Published online Jul 18, 2021. doi: 10.5500/wjt.v11.i7.303
Peer-review started: January 30, 2021
First decision: May 5, 2021
Revised: May 10, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: July 18, 2021
Processing time: 163 Days and 17.3 Hours
Focal segmental glomerulosclerosis (FSGS) is one of the most common glomerular diseases leading to renal failure. FSGS has a high risk of recurrence after kidney transplantation. Prevention of recurrent FSGS using rituximab and/or plasma
To assess the risk of recurrence of FSGS after transplantation using prophylactic rituximab with or without plasmapheresis, and plasmapheresis alone compared to the standard treatment group without preventive therapy.
This meta-analysis and systematic review were performed by first conducting a literature search of the MEDLINE, EMBASE, and Cochrane databases, from inception through March 2021; search terms included ‘FSGS,’ ’steroid-resistant nephrotic syndrome’, ‘rituximab,’ and ‘plasmapheresis,’. We identified studies that assessed the risk of post-transplant FSGS after use of rituximab with or without plasmapheresis, or plasmapheresis alone. Inclusion criteria were: Origi
Eleven studies, with a total of 399 kidney transplant recipients with FSGS, evaluated the use of rituximab with or without plasmapheresis; thirteen studies, with a total of 571 kidney transplant recipients with FSGS, evaluated plasma
Overall, the use of rituximab with or without plasmapheresis, or plasmapheresis alone, is not associated with a lower risk of FSGS recurrence after kidney trans
Core Tip: Focal segmental glomerulosclerosis (FSGS) is associated with a high risk of recurrence after kidney transplantation. Plasmapheresis and/or rituximab has been used to prevent recurrence with conflicting results. This meta-analysis is among the first to report that the use of preemptive rituximab, either alone or in combination with plasmapheresis, or plasmapheresis alone, did not alter the recurrence risk of FSGS after kidney transplantation.
- Citation: Boonpheng B, Hansrivijit P, Thongprayoon C, Mao SA, Vaitla PK, Bathini T, Choudhury A, Kaewput W, Mao MA, Cheungpasitporn W. Rituximab or plasmapheresis for prevention of recurrent focal segmental glomerulosclerosis after kidney transplantation: A systematic review and meta-analysis. World J Transplant 2021; 11(7): 303-319
- URL: https://www.wjgnet.com/2220-3230/full/v11/i7/303.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i7.303
Focal segmental glomerulosclerosis (FSGS) is an important glomerular cause of end-stage kidney disease, and is associated with a high risk of disease recurrence after kidney transplantation[1-5]. Approximately 30% of patients[6,7] develop recurrent FSGS following kidney transplantation, with studies reporting a range between 17% and 55%[8]. FSGS has been shown to negatively affect overall graft survival[9-12]. Although the exact pathogenesis of this disease is unknown, it is believed that circulating factors affecting podocytes and glomerular permeability may play an important role. FSGS recurrence presents early after kidney transplantation; thus, supporting the pathophysiological role of circulating factors.
Treatment for recurrent FSGS in kidney transplant recipients is difficult. Steroids have been used as the main therapy in adults. Unfortunately, only 50% of patients achieve remission following a course of steroid treatment[13]. Furthermore, a large proportion of patients relapse, eventually becoming either steroid-resistant, or steroid-dependent[14]. Plasmapheresis has been effectively used to treat recurrent FSGS after kidney transplantation, purportedly by removing pathophysiological circulating factors and inducing FSGS remission. Preemptive plasmapheresis following kidney transplantation has been proposed as a preventive measure for FSGS.
Rituximab is a monoclonal, chimeric antibody against CD20+ B lymphocytes, and has been used to both prevent and treat recurrent FSGS after kidney transplantation. In 2020, Hansrivijit and Ghahramani[15] reported promising outcomes after treatment of recurrent FSGS in kidney transplant recipients, using either a combination of rituximab and plasmapheresis, or plasma exchange alone. Their study demonstrated an overall remission rate of 72.7%, determined by a significant reduction in serum creatinine levels and the degree of proteinuria. Nevertheless, the efficacy of rituximab or plasmapheresis as a preventive measure for post-transplant recurrent FSGS remains controversial.
This systematic review and meta-analysis were conducted to explore the effectiveness of rituximab–with or without plasmapheresis–compared with plasmapheresis alone, for the prevention of recurrent FSGS after kidney transplantation.
This systematic review was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology guidelines[16]. A literature search was per
Studies were eligible for inclusion if they met the following criteria: (1) Original, published, randomized controlled cohort (either prospective or retrospective), case-control, or cross-sectional studies; (2) The odds ratio, relative risk, and standardized incidence ratio with 95% confidence intervals (CIs), or sufficient raw data to calculate these ratios, were provided; and (3) Subjects without interventions (controls) were used as comparators in cohort and cross-sectional studies.
Study eligibility was independently assessed by the investigators. Any disagree
Two investigators independently reviewed the titles and abstracts of all retrieved articles. Articles that did not fulfill the inclusion criteria were excluded. Only potentially relevant articles underwent full-text reviews to determine eligibility. A standardized data collection form was used to extract the following data: First author’s name, year of publication, year of study, country of origin, study design, source of population, number of subjects, baseline characteristics of the subjects, and effect estimates. This data extraction process was performed in duplicate to ensure accuracy.
All statistical analyses were performed using R version 3.2.0 (the R Foundation for Statistical Computing, Vienna, Austria). The pooled risk ratios for recurrent FSGS in the active intervention group compared with the no intervention group were calculated using the generic inverse method of DerSimonian and Laird[18]. A random effects model was utilized given the high likelihood of between-study variance due to differences in underlying population as well as methodology. Cochran’s Q-test, supplemented by the I2 statistic, was used to evaluate statistical heterogeneity. This statistic quantifies the proportion of total variation across studies due to true heterogeneity rather than chance. An I2 value of 0-25% represented insignificant heterogeneity, 25%-50% represented low heterogeneity, 50%-75% represented moderate heterogeneity, and > 75% represented high heterogeneity[19].
The initial search yielded 813 articles, all of which underwent both title and abstract reviews. Most were excluded at this step as they did not fulfill our inclusion criteria; i.e., they were case reports, letters to the editor, review articles, or interventional studies. A total of 38 studies underwent full-length article review. Of 17 were excluded, as they did not include controls or report the outcome of interest. A total of 21 observational studies, including 920 patients, met our inclusion criteria[8,20-39] and were included in the meta-analysis. Figure 1 outlines our search methodology and selection process. The baseline characteristics of the included studies are summarized in Tables 1-4 (detailed characteristics in Tables 3 and 4).
| Ref. | Country | Design | n (%) | Population | Age | PP protocol | Def of recurrence | Recurrence | Graft survival | Quality assessment |
| Kawaguchi et al[20], 1994 | Japan | Retrospective | 14 | FSGS children | 2-12 yr at FSGS Dx | 2-3 sessions immediately before KT (-5, -3, and -1 d) ATG 7-14 d pre-op | N/A | 3/8 (38%) vs 4/6 (67%) | 93% graft survival in overall cohort | Fair, 4-1-2 |
| Otsubo et al[21], 1999 | Japan | Retrospective | 37 | FSGS undergoing KT | 22 yr at KT | N/A | Clinical and biopsy in all cases | 4/19 (21%) vs 9/18 (50%) | 75%at 5 yr, 63% at 10 yr | Fair, 4-1-2 |
| Iguchi et al[32], 1997 | Japan | Prospective cohort | 11 | FSGS undergoing KT | 33.3 (20-43) yr | 3 sessions of pre-op PP within 3 d before KT | Clinical and/or pathologic | 1/3 (33%) vs 4/8 (50%) | 100% vs 63.6% | Fair, 4-2-2 |
| Ohta et al[33], 2001 | Japan | Retrospective | 21 | FSGS children | Age of FSGS onset 69.5 ± 36.4 mo (range 9-134 mo) | 1-2 sessions immediately before KT (-5, -3, and -1 d). Therapeutic PP until reduction of proteinuria | Clinical and/or pathologic | 5/15 (33%) vs 4/6 (67%) | 13/15 vs 3/5 (1 death with functioning graft in Non-PP) | Fair, 4-2-2 |
| Somers and Baum[34], 2009 | United States | Retrospective | 52 | FSGS children | 12.5 yr | N/A | N/A | 5/19 (26%) vs 18/33 (55%) | Overall, 11/52 graft loss | Fair, 4-1-2 |
| Gonzalez et al[35], 2011 | United States | Retrospective | 34 | FSGS children | Age at KT: 13 ± 5 yr. Age at FSGS diagnosis: 5.3 yr (n = 19, recurrence group), 6.9 yr (n = 15, no recurrence group) | 1-10 sessions | Clinical and/or pathologic | 9/17 (53%) vs 10/17 (59%) | Graft loss at 3 yr: 25% in recurrence group vs 20% in non-recurrence | High, 4-2-3 |
| Miyauchi et al[25], 2011 | Japan | Prospective cohort | 25 | FSGS undergoing KT | N/A | N/A | N/A | 3/9 (33%) vs 2/4 (50%) | N/A | Low, 3-1-1 |
| Park et al[26], 2014 | South Korea | Retrospective | 27 | FSGS undergoing KT | Age at KT: 39 ± 14 yr and 36 ± 11 yr | PP and IVGV infusion after each session of PP prior to transplantation | Clinical confirmed by biopsy | 1/4 (25%) vs 5/18 (27%) | FSGS with recurrence had less graft survival than those without recurrence (P = 0.01) | High, 4-2-3 |
| Okumi et al[27], 2015 | Japan | Retrospective | 38 | FSGS undergoing KT | N/A | N/A | N/A | 4/10 (40%) vs 2/5 (40%) | 5/38 graft loss overall | Low, 3-1-1 |
| Verghese et al[36], 2018 | United States | Retrospective | 57 | FSGS children | Age at KT: 13.2 ± 4.5 yr (after 2006 with PP) vs 10.4 ± 5.4 yr (before 2006, no PP) | LDKT: 3 sessions PP pre-op. DDKT: 1 session of PP pre-op. Post-op: 5 sessions of PP every other day starting POD1 | Biopsy; if unable to do biopsy, persistent nephrotic range proteinuria | 7/26 (27%) vs 8/31 (26%) | Death-censored graft survival not sig different (P = 0.61) | High, 4-2-3 |
| Koyun et al[37], 2019 | Turkey | Retrospective | 46 | FSGS children | Age at KT: 7.2 ± 1.2 yr (PP) vs 10.7 ± 4.5 yr (no PP) | LDKT: 2-5 sessions of PP pre-op. DDKT: 1 session of PP pre-op. Post-op: 5 session of early PP | N/A | 3/6 (50%) vs 5/40 (12.5%) | N/A | Low, 3-1-1 |
| Campise et al[38], 2019 | Italy | Retrospective | 73 | FSGS undergoing KT | Age at FSGS Dx: 27 (15-35) yr. Age at KT: 41 (38-52) yr | 2003-2008: post-transplant PP only 2008-2014: 1 session immediately before surgery and 3 sessions per week for 3 consecutive weeks from POD1 | Post-transplant proteinuria; confirmed by biopsy | Biopsy-proven: 5/21 (24%) vs 12/52 (23%) | Death-censored graft survival: 81% (17/21) vs 84% (44/52) (P = 0.7022) | High, 4-2-3 |
| Uffing et al[8], 2020 | United States, Europe, Brazil | Retrospective, multicenter | 176 | FSFS adults undergoing KT | Age at KT: 38 (29–47) yr. Age at FSGS Dx: 27 (17-40) yr | N/A | N/A | 9/22 (41%) vs 48/154 (31%) | Graft failure 15% w/o recurrence and 39% with recurrence | High, 4-2-3 |
| Ref. | Country | Design | n (%) | Population | Age | Rituximab dose and protocol | Concurrent PP | Def of recurrence | Recurrence | Graft survival | Follow-up duration | Quality assessment |
| Burke et al[22], 2009 | United States | Retrospective | 29 | FSGS undergoing KT | Age at KT: 6-21 yr | N/A | No | New onset proteinuria | 6/18 (33%) vs 8/11 (72%) | No significant difference in graft survival | N/A | Fair, 3-1-2 |
| Sagheshima et al[23], 2010 | United States | Prospective | 40 | FSGS undergoing KT | Age at KT: 4-24 yr | N/A | No | UPCR > 3.5 post-transplant | 8/29 (28%) vs 7/11 (64%) | N/A | N/A | Low, 3-1-1 |
| Fornoni et al[24], 2011 | United States | Retrospective | 41 | High-risk pediatric/young adult FSGS undergoing KT: (< 25 yr at FSGS Dx or progression to ESKD within 7 yr) | Age at KT: 15 ± 5.5 yr (rituximab), 12.3 ± 5.2 yr (control) | One dose of rituximab (375 mg/m2) within 24 h of kidney transplantation | No | UPCR > 3.5 within 30 d post-transplant or need for PP. Protocol biopsy in 20/27 (74%) | 7/27 (26%) vs 9/14 (64%) | 1-yr graft survival: 95.8% vs 85.7% (P = 0.26) | N/A | High, 4-1-3 |
| Miyauchi et al[25], 2011 | Japan | Prospective | 25 | FSGS undergoing KT | N/A | N/A | N/A | N/A | 2/12 (17%) vs 5/13 (38%) | N/A | N/A | Low, 3-1-1 |
| Park et al[26], 2014 | South Korea | Retrospective | 27 | FSGS undergoing KT | Age at KT: 39 ± 14 yr (n = 7, recurrence), 36 ± 11 yr (n = 20, no recurrence) | PP and IVGV infusion after each session of PP prior to transplantation, and RTX (375 mg/m2) was administeredwithin 1 wk prior to transplantation | Yes | Clinical confirmed by biopsy | 1/4 (25%) vs 5/18 (27%) | FSGS with recurrence had less graft survival than those without recurrence (P = 0.01) | N/A | High, 4-1-3 |
| Okumi et al[27], 2015 | Japan | Retrospective | 38 | FSGS undergoing KT | N/A | N/A | Yes | N/A | 5/23 (22%) vs 6/15 (40%) | 5/38 graft loss overall. Cr at yr 2 and 6 significantly lower in those who received both R + PP | N/A | Low, 3-1-1 |
| Futamura et al[28], 2016 | Japan | Retrospective | 28 | FSGS undergoing KT | N/A | N/A | Yes | N/A | 3/7 (43%) vs 5/21 (24%) | N/A | N/A | Low, 3-1-1 |
| Alasfar et al[29], 2018 | United States | Prospective | 64 | High-risk FSGS undergoing KT (2 of: white, age ≤ 30 at Dx, progression to ESKD ≤ 5 yr. Albumin < 3 g/dL during disease course, h/o failed KT due to recurrence) | Age at FSGS Dx: 29.9 ± 17.2. Age at KT: 38 ± 16.5 | Rituximab was given in 1 or 2 doses (375 mg/m2per dose) | Yes; 3-10 sessions of PP day-7 to POD 2 | Clinical and biopsy | 23/37 (62%) vs 14/27 (51%) | Trend toward better renal allograft survival in nonrecurrent group comparedto the recurrent group (P = 0.0662) | 29.5 mo | High, 4-1-3 |
| Lu et al[30], 2018 | United States | Retrospective | 55 | High-risk FSGS undergoing KT considered (age ≤ 25 at Dx, proteinuria ≥ 5 g/d, progression to ESKD ≤ 5-7 yr) | Age at KT: 44 | One dose of rituximab (375 mg/m2, max 100 mg) | No | Proteinuria and biopsy | 4/7 (57%) vs 6/48 (13%) | Graft loss: 1/7 (14%) vs 8/48 (17%) | N/A | Fair, 3-2-2 |
| Auñón et al[31], 2021 | Spain | Retrospective, multicenter | 34 (93 total cohort) | High-risk FSGS undergoing KT considered (hypoalbuminemia and NS at baseline); genetic form excluded | Age at KT: 35.0 ± 15.2 (R group), 42.4 ± 12.2 (non-R group) | Rituximab, 1 g at induction and 1 g on day 14 after transplantation | No | Recurrence of proteinuria, confirmed by biopsy | 6/12 (50%) vs 9/22 (41%) | 53.5% with recurrence vs 88.5% in non-recurrence group | N/A | High, 4-1-3 |
| Mukku et al[39], 2021 | United States | Retrospective | 18 | FSGS undergoing KT | Age at KT: 35 yr | N/A | Yes | Recurrence of proteinuria | 0/8 vs 3/10 (30%) | 8/8 vs 9/10 | 30 (1-36) mo | Low, 3-1-1 |
| Ref. | Country | Age | Genetic testing | Race | Time to ESKD | Repeat KT | Induction | IS | Donor types | Biopsy | Follow-up duration |
| Kawaguchi et al[20], 1994 | Japan | 2-12 yr at FSGS Dx | N/A | Asian | 12–117 mo | ATG only in PP group | CS, CsA, AZA/mizolibine | 13/14 living1/14 DDKT | N/A | N/A | |
| Otsubo et al[21], 1999 | Japan | 22 yr at KT | N/A | Asian | N/A | N/A | CS, CsA/Tac | CS, CsA/Tac, AZA/mizolibine | 34/37 LRKT, 4/37 DDKT | Per-cause biopsy | N/A |
| Iguchi et al[32], 1997 | Japan | 33.3 (20-43) yr | N/A | Asian | N/A | None | ATG during first 2 wk in PP group | CS, CsA, AZA | 100% LRKT | Intra-op biopsy (1 h) in all cases then as clinically indicated | N/A |
| Ohta et al[33], 2001 | Japan | Age of FSGS onset69.5 ± 36.4 mo (range 9-134 mo) | N/A | Asian | 51.8 ± 29.6 mo (range 7-120) | 1/21 | None | CS, CsA/Tac, AZA/mizolibine | 3/21 DDKT (14%) vs 18/21 (LRKT) | Intra-op biopsy (1 h) in all cases then as clinically indicated | 62.7 (PP group), 41.6 mo (non-PP group) |
| Somers and Baum[34], 2009 | Unite States | 12.5 yr (85% white) | N/A | 85% White | 3 yr (median) | N/A | N/A | CsA-based regimen | 42% living donor | N/A | N/A |
| Gonzalez et al[35], 2011 | United States | Age at KT: 13 ± 5 yr | NPHS2 mutation testing on 10 patients (9 tested negative, 1 with heterozygous mutation) | 29% White, 15% African, 44% Hispanic, 12% others | 4.2 yr (n = 19, recurrence group), 3.1 yr (n = 15, no recurrence group) | Recurrence in previous graft 5/34 | rATG (if ATN) or daclizumab | CS, CsA/Tac, MMF | 15/34 living, 19/34 DDKT | Per-cause biopsy | N/A |
| Miyauchi et al[25], 2011 | Japan | N/A | N/A | Asian | N/A | N/A | N/A | CS, CsA/Tac, AZA/mizolibine | N/A | N/A | N/A |
| Park et al[26], 2014 | South Korea | Age at KT: 39 ± 14 yr (n = 7, recurrence), 36 ± 11 yr (n = 20, no recurrence) | N/A | Asian | 46 ± 44 mo (n = 7, recur group), 68 ± 67 mo (n = 20, no recur group) | none | Basiliximab (20 mg) on days 0 and 4 | CS, CsA/Tac, MMF | 4/27 DDKT, 24/27 living (17/27 LRKT) | Per-cause biopsy | N/A |
| Okumi et al[27], 2015 | Japan | N/A | N/A | Asian | N/A | N/A | Basiliximab (after 2002) | CS, CsA/Tac, MMF | N/A | N/A | N/A |
| Verghese et al[36], 2018 | United States | Age at KT: 13.2 ± 4.5 yr (after 2006 with PP) vs 10.4 ± 5.4 yr (before 2006, no PP) | NPHS2 mutation testing (for those with NPHS2 homozygous mutation, PP not indicated) | N/A | N/A | N/A | 93% received lymphocyte depleting induction | Before 2006: AZA (90%), MMF (16%), CsA (97%), CS (97%). After 2006: AZA (12%), MMF (88%), CsA (62%)/Tac (38%), CS (12%) | DDKT 37% vs Living 63% | Per-cause biopsy | N/A |
| Koyun et al[37], 2019 | Turkey | Age at KT: 7.2 ± 1.2 yr (PP) vs 10.7 ± 4.5 yr (no PP) | Genetic testing (unspecified gene panel): 2/6 + in PP group vs 14/40+ in control group | N/A | N/A | N/A | N/A | N/A | DDKT 20%, Living 80% | N/A | N/A |
| Campise et al[38], 2019 | Italy | Age at FSGS Dx: 27 (15-35) yr. Age at KT: 41 (38-52) yr | Not done | 100% White | 5 (1-10) yr, 33% rapid (< 3 yr) progression to ESKD | (7/21) 33% in PP group; previous graft loss due to recurrence | Basiliximab (20 mg) on days 0 and 4 | CS, Tac, MMF | 100% DDKT | Per-cause biopsy | 45 (30-107) mo |
| Uffing et al[8], 2020 | Unites States, Europe, Brazil | Age at KT: 38 (29–47) yr. Age at FSGS Dx: 27 (17-40) yr | Not done in most patients | 56% White, 11% Black, 5% Hispanic, 5% Asian, 10% mixed, Other or unknown 14% | 38 (14–75) mo | 25%; prior graft loss due to FSGS 9% | rATG (42%), basiliximab (42%), daclizumab (3%), none (13%) | CS + Tac + MMF (72%), CS + CsA + MMF (17%), Tac + MMF (5%), other 6% | 67% DDKT, 22% LRKT, 15% LUKT | Per-cause biopsy | N/A |
| Ref. | Country | Age | Genetic testing | Race | Time to ESKD | Repeat KT | Induction | IS | DDKT | Follow-up duration |
| Burke et al[22], 2009 | United States | Age at KT: 6-21 yr | N/A | N/A | N/A | N/A | rATG or daclizumab | CS, Tac, MMF | N/A | N/A |
| Sagheshima et al[23], 2010 | United States | Age at KT: 4-24 yr | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fornoni et al[24], 2011 | United States | Age at KT: 15 ± 5.5 yr (rituximab), 12.3 ± 5.2 yr (control) | N/A | White 56%, Black 44% | 3.4 ± 2.0 yr (rituximab group), 3.3 ± 2.1 (control) | N/A | Combined thymoglobulin (1 mg/kg, 3–5 doses) and daclizumab. Alemtuzumab in one patient. | CS, Tac, MMF | Preemptive: 3/27 (11%) in rituximab group, 2/14 (14%) in non-rituximab group | N/A |
| Miyauchi et al[25], 2011 | Japan | FSGS undergoing KT | N/A | Asian | N/A | N/A | N/A | CS, CsA/Tac, AZA/mizolibine | N/A | N/A |
| Park et al[26], 2014 | South Korea | Age at KT: 39 ± 14 (n = 7, recurrence), 36 ± 11 (n = 20, no recurrence) | N/A | Asian | 46 ± 44 mo (n = 7, recur group), 68 ± 67 mo (n = 20, no recur group) | none | Basiliximab (20 mg) on days 0 and 4 | CS, CsA/Tac, MMF | 3/27 DDKT, 24/27 living | N/A |
| Okumi et al[27], 2015 | Japan | N/A | N/A | Asian | N/A | N/A | Basiliximab (after 2002) | CS, CsA/Tac, MMF | N/A | N/A |
| Futamura et al[28], 2016 | Japan | N/A | N/A | Asian | N/A | N/A | N/A | N/A | N/A | N/A |
| Alasfar et al[29], 2018 | United States | Age at FSGS Dx: 29.9 ± 17.2. Age at KT: 38 ± 16.5 | N/A | White 56%, Black 32%, Asian 7%, Hispanic 4% | 4 (0–9) yr | 37% (42/66 63% first transplant) | Depleting agent 92% | CS + Tac + MMF (92%), CS + CsA + MMF (8%) | DDKT 37%, LUKT 37%, LRKT 25% | 29.5 mo |
| Lu et al[30], 2018 | United States | Age at KT: 44 | N/A | White 64% | N/A | 0% | N/A | CS, Tac, MMF | N/A | N/A |
| Auñón et al[31], 2021 | Spain | Age at FSGS Dx: 24.5 ± 18.5 (rituximab group), 30 ± 13.7 (non-rituximab group). Age at KT: 35.0 ± 15.2 (R group), 42.4 ± 12.2 (non-R group) | Excluded suspected genetic causes of FSGS | N/A | 5.12 ± 4.44 (R group), 7.58 ± 7.11 (Non-R group) | 7/34 (21%); recurrence in previous graft 2/12 (16.7%) in R group vs 2/22 (9.1%) In non-R group | Rituximab group: rATG 16.7%, basiliximab 50%. Non-rituximab group: rATG 40.9%, basiliximab 22.7% | CS + Tac + MMF (93.3%) | 85.3% DDKT, 11.8% LRKT, 2.9% LUKT | N/A |
| Mukku et al[39], 2021 | United States | Age at KT: 35 yr | N/A | White 39%, Black 27% | N/A | 2/8 pre-emptive group vs 0/10 | rATG (61%), alemtuzumab (22%), basiliximab (17%) | CS + Tac + MMF (83%), CS + CsA + MMF (17%) | 89% DDKT | 30 (1-36) mo |
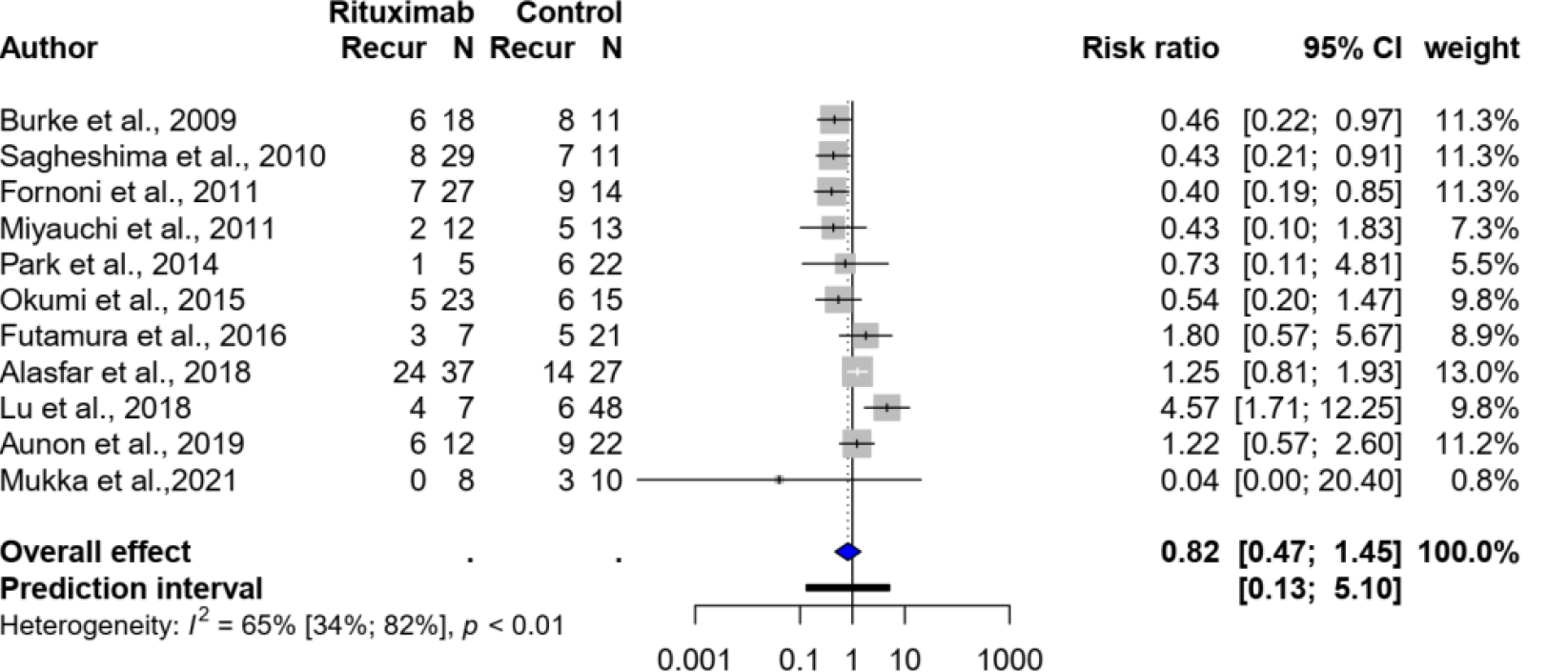
Eleven studies[22-31,39], with a total of 399 kidney transplant recipients with FSGS, evaluated the use of rituximab with or without plasmapheresis. There was no significant difference in recurrence between the group that received rituximab (with or without plasmapheresis) and the standard treatment group, with a pooled risk ratio of 0.82 (95%CI: 0.47-1.45, I2 = 65%). Figure 2 shows the forest plot.
Subgroup analysis, based on five studies[22-24,30,31] that evaluated preemptive rituximab use without concurrent plasmapheresis compared with no intervention, also showed no significant association; the pooled risk ratio was 0.82 (95%CI: 0.23-2.92, I2 = 81%).
Four studies[24,29-31] selected only patients deemed to be at high-risk of recur
Sensitivity analyses were also performed after excluding five studies[22,23,25,27,28,39] that did not report the rituximab dose or protocol; all were published as abstracts. The risk ratio was also not significant (risk ratio: 1.09, 95%CI: 0.37-3.19).
Thirteen studies[8,20,21,25-27,32-38], including 571 kidney transplant recipients with FSGS, evaluated the use of plasmapheresis alone. Compared with no plasmapheresis, plasmapheresis was not found to be associated with any significant difference in FSGS recurrence, with a pooled risk ratio of 0.85 (95%CI: 0.60-1.21, I2 = 23%, Figure 3). Subgroup analysis in pediatric patients also did not yield a significant association, with a pooled risk ratio of 0.86 (95%CI: 0.29-4.49, I2 = 63%).
Sensitivity analysis, after excluding three studies[25,27,34] that were published as abstracts and did not report the protocol or regimen of plasmapheresis, did not show a significant change in the risk ratio (1.07, 95%CI: 0.66-1.72, I2 =22%).
Although only five studies reported the timing of post-transplant recurrent FSGS, it appears that most cases occurred relatively early. Park et al[26] reported the time to recurrence in all 6 patients with recurrent FSGS: 3 patients experienced early recur
Some studies reported decreased allograft survival in patients who experienced FSGS recurrence compared to those who did not[8,26,31-33,35,39]. Allograft survival appears to depend on response to recurrent FSGS therapy, which variably consists of plasmapheresis with more intensive immunosuppressive regimens. Neither pre-emptive plasmapheresis or rituximab per se seems to have effects on allograft survival.
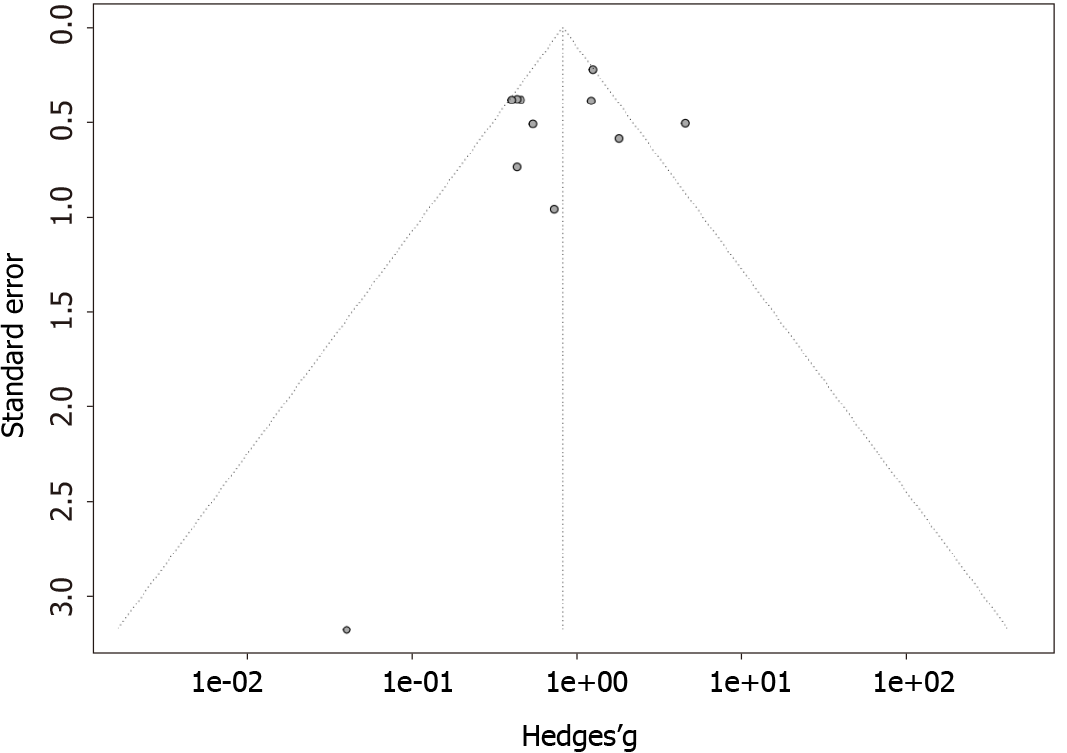
The funnel plots for the outcomes of rituximab and plasmapheresis are shown in Figures 4 and 5, respectively. They are symmetrical, and do not suggest the presence of publication bias in favor of positive studies. Egger’s asymmetry test yielded P-values of 0.56 and 0.83 for the rituximab and the plasmapheresis groups, respectively.
Primary FSGS often recurs after kidney transplantation, leading to graft loss and morbidity[6-8]. Multiple basic science and clinical studies have implicated circulating factors in the pathogenesis of recurrent FSGS[40-42]. The tendency of recurrent FSGS to present early and rapidly after kidney transplantation supports the pathophysio
Plasmapheresis is considered an effective treatment able to induce remission in established recurrent diseases[46]. Likewise, plasmapheresis has been used as a prevention of FSGS after kidney transplant. By rapidly removing pre-existing circu
More recently, rituximab has been effectively used to treat many glomerular diseases, including FSGS[47]. The exact mechanism of rituximab in the treatment of FSGS is unknown; however, it is believed that rituximab may have a B-cell-indepen
Our meta-analysis is among the first to report that the use of preemptive rituximab (either alone or in combination with plasmapheresis) or plasmapheresis alone did not alter the recurrence risk of FSGS after kidney transplantation. To increase power, we combined the patients who received rituximab alone and those who received both rituximab and plasmapheresis into the same group. This might have overestimated the effect of rituximab. However, sensitivity analyses in the subgroup that received rituximab alone or rituximab with plasmapheresis did not change the association so this is unlikely to be significant. The timing of recurrence was also not affected by the preventive measure. In contrast, rituximab and plasmapheresis have been shown to be effective for the treatment of recurrent FSGS after kidney transplantation. The efficacy and safety of combined rituximab and plasmapheresis in patients with recurrent FSGS was recently demonstrated in a meta-analysis, reporting that up to 72.7% of patients achieved remission[15]; of these, most patients achieved complete remission. The authors also described a significant reduction in serum creatinine levels (-0.65 mg/dL) and proteinuria (-4.79 g/d) following treatment[15].
Many studies suggest that recurrent FSGS in kidney transplant recipients is at least partially mediated by circulating factors and/or antibodies[43]. The ineffectiveness of prophylactic rituximab in the prevention of FSGS via suppression of antibody production, or plasmapheresis in the removal of pre-formed circulating factors, suggests either circulating factors may be inactive in quiescent FSGS or that removing the putative circulating factors may not be enough to prevent the immunologic cascades that trigger the onset of disease recurrence. It is possible that yet-to-be-identified B-cell-independent immunologic factors may trigger the onset of FSGS recurrence, which leads to production of circulating factors and stimulation of B cells, which are targeted by plasmapheresis and rituximab. The fact that patients who developed FSGS recurrence despite pre-emptive plasmapheresis or rituximab still responded well to plasmapheresis with or without rituximab supports that the initial triggering event is not the putative circulating factors per se and is likely B-cell independent.
Beyond plasmapheresis and rituximab, low-density lipoprotein (LDL) apheresis has been evaluated as a preventive strategy for recurrent FSGS in a Japanese study[49]. LDL apheresis removes plasma lipids, a source of oxidative stress, as well as multiple circulating humoral factors that contribute to disease recurrence. The authors reported no FSGS recurrence in five patients using this regimen of pre-transplant LDL apheresis, in addition to rituximab and basiliximab induction; however, this finding should be confirmed by larger studies.
The results of this meta-analysis should be interpreted with attention to the study limitations. First, all included studies were observational in design; thus, the risk of bias was present, and causality could not be established. Second, the sample size of most studies was small. Third, some studies did not report patient characteristics or prognostic factors. Fourth, the treatment regimen, dose of rituximab, and plasma
Efforts to elucidate the pathogenic mechanisms of FSGS are ongoing. Further clinical research is therefore required, both to accurately identify the subgroup of patients with FSGS who are at a higher risk for disease recurrence, as well as evaluate preventive interventions within this subgroup. At the time of writing, one ongoing randomized controlled trial (clinical trial number: NCT03763643) was identified, with the primary endpoint of preventing recurrent FSGS through the use of preemptive rituximab plus plasmapheresis or plasmapheresis alone.
In unselected patients with FSGS, preemptive rituximab with or without plasmaphe
Focal segmental glomerulosclerosis (FSGS) is one of the most common glomerular diseases leading to kidney failure. FSGS has a high risk of recurrence after kidney transplantation. Prevention of recurrent FSGS using rituximab and/or plasmapheresis has been evaluated in multiple small studies with conflicting results.
FSGS is associated with a high risk of recurrence after kidney transplantation. Plas
This meta-analysis was conducted to assess the effectiveness of rituximab–with or without plasmapheresis–compared with plasmapheresis alone, for the prevention of recurrent FSGS after kidney transplantation.
This meta-analysis and systematic review were performed by first conducting a literature search of the MEDLINE, EMBASE, and Cochrane databases, from inception through March 2021; search terms included ‘FSGS’, ’steroid-resistant nephrotic syndrome’, ‘rituximab’, and ‘plasmapheresis’. We identified studies that assessed the risk of post-transplant FSGS after use of rituximab with or without plasmapheresis, or plasmapheresis alone.
Eleven studies, with a total of 399 kidney transplant recipients with FSGS, evaluated the use of rituximab with or without plasmapheresis; thirteen studies, with a total of 571 kidney transplant recipients with FSGS, evaluated plasmapheresis alone. Post-transplant FSGS recurred relatively early. There was no significant difference in recurrence between the group that received rituximab (with or without plasmaphe
The use of rituximab with or without plasmapheresis, or plasmapheresis alone, is not associated with a lower risk of FSGS recurrence after kidney transplantation.
This meta-analysis is among the first to report that the use of preemptive rituximab, either alone or in combination with plasmapheresis, or plasmapheresis alone, did not alter the recurrence risk of FSGS after kidney transplantation.
| 1. | Bukosza EN, Kornauth C, Hummel K, Schachner H, Huttary N, Krieger S, Nöbauer K, Oszwald A, Razzazi Fazeli E, Kratochwill K, Aufricht C, Szénási G, Hamar P, Gebeshuber CA. ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Kwiatkowska E, Stefańska K, Zieliński M, Sakowska J, Jankowiak M, Trzonkowski P, Marek-Trzonkowska N, Kwiatkowski S. Podocytes-The Most Vulnerable Renal Cells in Preeclampsia. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Shuster S, Ankawi G, Licht C, Reiser J, Wang X, Wei C, Chitayat D, Hladunewich M. Fetal Renal Echogenicity Associated with Maternal Focal Segmental Glomerulosclerosis: The Effect of Transplacental Transmission of Permeability Factor suPAR. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Dumas De La Roque C, Prezelin-Reydit M, Vermorel A, Lepreux S, Deminière C, Combe C, Rigothier C. Idiopathic Nephrotic Syndrome: Characteristics and Identification of Prognostic Factors. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Frese J, Kettwig M, Zappel H, Hofer J, Gröne HJ, Nagel M, Sunder-Plassmann G, Kain R, Neuweiler J, Gross O. Kidney Injury by Variants in the COL4A5 Gene Aggravated by Polymorphisms in Slit Diaphragm Genes Causes Focal Segmental Glomerulosclerosis. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Chadban SJ. Glomerulonephritis recurrence in the renal graft. J Am Soc Nephrol. 2001;12:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Abbott KC, Sawyers ES, Oliver JD 3rd, Ko CW, Kirk AD, Welch PG, Peters TG, Agodoa LY. Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. Am J Kidney Dis. 2001;37:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Uffing A, Pérez-Sáez MJ, Mazzali M, Manfro RC, Bauer AC, de Sottomaior Drumond F, O'Shaughnessy MM, Cheng XS, Chin KK, Ventura CG, Agena F, David-Neto E, Mansur JB, Kirsztajn GM, Tedesco-Silva H Jr, Neto GMV, Arias-Cabrales C, Buxeda A, Bugnazet M, Jouve T, Malvezzi P, Akalin E, Alani O, Agrawal N, La Manna G, Comai G, Bini C, Muhsin SA, Riella MC, Hokazono SR, Farouk SS, Haverly M, Mothi SS, Berger SP, Cravedi P, Riella LV. Recurrence of FSGS after Kidney Transplantation in Adults. Clin J Am Soc Nephrol. 2020;15:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 9. | Artero M, Biava C, Amend W, Tomlanovich S, Vincenti F. Recurrent focal glomerulosclerosis: natural history and response to therapy. Am J Med. 1992;92:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 175] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, Ouseph R, Peddi VR, Pelz CJ, Roza AM, Vincenti F, George V. Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR). Transplantation. 1999;68:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Vallianou K, Marinaki S, Skalioti C, Lionaki S, Darema M, Melexopoulou C, Boletis I. Therapeutic Options for Recurrence of Primary Focal Segmental Glomerulonephritis (FSGS) in the Renal Allograft: Single-Center Experience. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Lanaret C, Anglicheau D, Audard V, Büchler M, Caillard S, Couzi L, Malvezzi P, Mesnard L, Bertrand D, Martinez F, Pernin V, Ducloux D, Poulain C, Thierry A, Del Bello A, Rerolle JP, Greze C, Uro-Coste C, Aniort J, Lambert C, Bouvier N, Schvartz B, Maillard N, Sayegh J, Oniszczuk J, Morin MP, Legendre C, Kamar N, Heng AE, Garrouste C. Rituximab for recurrence of primary focal segmental glomerulosclerosis after kidney transplantation: Results of a nationwide study. Am J Transplant. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Cattran DC, Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Moranne O, Watier L, Rossert J, Stengel B; GN-Progress Study Group. Primary glomerulonephritis: an update on renal survival and determinants of progression. QJM. 2008;101:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Hansrivijit P, Ghahramani N. Combined rituximab and plasmapheresis or plasma exchange for focal segmental glomerulosclerosis in adult kidney transplant recipients: a meta-analysis. Int Urol Nephrol. 2020;52:1377-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17252] [Article Influence: 663.5] [Reference Citation Analysis (0)] |
| 17. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 13629] [Article Influence: 851.8] [Reference Citation Analysis (1)] |
| 18. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 31211] [Article Influence: 780.3] [Reference Citation Analysis (1)] |
| 19. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48612] [Article Influence: 2113.6] [Reference Citation Analysis (4)] |
| 20. | Kawaguchi H, Hattori M, Ito K, Takahashi K, Ota K. Recurrence of focal glomerulosclerosis of allografts in children: the efficacy of intensive plasma exchange therapy before and after renal transplantation. Transplant Proc. 1994;26:7-8. [PubMed] |
| 21. | Otsubo S, Tanabe K, Tokumoto T, Ishikawa N, Shinmura H, Oshima T, Shimizu T, Harano M, Inui M, Shiraga H, Ito K, Fuchinoue S, Nihei H, Toma H. Long-term outcome in renal transplant recipients with focal and segmental glomerulosclerosis. Transplant Proc. 1999;31:2860-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Burke GW, Sageshima J, Fornoni A, Chen L, Abitbol C, Chandar J, Kupin W, Guerra G, Roth D, Shariatmadar S, Zilleruelo G, Ciancio G. Rituximab induction in high risk predominantly pediatric kidney transplant recipients may decrease the incidence and severity of recurrence of focal segmental glomerulosclerosis. Pediatr Transplan. 2009;94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Sagheshima J, Fornoni A, Wei C, Saenz M, Li J, Mattiazzi A, Ladino M, Kamalaveni P, Ricordi C, Rastaldi MP, Mundel P, Reiser J, Burke GW. Effect of rituximab on the regulation of sphingomyelinase-like phosphodiesterase 3b-precursor to prevent FSGS recurrence after renal transplantation. Am J Transplant. 2010;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW 3rd. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 415] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 25. | Miyauchi Y, Shirakawa H, Shimizu T, Omoto K, Ishida H, Tanabe K. Excellent outcomes of rituximab administration plus plasmapheresis as prophylactic treatment prior to kidney transplantation in patients with focal segmental glomerulosclerosis. Am J Transplant. 2011;427. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Park HS, Hong Y, Sun IO, Chung BH, Kim HW, Choi BS, Park CW, Jin DC, Kim YS, Yang CW. Effects of pretransplant plasmapheresis and rituximab on recurrence of focal segmental glomerulosclerosis in adult renal transplant recipients. Korean J Intern Med. 2014;29:482-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Okumi M, Miyauchi Y, Yagisawa T, Unagami K, Toki D, Omoto K, Ishida H, Tanabe K. Excellent outcomes of prophylactic rituximab administration with plasmapheresis in kidney transplant recipients with focal segmental glomerulosclerosis. Am J Transplant. 2015;. |
| 28. | Futamura K, Okada M, Nagai T, Yamamoto T, Hiramitsu T, Tsujita M, Goto N, Narumi S, Watarai Y. Recurrent focal segmental glomerular sclerosis after renal transplantation; prevention and treatment with Rituximab. Transplant. 2016;S658. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Alasfar S, Matar D, Montgomery RA, Desai N, Lonze B, Vujjini V, Estrella MM, Manllo Dieck J, Khneizer G, Sever S, Reiser J, Alachkar N. Rituximab and Therapeutic Plasma Exchange in Recurrent Focal Segmental Glomerulosclerosis Postkidney Transplantation. Transplantation. 2018;102:e115-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Lu Y, Lyons J, Tischer S, Woodside K, Park J. Efficacy and safety of a single-dose rituximab for prevention of focal segmental glomerulosclerosis recurrence after kidney transplant. Am J Transplant. 2018;801. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Auñón P, Polanco N, Pérez-Sáez MJ, Rodrigo E, Sancho A, Pascual J, Andrés A, Praga M. Pre-emptive rituximab in focal and segmental glomerulosclerosis patients at risk of recurrence after kidney transplantation. Clin Kidney J. 2021;14:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Iguchi Y, Tanabe K, Yagisawa T, Fuchinoue S, Kawai T, Kawaguchi H, Takahashi K, Ito K, Toma H, Agishi T, Ota K. Plasmapheresis for prevention of recurrent focal segmental glomerulosclerosis of kidney allograft in adult recipients. Ther Apher. 1997;1:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Ohta T, Kawaguchi H, Hattori M, Komatsu Y, Akioka Y, Nagata M, Shiraga H, Ito K, Takahashi K, Ishikawa N, Tanabe K, Yamaguchi Y, Ota K. Effect of pre-and postoperative plasmapheresis on posttransplant recurrence of focal segmental glomerulosclerosis in children. Transplantation. 2001;71:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Somers MJG, Baum MA. Pre-transplant conditioning with plasmapheresis and cyclosporine infusion reduces recurrence of focal segmental glomerulosclerosis (fsgs) in children. Pediatr Transplant. 2009;96. |
| 35. | Gonzalez E, Ettenger R, Rianthavorn P, Tsai E, Malekzadeh M. Preemptive plasmapheresis and recurrence of focal segmental glomerulosclerosis in pediatric renal transplantation. Pediatr Transplant. 2011;15:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Verghese PS, Rheault MN, Jackson S, Matas AJ, Chinnakotla S, Chavers B. The effect of peri-transplant plasmapheresis in the prevention of recurrent FSGS. Pediatr Transplant. 2018;22:e13154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Koyun M, Çomak E, Akman S. Peri-transplant Plasmapheresis in FSGS. Pediatr Transplant. 2019;23:e13322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Campise M, Favi E, Messa P. Clinical Outcomes of Prophylactic and Therapeutic Plasmapheresis in Adult Deceased-Donor Kidney Transplant Recipients With Primary Focal Segmental Glomerulosclerosis. Exp Clin Transplant. 2019;17:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Mukku VK, Hussain S, Mujtaba MA. 201 Overview of Recurrence of Focal Segmental Glomerulosclerosis in Renal Transplant Patients and Effectiveness of Preemptive Plasma Exchange and Rituximab in Preventing Recurrence. Am J Kidney Dis. 2021;630. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 501] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 41. | Le Berre L, Godfrin Y, Günther E, Buzelin F, Perretto S, Smit H, Kerjaschki D, Usal C, Cuturi C, Soulillou JP, Dantal J. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J Clin Invest. 2002;109:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, Burke GW, Bruneval P, Naesens M, Jackson A, Alachkar N, Canaud G, Legendre C, Anglicheau D, Reiser J, Sarwal MM. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6:256ra136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 43. | Kienzl-Wagner K, Waldegger S, Schneeberger S. Disease Recurrence-The Sword of Damocles in Kidney Transplantation for Primary Focal Segmental Glomerulosclerosis. Front Immunol. 2019;10:1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366:1648-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 45. | Kienzl-Wagner K, Rosales A, Scheidl S, Giner T, Bösmüller C, Rudnicki M, Oberhuber R, Margreiter C, Soleiman A, Öfner D, Waldegger S, Schneeberger S. Successful management of recurrent focal segmental glomerulosclerosis. Am J Transplant. 2018;18:2818-2822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Kashgary A, Sontrop JM, Li L, Al-Jaishi AA, Habibullah ZN, Alsolaimani R, Clark WF. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: A systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 2016;17:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Santos JE, Fiel D, Santos R, Vicente R, Aguiar R, Santos I, Amoedo M, Pires C. Rituximab use in adult glomerulopathies and its rationale. J Bras Nefrol. 2020;42:77-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Gauckler P, Shin JI, Alberici F, Audard V, Bruchfeld A, Busch M, Cheung CK, Crnogorac M, Delbarba E, Eller K, Faguer S, Galesic K, Griffin S, Hrušková Z, Jeyabalan A, Karras A, King C, Kohli HS, Maas R, Mayer G, Moiseev S, Muto M, Odler B, Pepper RJ, Quintana LF, Radhakrishnan J, Ramachandran R, Salama AD, Segelmark M, Tesař V, Wetzels J, Willcocks L, Windpessl M, Zand L, Zonozi R, Kronbichler A; RITERM study group. Rituximab in adult minimal change disease and focal segmental glomerulosclerosis - What is known and what is still unknown? Autoimmun Rev. 2020;19:102671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 49. | Sannomiya A, Murakami T, Koyama I, Nitta K, Nakajima I, Fuchinoue S. Preoperative Low-Density Lipoprotein Apheresis for Preventing Recurrence of Focal Segmental Glomerulosclerosis after Kidney Transplantation. J Transplant. 2018;2018:8926786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ban TH, Rijkse E, Rostaing L S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY