analysis of Clinical Outcomes. Liver Transpl. 2021;27:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Warrens AN, Birch R, Collett D, Daraktchiev M, Dark JH, Galea G, Gronow K, Neuberger J, Hilton D, Whittle IR, Watson CJ; Advisory Committee on the Safety of Blood, Tissues and Organs, UK. Advising potential recipients on the use of organs from donors with primary central nervous system tumors. Transplantation. 2012;93:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | European Directorate for the Quality of Medicines & HealthCare. Guide to the Quality and Safety of Organs For Transplantation. Strasbourg: Council of Europe, 2018. |
| 7. | Subramanian A, Harris A, Piggott K, Shieff C, Bradford R. Metastasis to and from the central nervous system--the 'relatively protected site'. Lancet Oncol. 2002;3:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Armanios MY, Grossman SA, Yang SC, White B, Perry A, Burger PC, Orens JB. Transmission of glioblastoma multiforme following bilateral lung transplantation from an affected donor: case study and review of the literature. Neuro Oncol. 2004;6:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Jimsheleishvili S, Alshareef AT, Papadimitriou K, Bregy A, Shah AH, Graham RM, Ferraro N, Komotar RJ. Extracranial glioblastoma in transplant recipients. J Cancer Res Clin Oncol. 2014;140:801-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Jonas S, Bechstein WO, Lemmens HP, Neuhaus R, Thalmann U, Neuhaus P. Liver graft-transmitted glioblastoma multiforme. A case report and experience with 13 multiorgan donors suffering from primary cerebral neoplasia. Transpl Int. 1996;9:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Buell JF, Trofe J, Sethuraman G, Hanaway MJ, Beebe TM, Gross TG, Alloway R, First MR, Woodle ES. Donors with central nervous system malignancies: are they truly safe? Transplantation. 2003;76:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Penn I. Transmission of cancer from organ donors. Ann Transplant. 1997;2:7-12. [PubMed] |
| 13. | Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Deceased donors with a past history of malignancy: an organ procurement and transplantation network/united network for organ sharing update. Transplantation. 2007;84:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Chui AK, Herbertt K, Wang LS, Kyd G, Hodgeman G, Verran DJ, DeLeon C, Sheil AG. Risk of tumor transmission in transplantation from donors with primary brain tumors: an Australian and New Zealand registry report. Transplant Proc. 1999;31:1266-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Pokorna E, Vítko S. The fate of recipients of organs from donors with diagnosis of primary brain tumor. Transpl Int. 2001;14:346-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Watson CJ, Roberts R, Wright KA, Greenberg DC, Rous BA, Brown CH, Counter C, Collett D, Bradley JA. How safe is it to transplant organs from deceased donors with primary intracranial malignancy? Am J Transplant. 2010;10:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Lee MS, Cho WH, Ha J, Yu ES, Jeong YS, Oh JS, Lee JR, Lee JM. Safety of Donation From Brain-dead Organ Donors With Central Nervous System Tumors: Analysis of Transplantation Outcomes in Korea. Transplantation. 2020;104:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 18. | Louis DN, Ohgaki H, Wisteler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Refeinberger G, von Deimling A. WHO Classification of Tumors of the Central Nervous System. Revised 4th ed. Lyon: IARC, 2016: 16-74. |
| 19. | Liwnicz BH, Rubinstein LJ. The pathways of extraneural spread in metastasizing gliomas: a report of three cases and critical review of the literature. Hum Pathol. 1979;10:453-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 14289] [Article Influence: 1428.9] [Reference Citation Analysis (1)] |
| 21. | James TG, Pagel W. Oligodendroglioma with extracranial metastases. Br J Surg. 1951;39:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Nalesnik MA. Donors With Central Nervous System Cancer: Proceed With Vigilance. Transplantation. 2020;104:458-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Penn I. Questions about the use of organ donors with tumors of the central nervous system. Transplantation. 2000;70:249-250. [PubMed] |
| 24. | Nalesnik MA, Woodle ES, Dimaio JM, Vasudev B, Teperman LW, Covington S, Taranto S, Gockerman JP, Shapiro R, Sharma V, Swinnen LJ, Yoshida A, Ison MG. Donor-transmitted malignancies in organ transplantation: assessment of clinical risk. Am J Transplant. 2011;11:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Perry A. Metastatic Oligodendroglioma: A Mini-Epidemic? Adv Anat Pathol. 2004;11:325. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Cunha MLVD, Maldaun MVC. Metastasis from glioblastoma multiforme: a meta-analysis. Rev Assoc Med Bras (1992). 2019;65:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Parajuli S, United States; Yu F, China A-Editor: Zhu JQ, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ