Published online Sep 18, 2021. doi: 10.5500/wjt.v11.i9.372
Peer-review started: January 29, 2021
First decision: April 6, 2021
Revised: April 10, 2021
Accepted: August 19, 2021
Article in press: August 19, 2021
Published online: September 18, 2021
Processing time: 228 Days and 13.6 Hours
The increased awareness of systemic sclerosis (SS) and its pathogenetic back
Core Tip: The current progress in the management of systemic sclerosis has its impact on improving patient’s survival and quality of life. Patients developed scleroderma renal crisis have greatly managed after commencing angiotensin converting enzyme inhibitors. Moreover, scleroderma patient with kidney failure has a marvelous thera
- Citation: Abbas F, El Kossi M, Shaheen IS, Sharma A, Halawa A. Journey of a patient with scleroderma from renal failure up to kidney transplantation. World J Transplant 2021; 11(9): 372-387
- URL: https://www.wjgnet.com/2220-3230/full/v11/i9/372.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i9.372
Scleroderma or systemic sclerosis (SS) is an autoimmune disorder that comprises vasculopathy, inflammatory changes, deposited collagen and fibrotic alterations involving the skin and vital organs. The involved organs are usually related to the current types of autoantibody found in SS patients. However, two types have been considered with SS cutaneous involvement: Limited cutaneous SS (lcSS) with thickened skin involving the elbow and knee joints, and the diffuse type (dcSS) with widespread skin affection. SS is primarily seen in females, with a prevalence rate of 7-489 case(s) per million population (PMP) and an incidence of 0.6-122 case(s) PMP/year, with geographic variability[1-3]. Systemic SS involvement can be observed as pul
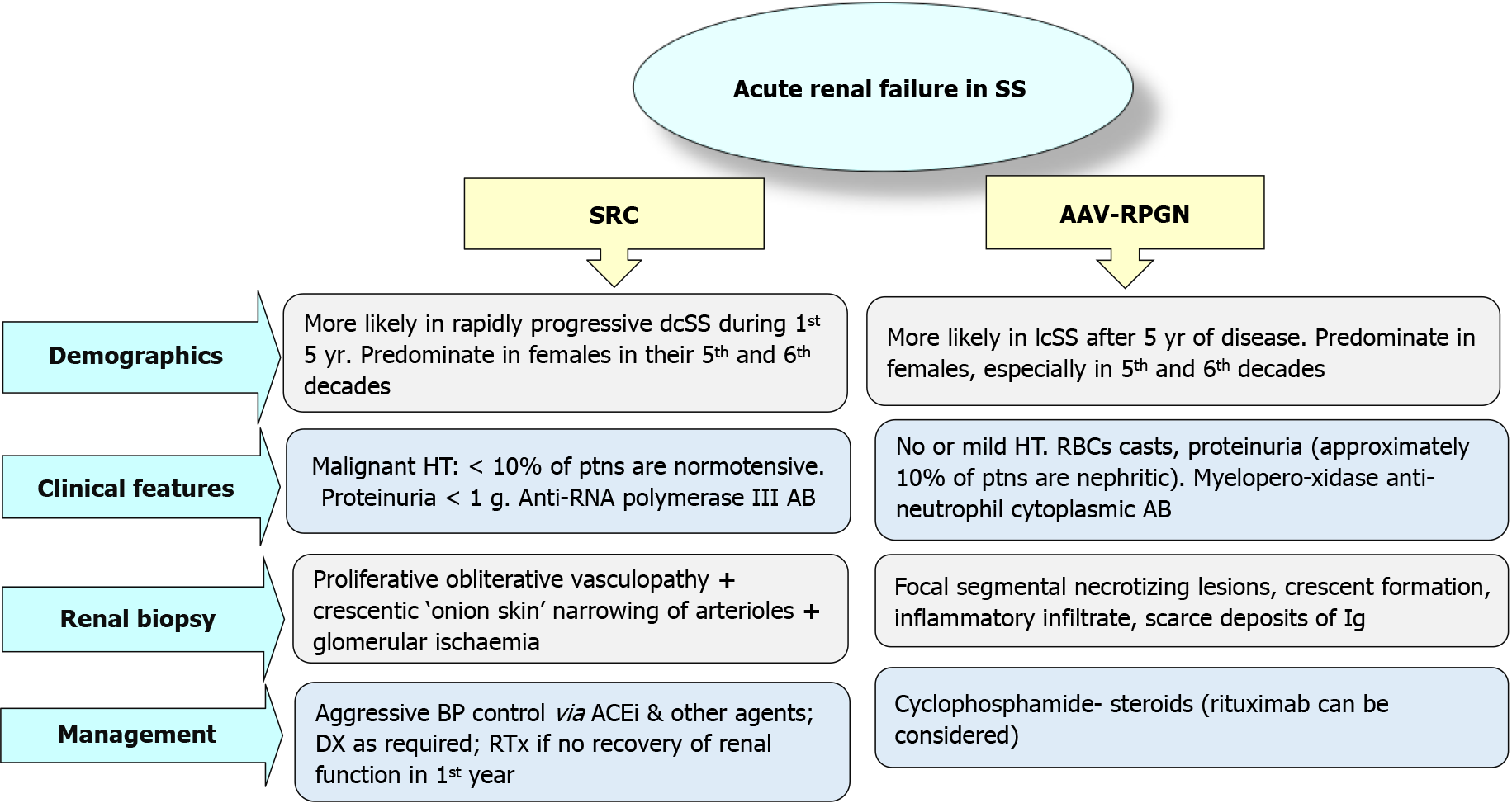
Microangiopathic hemolytic anemia (MAHA) can be seen in almost half of cases that can be manifested by a proteinuria/hematuria syndrome with red blood cells fragmentations[7,8]. SRC is more commonly observed with the diffuse type of SS as compared with the limited one, particularly with the rapidly progressive dcSS in the first 3-5 years of disease onset. Predictors of SRC may include the following: (1) Anti-RNA polymerase III autoantibodies; (2) Tendon friction rub, and synovitis[9]; and (3) Steroid therapy (> 7.5 mg/d) may induce a dose-related impact on the SRC evolution risk[7,10].
Furthermore, and despite controversial, angiotensin converting enzyme inhibitors (ACEi) therapy before the sudden rise in BP and SCr level elevations may be accompanied with a higher risk of dialysis (DX) or mortality rates (MR)[7,10,11].
The characteristic features of SRC may include: (1) New onset; (2) Moderate/severe HT; (3) Acute rise in SCr[12,13]; and/or (4) Almost half of cases may show MAHA[7,14].
On contrary to this definition, cases with an acute rise in SCr with normal BP are named the normotensive renal crises (10% of cases)[14]. With absence of a definite etiology, kidney biopsy may be warranted to settle the diagnosis and clarify the prog
Incidence: Age- and sex-adjusted incidence of renal replacement therapy (RRT) for scleroderma-induced end-stage renal disease (ESRD) in the period from 2002 to 2013 approached only 0.18 PMP with insignificant decline in SS incidence by time. Scle
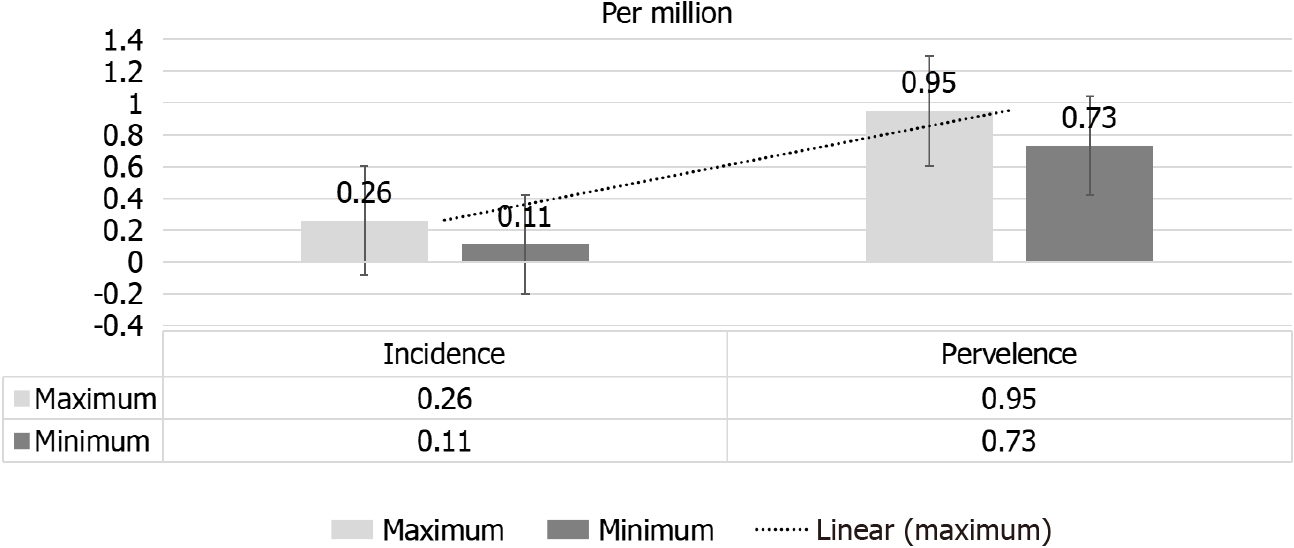
On the other hand, a significant rise in SS prevalence from 0.80 pmp in 2002 to 0.89 pmp in 2013. A higher prevalence of scleroderma in North America and Australia as compared to Europe or Asia has been observed[17,18]. In view of the improving patients’ outcome and increased awareness of the nature of the disease, an increased prevalence of SS has been reported[2] this is despite the lower incidence of SRC that has been given by a more recent report[19]. A significant decline in RRT-dependent SS patients in Australia and New Zealand in the period between 2002 to 2013, from 0.51 pmp to 0.18 pmp[20]. However, Hruskova et al[16], observed an insignificant nominal decline in incidence of RRT-dependent SS patients[16]. The observed fluctuation in incidence has been expected considering the rarity of this disease. Between 2002 and 2013, the range of adjusted annual incidence and prevalence rates of RRT for SS-induced ESRD were 0.11-0.26 and 0.73-0.95 pmp), respectively[16] (Figure 2).
Pathophysiology: The vasculopathy-induced decline in kidney perfusion as well as the activated endothelial cell are considered the main contributors in SRC deve
More than 80% of SRC patients may exhibit the diffuse type of cutaneous involvement, particularly that is characterized by a rapidly progressive behavior. Previous data have recognized the predominant indicators of SRC evolution as follows: (1) Newly diagnosed anemia; (2) Cardiac involvement (e.g., pericarditis and congestive heart failure); (3) Rapidly developed skin thickening; (4) Systemic inflammations: Arth
Recognition of acute renal failure (ARF) as a sequala of SS is not always clear. About 10%-20% of SS patients could be presented with normal BP[14], moreover, SRC could be their firstly observed manifestation of SS[8]. Differential diagnosis (DD) may include: (1) Lupus Nephritis[21]; (2) Thrombotic thrombocytopenic purpura[28]; (3) Crescentic rapidly progressive glomerulonephritis (RPGN); and (4) Anti-neutrophil cytoplasmic antibody (ANCA)-related glomerulonephritis (GN).
Other DD may include membranous and membranoproliferative GN, other vas
Almost 90%-95% of SS patients may experience circulating antinuclear antibodies that could be detected via one of the following: Immunofluorescence, enzyme-linked immunosorbent assay, immunodiffusion, in addition to immunoblotting. A variety of antinuclear antibody specifically related to SS including antibodies to topoisomerase (anti-TOPO I), kinetochore proteins, RNA polymerase enzyme (anti-RNAP III), ribonuclear proteins and nucleolar antigens. Clinically, these autoantibodies specified to SS disease could be currently linked to distinct clinical criteria. So, the identification of a particular antibody could be crucial in anticipating certain organ affection that would be reflected on its timely control[29].
Kidney biopsy is not usually mandated for a patient presented with classic clinical criteria that include a newly presented and symptomatizing HT, elevated serum creatinine levels and a normal urine sediment. However, with a normotensive patient and raised creatinine levels with/without active urine sediment, a kidney biopsy may provide a suitable diagnostic and therapeutic guide particularly if ANCA-positive RPGN was a possibility and to exclude other comorbidities[21]. Moreover, a kidney biopsy has a prognostic implication for dialysis dependent SRC patients regarding enrolment in a kidney transplant (KTx) list. The current recommendation is to postpone KTx up to 18-24 mo after commencing DX if signs of kidney function recovery were not observed along 12 mo. A potential kidney donor should be screened for a timely provided transplant and better quality of life[12].
The 5-years patient’s outcome in SRC has not improved after the advent of ACEi therapy[7,8,14], more plans to improve SRC outcomes are currently warranted. Pilot reports with ET receptors antagonists (ERAs) therapy have reported a reasonable safety and potential efficacy to proceed to randomized controlled trials to recognize the feasibility of ERA in limiting DX requirements and improve patient’s survival[12].
To mitigate the risk of SRC evolution, the following measures have been suggested.
BP monitoring, SCr concentrations and periodic urinalysis for patients with the following criteria: (1) Tendon friction rub[26]; (2) Large joints contracture[27]; (3) Arthralgia/synovitis[9,10,25]; (4) Steroid therapy[10,25]; (5) Early, diffuse skin involve
The least dose of steroids for the minimal period allowed to manage the inflammatory manifestations[27,33] should be utilized.
Manage essential HT via non-ACEi regimen with calcium channel blockers (CCB) included as much as possible[33].
Start CCB for peripheral vasculopathy[33].
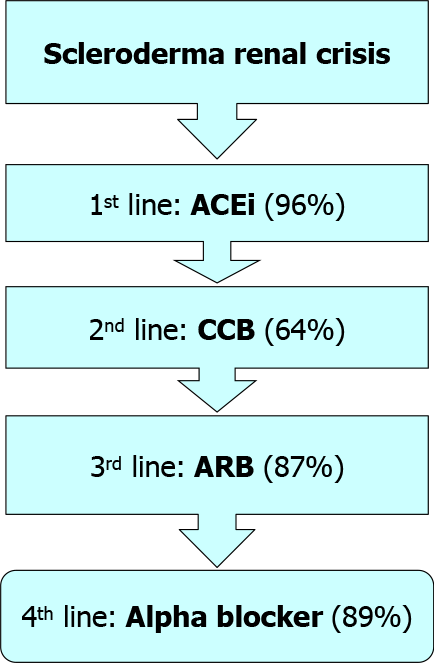
It is noteworthy to mention that SRC therapeutic algorithm is currently stable for a long time. The current algorithm is simple (Figure 4), as same agents have been administrated to mild as well as sever cases with better experts’ agreements from 66% to 81%. Since the advent of ACEi, no fundamental changes have been introduced into SRC therapeutic strategies[34]. Tight and rapid blood pressure management can be achieved via the addition of other antihypertensive agents. In this concept, angiotensin receptor blockers (ARBs) have been replaced by the CCBs as a second therapeutic line. Forty percent of experts would prefer keeping ACEi-despite the associated increased risk of fetal anomalies in pregnant women-if there is a history of SRC to avoid an increased risk of SRC recurrence in case of withdrawal of these agents[35].
Renal vasculopathy per se cannot be considered a risk factor for SRC evolution. Owing to the growing awareness of SRC prophylactic measures, prevalence rates have been declined. Prophylactic measures against SRC development might include tight BP control in patients with early dcSS and rapid progress of skin manifestations, particularly with associated anti-RNAP III antibodies. Furthermore, the finding of active inflammation as evidenced by the presence of tendon friction rub and/or arthritis should pay patient’s and his physician’s attention to an increasing risk of SRC development. Given the robust association between steroid use and the evolution of SRC, this type of therapy should be limited to its lowest dosage with the possible accepted shortest period of therapy. However, the 5-years patients’ survival of 50%-70% has been reported by many studies, this high percentage should be improved. Current management primarily depends on an early diagnosis, tight control of BP via ACEi and other agents and/or DX therapy whenever required.
For earlier SRC detection, risky patients should be asked to provide three BP readings at home at least every week, with a higher allowed level of BP > 140-150/90 mmHg. Repeat measuring after one hour, if still high, patient should contact his physician and SCr concentration should be provided with a reasonable dose of ACEi should be instituted, and patient hospitalization may be considered. However, using these agents prior to the onset of SRC may be associated with a higher risk of mortality in dcSS patients within the first 4-5 years[7,10]; ACEi therapy at this period is not currently advised. Regarding SRC in lcSS patients, safety of these agents still uncertain in view of rarity of cases and data sparsity. A retrospective study of Italian SS patients (410 with SS < 5 years), postulated that dihydropyridine CCB agents may be associated with a lower risk of SRC evolution (P < 0.001)[12,33].
Many studies in the literature have reported poor outcome for SS patients with ESRD on dialysis[25]. For example, the French “REIN” registry, 98 SS patients dialyzed between 2001 and 2013, 81% developed ESRD secondary to SRC, while patients’ survival was reported to be 75%, 55% and 32% within 1, 3- and 5-years respectively[36].
ACEi have greatly improved SS patients’ outcome[37]. One report studied 145 ACEi-treated SRC patients has showed the following: (1) 61% showed good outcome: 38%: No need for DX and 23% commenced temporary dialysis; and (2) 38% showed poor outcome: 19% was survived on DX and 19% died within 1st 6 mo[38].
A non-invasive prognostic technique is to estimate the N-terminal pro-b-type natriuretic peptide (NT-proBNP) to predict the need of DX in SRC patients. It has been shown that SRC patients requiring permanent, transient, or no DX have exhibited NT-proBNP levels of 3373 pg/mL, 1729 pg/mL, and 119 pg/mL, resp[39]. However, the role of NT-proBNP renal clearance has not been settled and well-controlled pros
Whilst Hruskova et al[16] reported that SS patients were less vulnerable for peritoneal dialysis (PD) than hemodialysis (HD) therapy as compared to matched controls, registries coming from Australian and New Zealand reported more common use of PD in SS patients as compared to patients with other etiologies of ESRD. This finding may be explained by the more frequency of PD therapy in Europe[40]. Optimal option, however, still uncertain[16,41].
The unfavorable outcome for SS patients on RRT therapy has been observed in several reports[40,42], moreover, RRT for this cohort of patients was an independent predictor of mortality[40]. Recent reports agreed with these findings particularly among dia
Data from two large studies (more than 100 SRC cases on DX) showed that kidney function has recovered in 40%-50% of cases within 8 mo in the first study, and within 11 mo in the other one. On the other hand, the Australian/New Zealand DX and Tx registries have observed that only 10% of cases have recovered a reasonable kidney function to be withdrawn from DX, and recovery was observed within the 1st 12-18 mo after commencing DX. A given explanation to the diminished recovery rates was that the cases with earlier kidney recovery (< 3 mo of DX institution) have been excluded[12] (Figure 5).
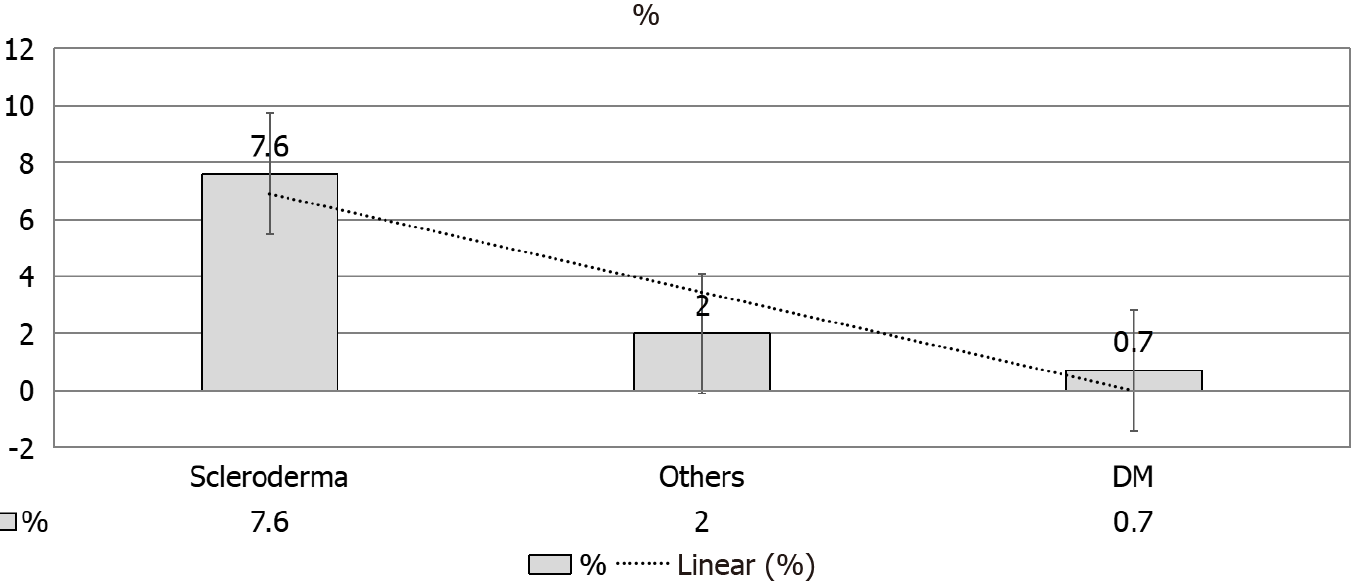
Renal recovery in this study[40] agreed with Hruskova et al[16] (7.6%). The latter study has reported a higher recovery rate in SS-induced ESRD patients as compared to other etiologies of ESRD (Figure 5). Of note, autoimmune disease may show a higher rate of recovery as compared to other primary renal diseases[43]. The robust possi
RRT, either HDX or PD as well as KTx are all potentially offered to SRC-induced ESRD patients. The latter option i.e., KTx is known to be the best therapeutic one for this cohort of ESRD patients with the best offered outcome[44]. A thorough evaluation of the KTx recipients (KTRs) regarding stabilization of BP, various co-morbidities, and the possibility of renal recovery. The latter may necessitate basal data that can be obtained from a kidney biopsy. In addition, assessment of SRC disease activity may warrant an estimation of renin and ET-1 levels[12].
The introduction of ACEi in SS therapy has greatly alleviated the SCR-related poor kidney and overall outcome, with an expected reversal of this serious syndrome[41,45,46]. Nevertheless, almost half of these patients still requiring long-term RRT including KTx[47]. As compared to other primary kidney diseases, old reports have observed poorer patient and allograft survival[47]. However, Bertrand et al[36] presented an observational study including 34 patients with SS who received KTx and uniquely reported the evolution of post-transplant extrarenal involvement[36].
The proper time of renal transplantation for patients with SS requiring RRT still uncertain. SS patients are mostly experienced SRC, with about 1%-5% of them showing ANCA-associated vasculitis or MAHA[12]. Depending on the observation that 25% of patients with SRC/ESRD may recover kidney function within almost one year of DX[8,11,25,41], four articles have been published showing their experience in postponing dialysis until the point of time at which the recovery of kidney function is not certain and KTx is currently indicated[40,44,48,49].
The relative consideration of scleroderma as a highly probable disease of renal recovery, even with prolonged dialysis[50], leads to the recommendation by some experts that dialysis should be continued for at least two years before an attempt to offer a kidney to an SS patient[51]. On the other hand, Canadian guidelines admitted two conditions for offering a KTx: (1) Six months-at least-free of cytotoxic medications should be elapsed prior to any attempt of KTx; and (2) Limitation of the extrarenal manifestations[16,52].
Renal recovery, however, has been reported to be as greater as 38% in previous studies[20].
Richardson[53] were firstly performed a KTx to an SS patient[53]. They were generally considering KTx a safe procedure as long as the kidney was the primary organ involved with relative stability of other lesions[36].
The role of immunosuppressive agents in KTx is crucial in improving the systemic manifestations in SS patients. However, there is no consensus in this particular setting. Ruiz et al[54] (1991) have postulated that CyA should be excluded from the immuno
Nevertheless, owing to the relatively small number of the studied patients, an ideal protocol for immunosuppression cannot be established yet. A reasonable and com
Gibney et al[44] have reported the development of skin lesions in four SS KTRs, with noticeable improvement according to the “Rodnan score”. Considering the intensity of disease activity prior to and after KTx, this study lacks the clinico-biological data base owing to its retrospective nature[44].
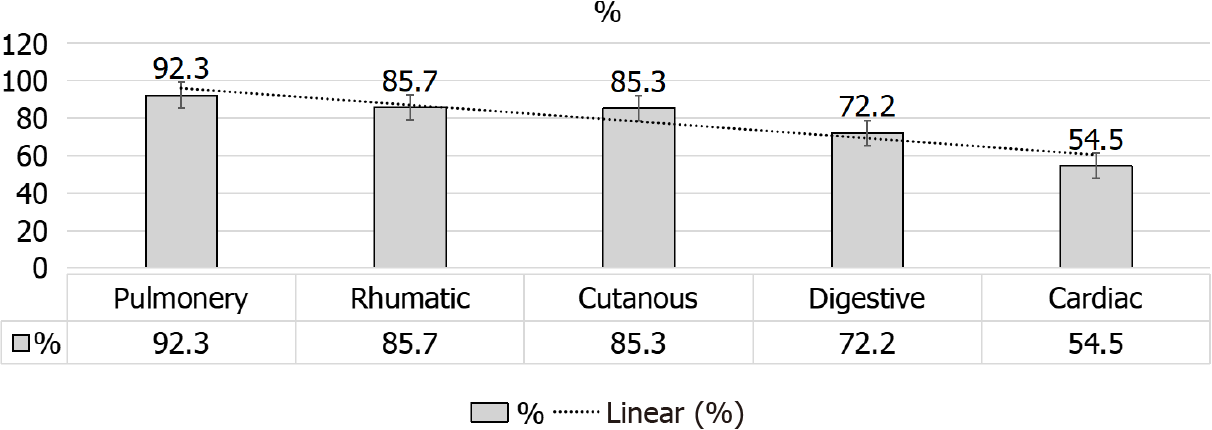
However, Bertrand et al[36], study provides-for the 1st time-broad data base about the extrarenal manifestations during and post KTx. Despite the observed general stability of this disorder, the provided data shed the light on the importance of the cardiac and GI involvement that may getting worse after KTx (Figure 6). Accordingly, close monitoring of extrarenal manifestations would be crucial prior to and after KTx, up to the extent that stabilized extrarenal manifestations is a robust indication to proceed to KTx. This concept might be intensified by the multicenter nature of Bertrand et al[36], study. In addition, pulmonary involvement in an SS patient was considered as a post-transplant independent risk factor of death in this study. However, Pulmonary involvement in SS patients could be classified into two main categories: (1) Primary pulmonary affection (i.e., lung parenchymal disease and pulmonary HT, PH); and (2) Secondary pulmonary involvement (i.e., airway disease owing to bronco-aspiration that usually results from gastro-esophageal reflux disease, drug-induced lung toxicity and infectious causes)[55-57].
In non-transplant cohort, the associated parenchymal pulmonary disease, PH, and kidney involvement is complicated by a higher MR[58]. An associated interstitial lung disease or PH is responsible for 60% of the total MR in this cohort[57]. However, Bertrand et al[36] observed that in the transplant cohort, pulmonary affection appears to exert a similar impact on MR. Accordingly, a particular caution prior to KTx must be directed to explore and evaluate the presence of parenchymal pulmonary disease or PH that may preclude a KTx[36].
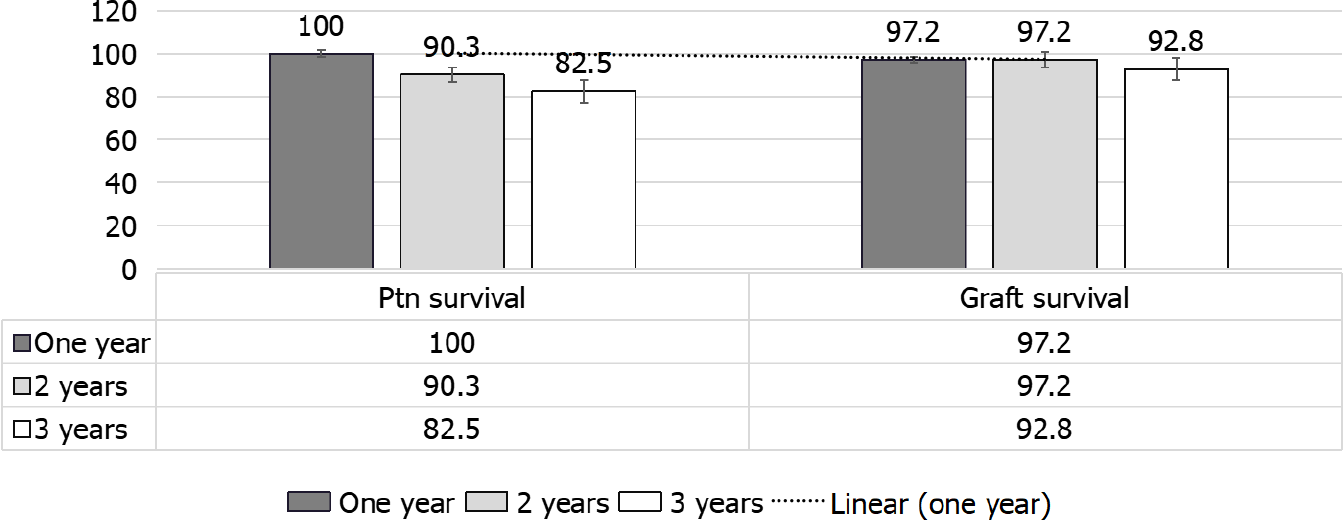
Whilst the patient survival was 100%, 90.3% and 82.5 %, the death-censored allo
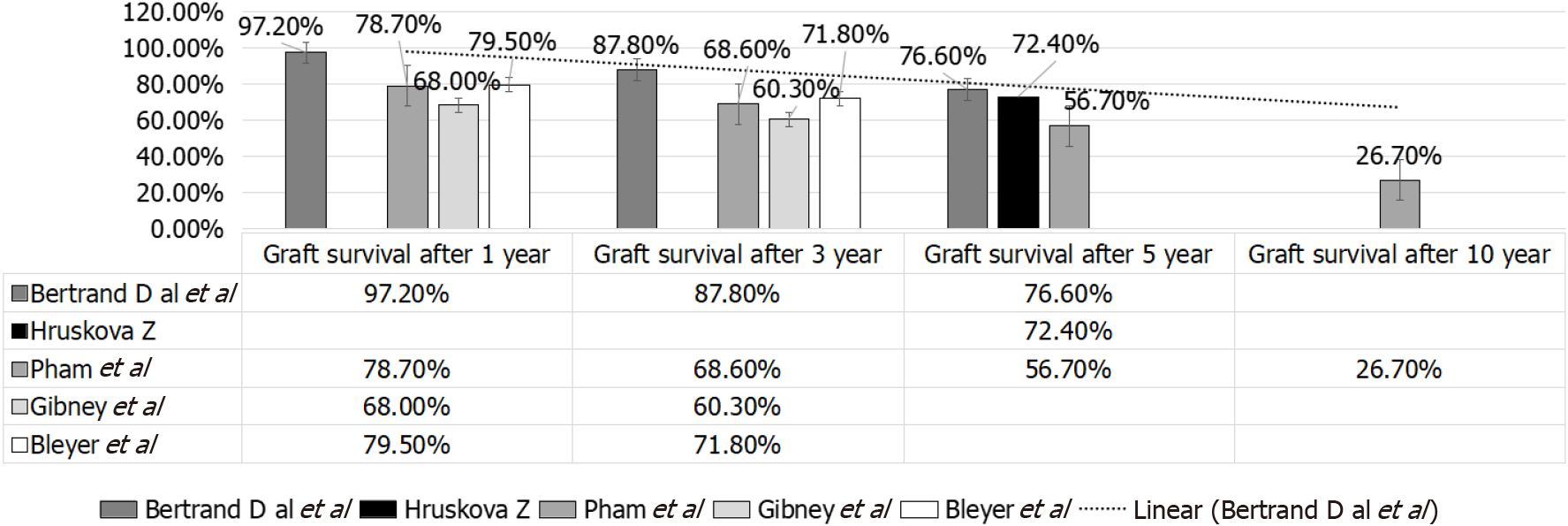
In Bertrand et al[36] study, no early deaths with a functioning graft have been observed, and the primary non-functioning graft due to possible recurrent SRC has been reported in only one patient[36]. In Pham et al[59], on the other hand, the non-death-censored graft outcome at 1, 3, 5 and 10 years, resp, were 78.7%, 68.6%, 56.7% and 26.7% (UNOS: 1987-2004) that was far lower than that given by Bertrand et al[36]. Moreover, Bertrand et al[36], reported graft survival in SS patients that was far less than that observed in other primary renal disorders (79.5% and 71.8% after 1 and 3 years)[47] (Table 1 and Figure 8). Of note European and United States reports have reported poorer graft outcome as compared to that observed with other primary renal diseases[47].
In Bertrand et al[36], study, the death-censored graft outcome was excellent and comparable to that was reported by the global French cohort of KTx from 1993 to 2010, 91.2% after 1 year, and 79.7% after 5 years resp, (Agence de Biomédecine, annual report)[36]. They depended on the given data base that were more recent (1987-2012) as compared to that in prior literature, that may partially explain their better results. In fact, more potent immunosuppression regimen is more beneficial not only for rejection, but also for SS management, in addition to the better KTRs selection, taken together may improve graft outcome[36]. Bertrand et al[36], study was limited by the number of the included KTRs. Nevertheless, crucial information particularly that related to extrarenal manifestation in SS patients and its development after KTx were lacking.
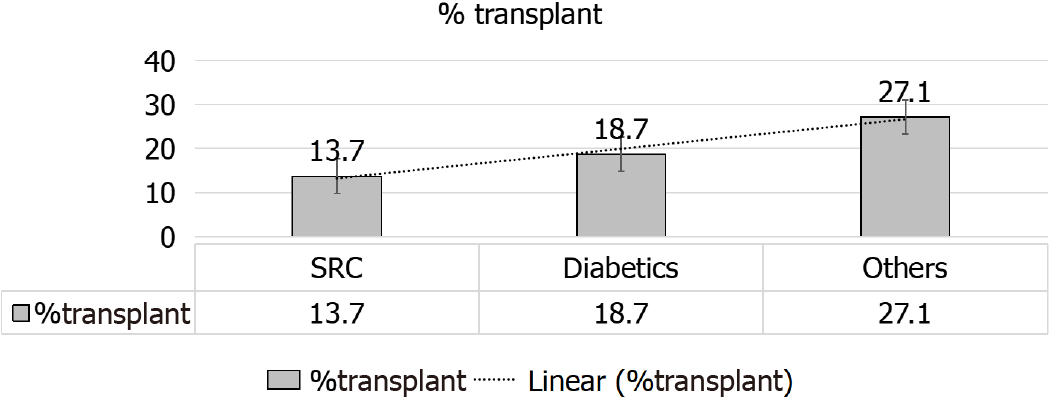
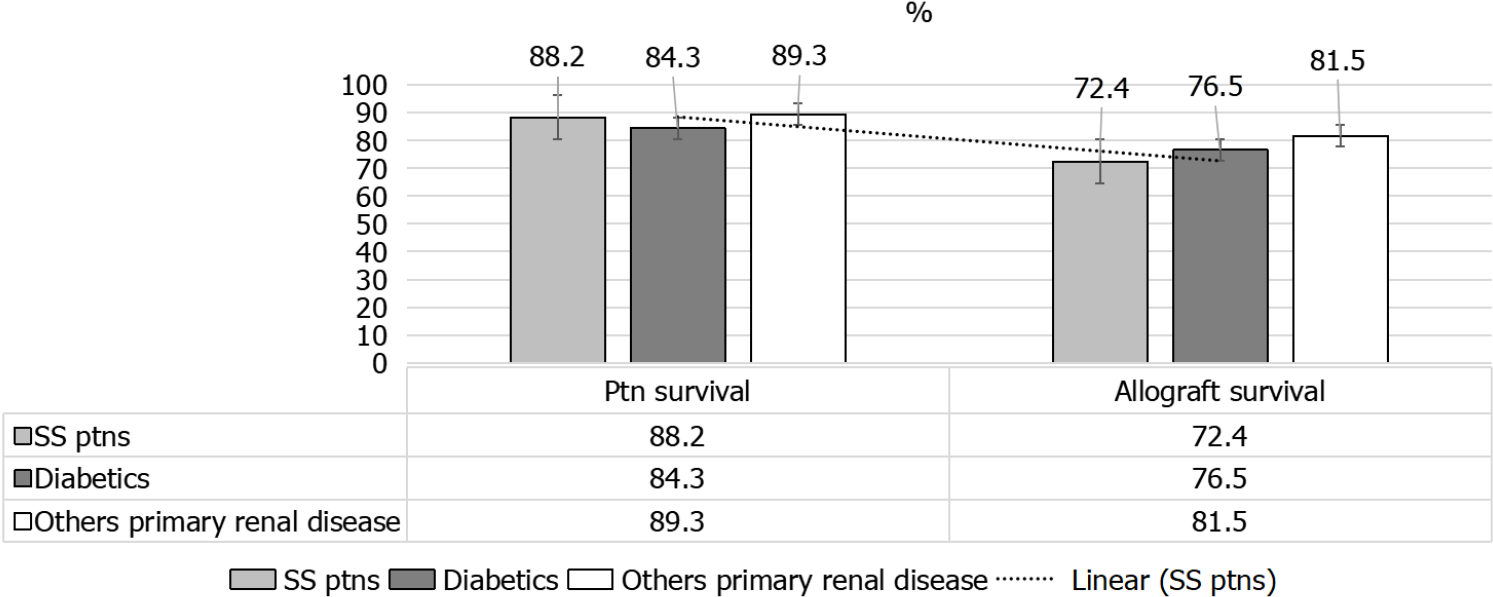
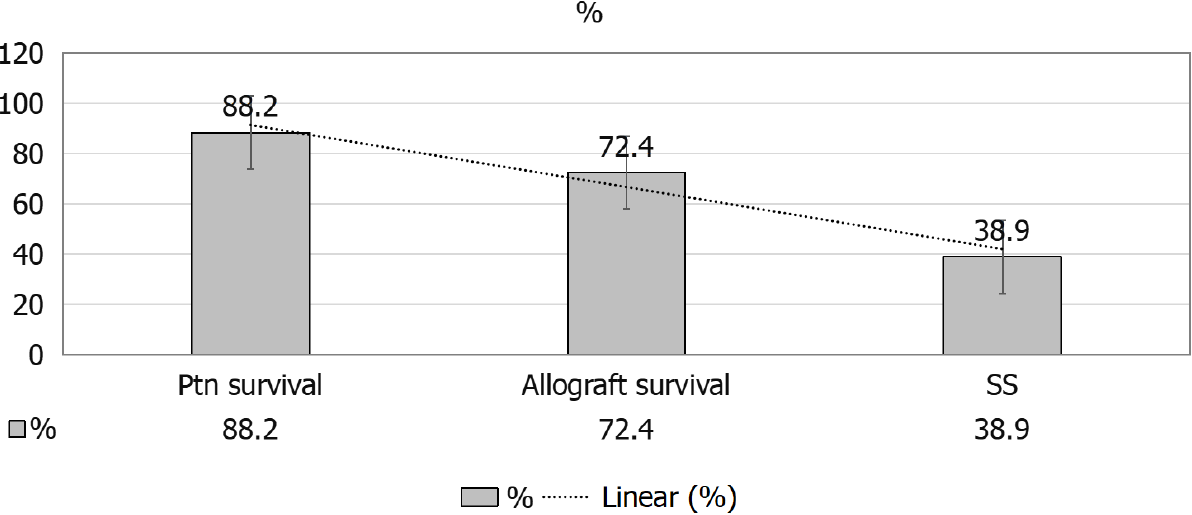
A given comparison by Hruskova et al[16] (2018), for SRC patients’ outcomes[16], as compared to diabetics and patients with other primary renal disease has showed the following: (1) Less percentage of KTx for patients with SRC, 13.7% as compared to patients with diabetes mellitus, 18.7%, and those with other primary renal disease, 27.1% (both P < 0.001) (Figure 9); (2) Patients and allograft survival were comparable to that in other cohorts, the 5-years patient and graft survival after they receive their 1st KTx, were (88.2% and 72.4%) for SS patients and (84.3% and 76.5%) for matched control diabetics and (89.3% and 81.5%), for other primary renal diseases, respectively, matched on sex and age at KTx (Figure 10); and (3) The 5-years survival probability from day 91 of RRT in SS patients was 38.9%, as compared to the 5-years post-transplant patients’ survival and allograft survival that approaches 88.2% and 72.4%, respectively[16] (Figure 11).
Whilst earlier case studies reported high rate of post-transplant SRC recurrence (20%-50%), more recent registries documented much less rates (1.9%-2.1%)[40,44,59]. Analysis of 260 KTx(s) in the period of 1987-2004 in SRC KTRs registered in the UNOS reported that only 1.9% of KTRs developed SRC recurrence-related graft failure between 70 and 805 days after recurrence[44]. Risk factors for SRC prediction of recurrence in the renal allograft still uncertain, many selection biases may be altering[40,44,59]. Considering the 5 well-studied cases with recurrent SRC in the literature, disease activity was associated with the following: (1) Cutaneous tightness (4 cases); (2) Anemia (2 cases); and (3) Pleuro-pericarditis and pericardial effusion (2 cases)[59].
In post-transplant SRC recurrence, less than two weeks have been elapsed from the timing of SRC development until an ESRD established. Nevertheless, an aggressive evolution of ESRD is not always associated with in SRC recurrence. However, con
In Bertrand et al[36], study, 3 patients with suspected recurrent SRC (8.3%), one recurrent case was complicated by graft loss. All the recurrent cases were on CNI, steroids and ACEi. In follow up biopsies, no subclinical vascular alteration has been observed. Of note, only 6 cases with recurrent SRC have been reported in the li
Whilst UNOS may under-estimate the actual rate of SRC recurrence, published series may over-estimate SRC recurrence, considering the publication bias of recording serious cases with worst outcome. In addition, two potential diagnoses must be differentiated from the recurrent SCR: (1) Acute/chronic AMR; and (2) CNI toxicity[54]. Consequently, it is difficult to conclude a definite diagnosis particularly with the retrospective nature of the current reports. The SRC prediction in the non-transplant cohort is quite certain[60-63]. Recognition of RNA polymerase III could be a helpful screening technique in the setting of high-risk patients of recurrence[34,36,64].
The finding that mTOR inhibitors may impede collagen produced from the dermal fibroblasts in vitro, may suggest a potential therapeutic role of mTORi in the cuta
In the same setting CyA safety has been examined in an open-label study against placebo and declared an improved skin score; UCLA skin score declined by 35% in six out of ten CyA-treated patients but still stationery in control group. Of note, transient decline in kidney function in many patients (21%) can be reversed via dose reduction[67]. So, an mTORi-based regimen may be suggested against CNI-based regimen for SRC KTRs candidates, however, evidence base still lacking[57]. ACEi has its crucial role as a renoprotective agent among KTRs[68]. Post-transplant SRC recurrence has been observed in KTRs who have been switched from the ACEi “captopril” to the ARB “losartan”[69], however, no sufficient evidence supporting the role of ARBs in therapy/prevention of SRC. A non-dihydropyrimide CCB agent can be administered to SS KTRs, so that CNI dosage can be reduced[12,68].
SS is a multisystem disorder that can be clinically encountered in several stages. In contrary to the reported poor survival of SS patients maintained on RRT in comparison to other groups, these patients may show the highest likelihood of renal recovery permitting their withdrawal from dialysis. Recent data in the literature are in favor of better outcome of SS patients receiving a KTx as compared to the previous results. Furthermore, these results were comparable to KTRs in other groups of patients. A particular insight, however, should be focused on the extrarenal manifestations of this disease, especially those related to the pulmonary involvement, an independent risk factor of death in this cohort. Furthermore, the post-transplant cardiac and GI involvement should be closely monitored as they may getting worse. In view of the comparable patients and allograft survival rates that have been observed in trans
| 1. | Mayes MD, Lacey JV Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, Schottenfeld D. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 589] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 2. | Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol. 2012;24:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Steen VD, Syzd A, Johnson JP, Greenberg A, Medsger TA Jr. Kidney disease other than renal crisis in patients with diffuse scleroderma. J Rheumatol. 2005;32:649-655. [PubMed] |
| 5. | Denton CP. Renal manifestations of systemic sclerosis--clinical features and outcome assessment. Rheumatology (Oxford). 2008;47 Suppl 5:v54-v56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Caron M, Hudson M, Baron M, Nessim S, Steele R; Canadian Scleroderma Research Group. Longitudinal study of renal function in systemic sclerosis. J Rheumatol. 2012;39:1829-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Hudson M, Baron M, Tatibouet S, Furst DE, Khanna D; International Scleroderma Renal Crisis Study Investigators. Exposure to ACE inhibitors prior to the onset of scleroderma renal crisis-results from the International Scleroderma Renal Crisis Survey. Semin Arthritis Rheum. 2014;43:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Denton CP, Lapadula G, Mouthon L, Müller-Ladner U. Renal complications and scleroderma renal crisis. Rheumatology (Oxford). 2009;48 Suppl 3:iii32-iii35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Avouac J, Walker UA, Hachulla E, Riemekasten G, Cuomo G, Carreira PE, Caramaschi P, Ananieva LP, Matucci-Cerinic M, Czirjak L, Denton C, Ladner UM, Allanore Y; EUSTAR collaborators*; EUSTAR collaborators. Joint and tendon involvement predict disease progression in systemic sclerosis: a EUSTAR prospective study. Ann Rheum Dis. 2016;75:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Teixeira L, Mouthon L, Mahr A, Berezné A, Agard C, Mehrenberger M, Noël LH, Trolliet P, Frances C, Cabane J, Guillevin L; Group Français de Recherche sur le Sclérodermie (GFRS). Mortality and risk factors of scleroderma renal crisis: a French retrospective study of 50 patients. Ann Rheum Dis. 2008;67:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Woodworth TG, Suliman YA, Li W, Furst DE, Clements P. Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol. 2016;12:678-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Bussone G, Bérezné A, Pestre V, Guillevin L, Mouthon L. The scleroderma kidney: progress in risk factors, therapy, and prevention. Curr Rheumatol Rep. 2011;13:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mouthon L, Bussone G, Berezné A, Noël LH, Guillevin L. Scleroderma renal crisis. J Rheumatol. 2014;41:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Shanmugam VK, Steen VD. Renal manifestations in scleroderma: evidence for subclinical renal disease as a marker of vasculopathy. Int J Rheumatol. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
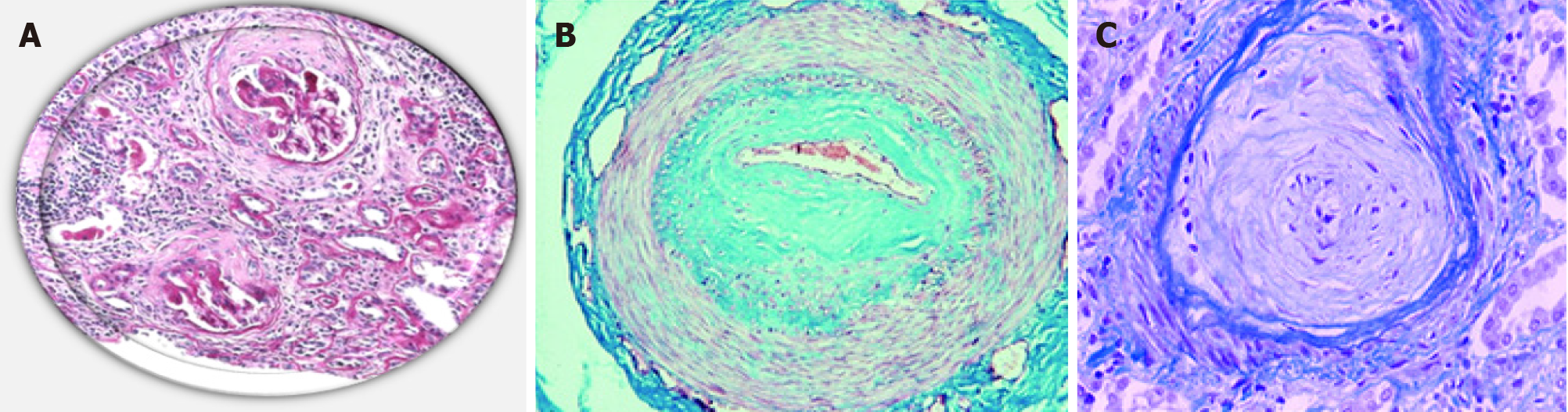
| 15. | Batal I, Domsic RT, Medsger TA, Bastacky S. Scleroderma renal crisis: a pathology perspective. Int J Rheumatol. 2010;2010:543704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Hruskova Z, Pippias M, Stel VS, Abad-Díez JM, Benítez Sánchez M, Caskey FJ, Collart F, De Meester J, Finne P, Heaf JG, Magaz A, Palsson R, Reisæter AV, Salama AD, Segelmark M, Traynor JP, Massy ZA, Jager KJ, Tesar V. Characteristics and Outcomes of Patients With Systemic Sclerosis (Scleroderma) Requiring Renal Replacement Therapy in Europe: Results From the ERA-EDTA Registry. Am J Kidney Dis. 2019;73:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Ranque B, Mouthon L. Geoepidemiology of systemic sclerosis. Autoimmun Rev. 2010;9:A311-A318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Shanmugam VK, Steen VD. Renal disease in scleroderma: an update on evaluation, risk stratification, pathogenesis and management. Curr Opin Rheumatol. 2012;24:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | ANZDATA. Report of renal failure treatment in Australia and New Zealand from the dialysis and transplant registry (ANZDATA). Nephrology. 1995;1:105-111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Soukup T, Toms J, Oreska S, Honsova E, Safranek R. Renal Involvement in Systemic Sclerosis, 9 July 2019. [cited 10 January 2021]. Available from: https://www.researchgate.net/publication/334503858_Renal_Involvement_in_Systemic_Sclerosis. |
| 22. | Mouthon L, Mehrenberger M, Teixeira L, Fakhouri F, Bérezné A, Guillevin L, Noël LH. Endothelin-1 expression in scleroderma renal crisis. Hum Pathol. 2011;42:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Penn H, Quillinan N, Khan K, Chakravarty K, Ong VH, Burns A, Denton CP. Targeting the endothelin axis in scleroderma renal crisis: rationale and feasibility. QJM. 2013;106:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Guillevin L, Bérezné A, Seror R, Teixeira L, Pourrat J, Mahr A, Hachulla E, Agard C, Cabane J, Vanhille P, Harle JR, Deleveaux I, Mouthon L. Scleroderma renal crisis: a retrospective multicentre study on 91 patients and 427 controls. Rheumatology (Oxford). 2012;51:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Sobanski V, Dauchet L, Lefèvre G, Lambert M, Morell-Dubois S, Sy T, Hachulla E, Hatron PY, Launay D, Dubucquoi S. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: New data from a French cohort and a systematic review and meta-analysis. Arthritis Rheumatol. 2014;66:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Doré A, Lucas M, Ivanco D, Medsger TA Jr, Domsic RT. Significance of palpable tendon friction rubs in early diffuse cutaneous systemic sclerosis. Arthritis Care Res (Hoboken). 2013;65:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | DeMarco PJ, Weisman MH, Seibold JR, Furst DE, Wong WK, Hurwitz EL, Mayes M, White B, Wigley F, Barr W, Moreland L, Medsger TA Jr, Steen V, Martin RW, Collier D, Weinstein A, Lally E, Varga J, Weiner SR, Andrews B, Abeles M, Clements PJ. Predictors and outcomes of scleroderma renal crisis: the high-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis trial. Arthritis Rheum. 2002;46:2983-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Keeler E, Fioravanti G, Samuel B, Longo S. Scleroderma renal crisis or thrombotic thrombocytopenic purpura: seeing through the masquerade. Lab Med. 2015;46:e39-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Stochmal A, Czuwara J, Trojanowska M, Rudnicka L. Antinuclear Antibodies in Systemic Sclerosis: an Update. Clin Rev Allergy Immunol. 2020;58:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 30. | Maurer B, Graf N, Michel BA, Müller-Ladner U, Czirják L, Denton CP, Tyndall A, Metzig C, Lanius V, Khanna D, Distler O; EUSTAR co-authors. Prediction of worsening of skin fibrosis in patients with diffuse cutaneous systemic sclerosis using the EUSTAR database. Ann Rheum Dis. 2015;74:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Nikpour M, Hissaria P, Byron J, Sahhar J, Micallef M, Paspaliaris W, Roddy J, Nash P, Sturgess A, Proudman S, Stevens W. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther. 2011;13:R211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Hesselstrand R, Scheja A, Wuttge DM. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand J Rheumatol. 2012;41:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Montanelli G, Beretta L, Santaniello A, Scorza R. Effect of dihydropyridine calcium channel blockers and glucocorticoids on the prevention and development of scleroderma renal crisis in an Italian case series. Clin Exp Rheumatol. 2013;31:135-139. [PubMed] |
| 34. | Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1233] [Cited by in RCA: 1156] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 35. | Fernández-Codina A, Walker KM, Pope JE; Scleroderma Algorithm Group. Treatment Algorithms for Systemic Sclerosis According to Experts. Arthritis Rheumatol. 2018;70:1820-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 36. | Bertrand D, Dehay J, Ott J, Sberro R, Brunelle C, Kamar N, Colosio C, Chatelet V, Albano L, Girerd S, Audard V, Barbet C, Dantal J, Ducloux D, Durrbach A, Garrigue V, Hazzan M, Heng AE, Mariat C, Merville P, Rerolle JP, Moulin B, Guerrot D. Kidney transplantation in patients with systemic sclerosis: a nationwide multicentre study. Transpl Int. 2017;30:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Denton CP, Black CM. Scleroderma--clinical and pathological advances. Best Pract Res Clin Rheumatol. 2004;18:271-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Steen VD, Medsger TA Jr. Long-term outcomes of scleroderma renal crisis. Ann Intern Med. 2000;133:600-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Schioppo T, Artusi C, Ciavarella T, Ingegnoli F, Murgo A, Zeni S, Chighizola C, Meroni PL. N-TproBNP as biomarker in systemic sclerosis. Clin Rev Allergy Immunol. 2012;43:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Siva B, McDonald SP, Hawley CM, Rosman JB, Brown FG, Wiggins KJ, Bannister KM, Campbell SB, Johnson DW. End-stage kidney disease due to scleroderma--outcomes in 127 consecutive ANZDATA registry cases. Nephrol Dial Transplant. 2011;26:3165-3171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 41. | Brown N, Summers A, Venning MC, Bruce IN. The challenges of dialysis in systemic sclerosis: between the devil and the deep blue sea? Case Rep Nephrol. 2012;2012:865193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Abbott KC, Trespalacios FC, Welch PG, Agodoa LY. Scleroderma at end stage renal disease in the United States: patient characteristics and survival. J Nephrol. 2002;15:236-240. [PubMed] |
| 43. | Hruskova Z, Stel VS, Jayne D, Aasarød K, De Meester J, Ekstrand A, Eller K, Heaf JG, Hoitsma A, Martos Jimenéz C, Ravani P, Wanner C, Tesar V, Jager KJ. Characteristics and Outcomes of Granulomatosis With Polyangiitis (Wegener) and Microscopic Polyangiitis Requiring Renal Replacement Therapy: Results From the European Renal Association-European Dialysis and Transplant Association Registry. Am J Kidney Dis. 2015;66:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Gibney EM, Parikh CR, Jani A, Fischer MJ, Collier D, Wiseman AC. Kidney transplantation for systemic sclerosis improves survival and may modulate disease activity. Am J Transplant. 2004;4:2027-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Steen VD, Costantino JP, Shapiro AP, Medsger TA Jr. Outcome of renal crisis in systemic sclerosis: relation to availability of angiotensin converting enzyme (ACE) inhibitors. Ann Intern Med. 1990;113:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 259] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Shor R, Halabe A. New trends in the treatment of scleroderma renal crisis. Nephron. 2002;92:716-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Bleyer AJ, Donaldson LA, McIntosh M, Adams PL. Relationship between underlying renal disease and renal transplantation outcome. Am J Kidney Dis. 2001;37:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Chang YJ, Spiera H. Renal transplantation in scleroderma. Medicine (Baltimore). 1999;78:382-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Woodworth TG, Furst DE. Timely renal transplantation for scleroderma end-stage kidney disease patients can improve outcomes and quality of life. Ann Transl Med. 2019;7:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Teixeira L, Mahr A, Berezné A, Noël LH, Guillevin L, Mouthon L. Scleroderma renal crisis, still a life-threatening complication. Ann N Y Acad Sci. 2007;1108:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, Rush D, Cole E; Kidney Transplant Working Group of the Canadian Society of Transplantation. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173:1181-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Richardson JA. Hemodialysis and kidney transplantation for renal failure from scleroderma. Arthritis Rheum. 1973;16:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Ruiz JC, Val F, de Francisco AL, de Bonis E, Zubimendi JA, Prieto M, Canga E, Arias M. Progressive systemic sclerosis and renal transplantation: a contraindication to ciclosporin. Nephron. 1991;59:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Morales-Cárdenas A, Pérez-Madrid C, Arias L, Ojeda P, Mahecha MP, Rojas-Villarraga A, Carrillo-Bayona JA, Anaya JM. Pulmonary involvement in systemic sclerosis. Autoimmun Rev. 2016;15:1094-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Desbois AC, Cacoub P. Systemic sclerosis: An update in 2016. Autoimmun Rev. 2016;15:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 57. | Hant FN, Herpel LB, Silver RM. Pulmonary manifestations of scleroderma and mixed connective tissue disease. Clin Chest Med. 2010;31:433-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Bauer PR, Schiavo DN, Osborn TG, Levin DL, St Sauver J, Hanson AC, Schroeder DR, Ryu JH. Influence of interstitial lung disease on outcome in systemic sclerosis: a population-based historical cohort study. Chest. 2013;144:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Pham PT, Pham PC, Danovitch GM, Gritsch HA, Singer J, Wallace WD, Hayashi R, Wilkinson AH. Predictors and risk factors for recurrent scleroderma renal crisis in the kidney allograft: case report and review of the literature. Am J Transplant. 2005;5:2565-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Steen VD, Medsger TA Jr, Osial TA Jr, Ziegler GL, Shapiro AP, Rodnan GP. Factors predicting development of renal involvement in progressive systemic sclerosis. Am J Med. 1984;76:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 166] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Steen VD, Medsger TA Jr. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 1998;41:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Lee AT, Burnet S. Corticosteroid-induced scleroderma renal crisis. Med J Aust. 2002;177:459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 63. | Steen VD. Scleroderma renal crisis. Rheum Dis Clin North Am. 2003;29:315-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Emilie S, Goulvestre C, Bérezné A, Pagnoux C, Guillevin L, Mouthon L. Anti-RNA polymerase III antibodies are associated with scleroderma renal crisis in a French cohort. Scand J Rheumatol. 2011;40:404-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem. 2004;279:23166-23175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Su TI, Khanna D, Furst DE, Danovitch G, Burger C, Maranian P, Clements PJ. Rapamycin versus methotrexate in early diffuse systemic sclerosis: results from a randomized, single-blind pilot study. Arthritis Rheum. 2009;60:3821-3830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Clements PJ, Lachenbruch PA, Sterz M, Danovitch G, Hawkins R, Ippoliti A, Paulus HE. Cyclosporine in systemic sclerosis. Results of a forty-eight-week open safety study in ten patients. Arthritis Rheum. 1993;36:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 83] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Mangray M, Vella JP. Hypertension after kidney transplant. Am J Kidney Dis. 2011;57:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Cheung WY, Gibson IW, Rush D, Jeffery J, Karpinski M. Late recurrence of scleroderma renal crisis in a renal transplant recipient despite angiotensin II blockade. Am J Kidney Dis. 2005;45:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013;65:1953-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhou Z S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY