Published online Jun 18, 2021. doi: 10.5500/wjt.v11.i6.244
Peer-review started: April 7, 2021
First decision: May 5, 2021
Revised: May 12, 2021
Accepted: June 1, 2021
Article in press: June 1, 2021
Published online: June 18, 2021
Processing time: 65 Days and 22.5 Hours
Bartonellosis is a rare but challenging condition to diagnose with a spectrum of clinical presentations in the immunocompromised host.
To further characterize the presentation of Bartonella henselae (B. henselae) infec
We conducted a single-center retrospective study of all B. henselae testing for 5012 transplant recipients receiving care at a single institution between 2011 and 2018.
We identified 38 patients who underwent testing for B. henselae, and three of 38 were found to have bartonellosis. Two of the patients were renal transplant recipients who presented with visceral bartonellosis and symptoms concerning for post-transplant lymphoproliferative disorder. One autologous stem cell transplant recipient presented with cat scratch disease. We detail the clinical courses of these three cases and review the literature concerning the clinical presentations, differential diagnosis, and limitations of diagnostic tests for B. henselae infections in transplant recipients.
Although the incidence of B. henselae infection in transplant recipients is unknown, it merits inclusion in the differential diagnosis for fever of unknown origin in this population.
Core Tip: Bartonellosis is a rare but challenging condition to diagnose. We conducted a single-center retrospective study of all Bartonella henselae (B. henselae) testing for 5012 transplant recipients receiving their care at our institution between 2011 and 2018. We identified 38 patients who underwent testing for B. henselae, and three of 38 were found to have bartonellosis. Two of the patients were renal transplant recipients and presented with symptoms concerning for post-transplant lymphoproliferative disorder. One stem cell transplant recipient presented with cat scratch disease. We detail the clinical courses of these three cases and review the literature concerning B. henselae infections in transplant recipients.
- Citation: Pischel L, Radcliffe C, Vilchez GA, Charifa A, Zhang XC, Grant M. Bartonellosis in transplant recipients: A retrospective single center experience. World J Transplant 2021; 11(6): 244-253
- URL: https://www.wjgnet.com/2220-3230/full/v11/i6/244.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i6.244
Bartonella henselae (B. henselae) is a zoonotic pathogen whose wide range of clinical presentations is contingent upon the immune status of the human host. While immunocompetent hosts often present with cat scratch disease (CSD), a peripheral lymphadenitis, this is a less common presentation for solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) recipients[1]. Transplant recipients often present with granulomatous lesions of visceral organs or deep lymph nodes. This patient population may also present with bacillary angiomatosis-peliosis–a proliferation of vascular endothelial cells from angiogenic factors in the skin, liver, or spleen often associated with acquired immunodeficiency syndrome[1-5]. Findings may be localized to a specific organ or they may be disseminated, involving two or more organ systems.
Bartonellosis can present as fever and deep lymphadenopathy in the transplant population; however, the differential diagnosis for this symptom dyad is broad and includes post-transplant lymphoproliferative disease (PTLD), disseminated mycobacterial infections, invasive fungal infections, and several other etiologies[6-8]. B. henselae infection is an unusual culprit, and confirmation of the diagnosis is further comp
To better characterize the clinical presentation and diagnosis of B. henselae infections in SOT and HSCT recipients, we conducted a retrospective study of all B. henselae testing for 5012 transplant recipients who received their care at our institution between 2011 and 2018. We aimed to characterize the presenting symptoms and detail the diagnostic methods required to establish the diagnosis of bartonellosis in this population.
This study was conducted at our tertiary care transplant center at Yale New Haven Hospital in New Haven, Connecticut. Electronic medical records for all adult SOT and HSCT recipients (heart, lung, kidney, pancreas, liver, cord blood, and autologous or allogeneic bone marrow transplant recipients) who received their care at our institution between 2011 and 2018 were queried to identify all transplant recipients for which B. henselae immunoglobulin (Ig)M titers, IgG titers, and/or serum polymerase chain reaction (PCR) were obtained during both inpatient and outpatient clinical courses. The electronic medical records of all transplant recipients with positive tests were reviewed for symptoms compatible with either classic CSD or bartonellosis involving viscera, nervous tissue, or any other organ system[11,12]. Patients with fever, lymphadenopathy, skin findings, neurological deficits, ocular findings, hepatosplenic lesions, or possible osteomyelitis temporally associated with positive B. henselae tests were included for further analysis. Patients were excluded if they were less than 18 years old. Two authors (LP, MG) verified whether cases should be included or excluded. Data was stored in a secure, encrypted fashion during this study. The Yale University Institutional Review Board reviewed and approved our study protocol, and the need for informed consent was waived.
In total, 38 of 5012 (0.8%) SOT and HSCT recipients were tested for B. henselae. Positive IgG or IgM titers were identified in 6 of 38 (16%) patients. Three patients were excluded as follows: (1) One patient was excluded as the diagnosis of CSD was 4 mo before autologous stem cell transplantation; (2) A 26-year-old female status-post bilateral lung transplant recipient treated for culture-negative chronic osteomyelitis of multiple vertebrae with B. henselae serum IgG detected (1:128) who improved on vancomycin and ciprofloxacin therapy; convalescent titers were not drawn; and (3) A 76-year-old male liver transplant recipient who presented with malaise and myalgias and was found to have positive B. henselae (IgG 1:256) and babesia (IgG 1:512) serology whose symptoms resolved after 10 d of azithromycin and atovaquone followed by three weeks of doxycycline. The cause of his symptoms was attributed to babesiosis. The 26-year-old female and 76-year-old male were excluded due to competing diagnoses which better accounted for symptoms. The remaining three patients were included as cases in the present study (Table 1), and their clinical courses are detailed below.
| Age/sex | Transplant type | Time since transplant | Immunosuppressive medications | Presenting symptoms | Confirmatory studies | Treatment | Outcome |
| 26/F | Kidney | 13 mo | Prednisone, mycophenolate, tacrolimus | Fever, chills, cough, diarrhea | Liver biopsy & IHC stain, tissue PCR, serology | Azithromycin/doxycycline | Success |
| 44/M | Kidney-pancreas | 14 yr | Prednisone, cyclosporine | Fever, night sweats, weight loss | Serology, serum PCR, splenic biopsy & Warthin-Starry stain | Azithromycin/doxycycline | Success |
| 21/M | Autologous stem cell transplant | 6 yr | None | Supraclavicular and axillary lymphadenopathy | Excisional lymph node biopsy, serology | None | Success |
A 26-year-old woman with a history of hypoplastic kidney disease, and a failed living donor kidney transplant at age 14, presented with one month of fever. She had received a deceased donor kidney transplant 13 mo prior to presentation with alemtuzumab induction and had been taking prednisone, mycophenolate, and tacrolimus subsequently for immunosuppression. On admission, she reported daily fevers (Tmax 102.3 °F), chills, cough, and watery diarrhea. Her physical exam was unremarkable.
Initial laboratory testing revealed a white blood cell count of 8900 cells/mm3, hemoglobin of 6.8 g/dL, and platelet count of 320000/mm3. Epstein-Barr virus (EBV) plasma PCR was 670 copies/mL while cytomegalovirus, parvovirus, and BK virus were not detected by PCR. Human immunodeficiency virus (HIV), hepatitis serologies, and Histoplasma capsulatum urine antigen were negative.
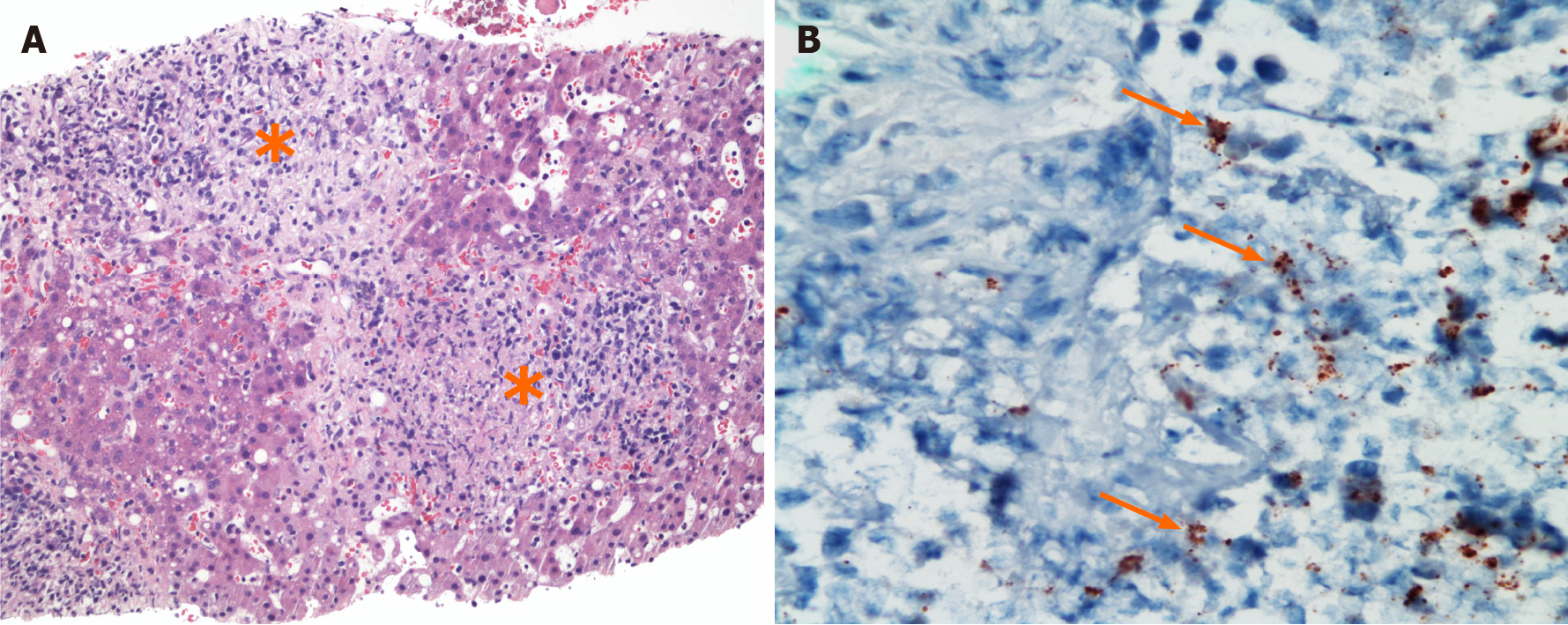
On hospital day (HD)-4, a contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed a poorly defined 2.5 cm hypoattenuated lesion in the right lobe of the liver and prominent gastrohepatic and retroperitoneal lymph nodes. On HD-5, further history revealed she owned a cat, and B. henselae serologies were sent. A CT-guided biopsy of the hepatic lesion revealed granulomatous hepatitis with numerous non-necrotizing granulomas (Figure 1A), focal abscess formation, and infiltrating lymphocytes with scattered CD20+ B lymphocytes and polytypic plasma cells. In situ hybridization for EBV was negative as were Gomori methenamine silver (GMS), Warthin-Starry, and acid-fast bacillus (AFB) stains.
Her hospital course was complicated by acute kidney injury which briefly required renal replacement therapy. An allograft biopsy showed chronic injury without evidence of acute cellular rejection, and there was evidence of membranoproliferative glomerulonephritis. On HD-10, a positron emission tomography-CT scan (PET-CT) identified the right liver lesion, a portocaval lymph node, and paraaortic lymph nodes as hypermetabolic. As suspicion for PTLD remained high, a bone marrow biopsy was collected on HD-14 and returned negative for PTLD.
On HD-15, B. henselae serology returned with a positive IgM titer of 1:64 and IgG > 1:1024. From her prior liver biopsy, immunohistochemical staining for B. henselae was positive (Figure 1B). She was diagnosed with hepatic bartonellosis, and oral azithro
She clinically improved and was discharged on HD-25 without continued need for dialysis. Six weeks after discharge, a repeat PET-CT showed resolution of the hypermetabolic areas. In total, she received 6.5 mo of azithromycin and doxycycline therapy. Four years after her diagnosis, she displays no signs or symptoms of relapse and continues to own a cat.
A 44-year-old man with well-controlled type 1 diabetes mellitus and dual kidney-pancreas transplant 14 years prior to admission presented with two months of fever, drenching night sweats, and a 30-pound weight loss. His immunosuppressive medications were cyclosporine and prednisone (5 mg daily). His physical exam was unrevealing.
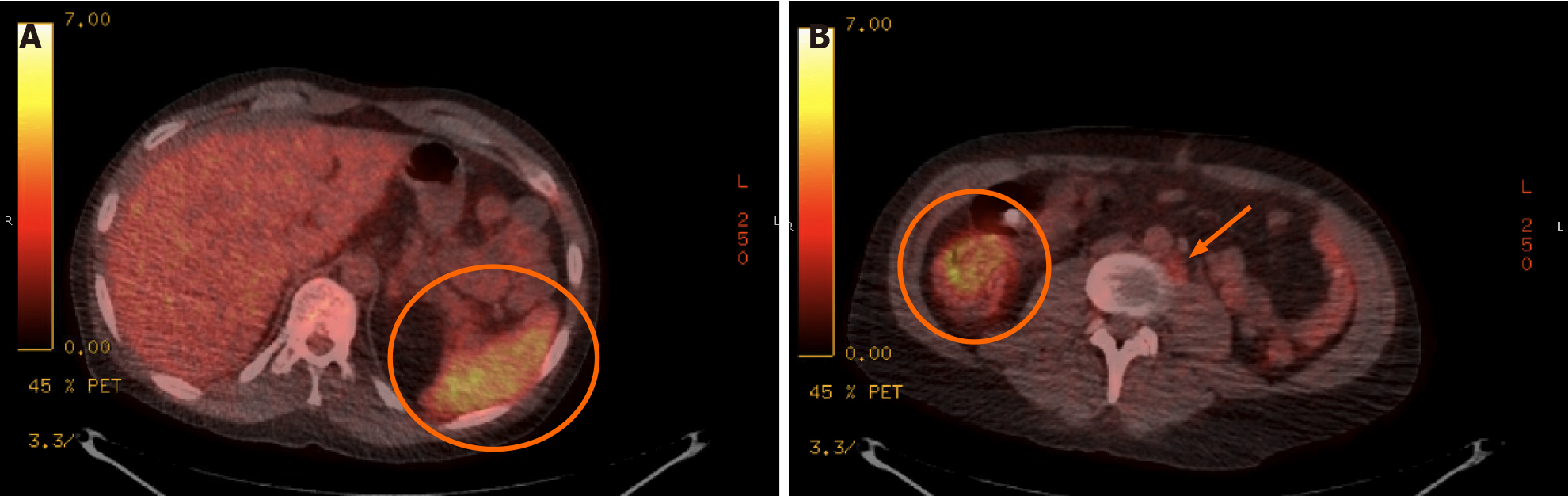
On admission, laboratory studies demonstrated no leukocytosis, mild anemia (9.8 g/dL), and EBV plasma PCR detected 8211 copies/mL (log 3.91). PET-CT scan on the day of admission showed multiple mildly hypermetabolic < 1 cm retroperitoneal lymph nodes, diffuse splenic uptake, and increased signal in the pericecal region and sigmoid colon (Figure 2). PTLD was suspected, but bone marrow biopsy on HD-2 showed normal trilineage hematopoiesis. T-cell receptor gene rearrangements from the peripheral blood and bone marrow were negative for lymphoproliferative disorders. Serum toxoplasma serologies were negative. He remained off antibiotics with daily fevers to 103 ⚬F. EBV viral load rose to 56000 copies/mL (log 4.75) on HD-3. He reported chronic headache the following day, and cerebrospinal fluid (CSF) collected via lumbar puncture showed normal cell count, glucose, and protein. John Cunningham virus PCR, Cryptococcus neoformans antigen, and CSF fungal cultures were negative; however, EBV was detected (log 2.2). The same day, he reported owning a cat.
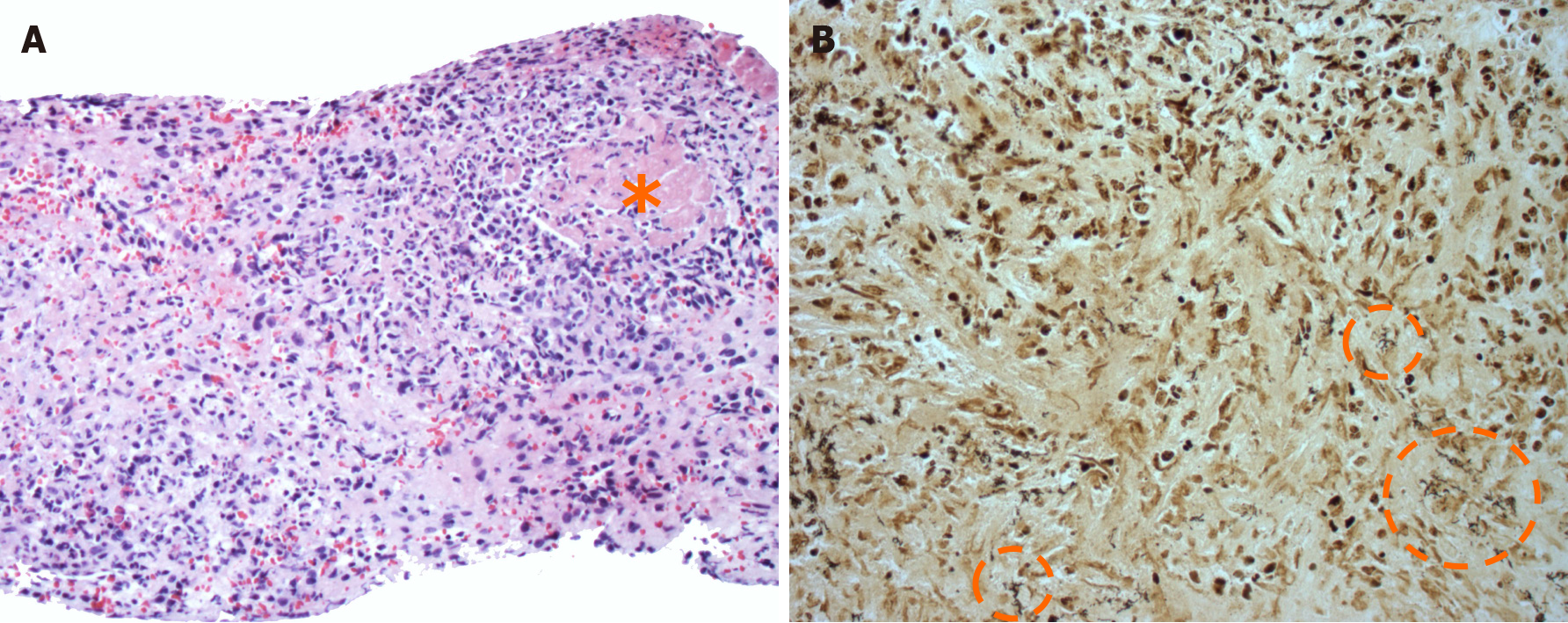
With continued concern for PTLD, the patient underwent a percutaneous core biopsy of the spleen. A histopathologic review showed clusters of small lymphocytes and necrotizing granulomas with neutrophil infiltration (Figure 3A) raising suspicion for disseminated bartonellosis. A Warthin-Starry stain was positive for individual and clustered extracellular coccobacilli within necrotic foci (Figure 3B). AFB, GMS, and Brown-Hopps Gram stains were negative on the splenic tissue while EBV-encoded DNA was diffusely positive. Azithromycin and doxycycline were initiated. Serologic titers from HD-14 returned positive for B. henselae with IgG 1:128 and IgM < 1:16. On HD-20, B. henselae serum PCR returned positive.
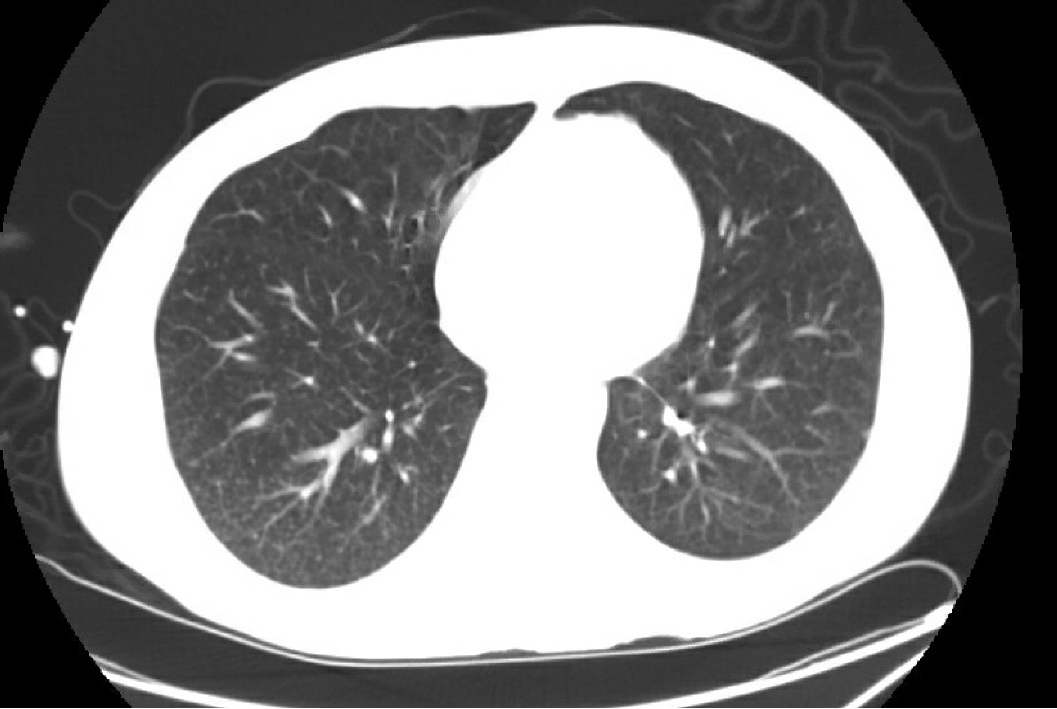
After the diagnosis, he developed a cough, and a chest CT demonstrated a miliary pattern (Figure 4). Bronchoalveolar lavage 11 d into therapy yielded abundant pulmonary macrophages but was negative for malignant cells or fungal organisms. Pathology from a transbronchial biopsy showed noncaseating granulomas with negative AFB and GMS. This was thought to be consistent with disseminated bartonellosis despite negative Warthin-Starry staining and 16S ribosomal RNA sequencing. The negative result may have been due to 11 days of azithromycin and doxycycline therapy prior to biopsy.
Azithromycin and doxycycline were continued for one year. With concern for concomitant PTLD, he received four doses of rituximab, and his EBV viral load decreased from log 5.06 to log 3.26. Serum B. henselae IgG titers rose to 1:256 and remained stable 19 mo after his initial presentation. He has been without signs or symptoms of a recurrence of disseminated bartonellosis for eight years.
A 21-year-old man status-post autologous stem cell transplant 6 years prior and off immunosuppressive therapy presented with an enlarged, firm 1 cm lymph node in his left supra-clavicular area. This lymph node was re-demonstrated on a PET-CT scan in addition to two lymph nodes in the left axilla. He had an excisional biopsy of the left supraclavicular node that showed necrotizing lymphadenitis and histologic features consistent with CSD. Multiple caseating granulomas, one large granuloma with prominent central necrosis, and some scattered neutrophils were noted. AFB, GMS, and Warthin-Starry stains were negative for microorganisms. B. henselae IgG serology was positive at 1:256 (IgM < 1:16). No convalescent serology was performed. No record of CSD treatment was noted, but nine months later he did not endorse lymphadenopathy. Five years after his initial presentation, he has not endorsed recurrence of his lymphadenopathy.
B. henselae is a fastidious, gram-negative coccobacillus often transmitted by direct inoculation from a feline host or via cat fleas (Ctenocephalides felis)[1]. To further characterize the presentation of B. henselae infections in SOT and HSCT recipients, we conducted a single-center retrospective study of all B. henselae testing for 5012 transplant patients receiving their care at our institution between 2011 and 2018. We identified two SOT recipients who were found to have infiltrative bartonellosis involving reticuloendothelial organs and one autologous stem cell transplant recipient with CSD. Of all patients reviewed, only one (Case 2) had disseminated disease. Both SOT patients with systemic symptoms presented with symptoms concerning for PTLD and had hepatosplenic involvement. Fortunately, all patients in our study had positive outcomes.
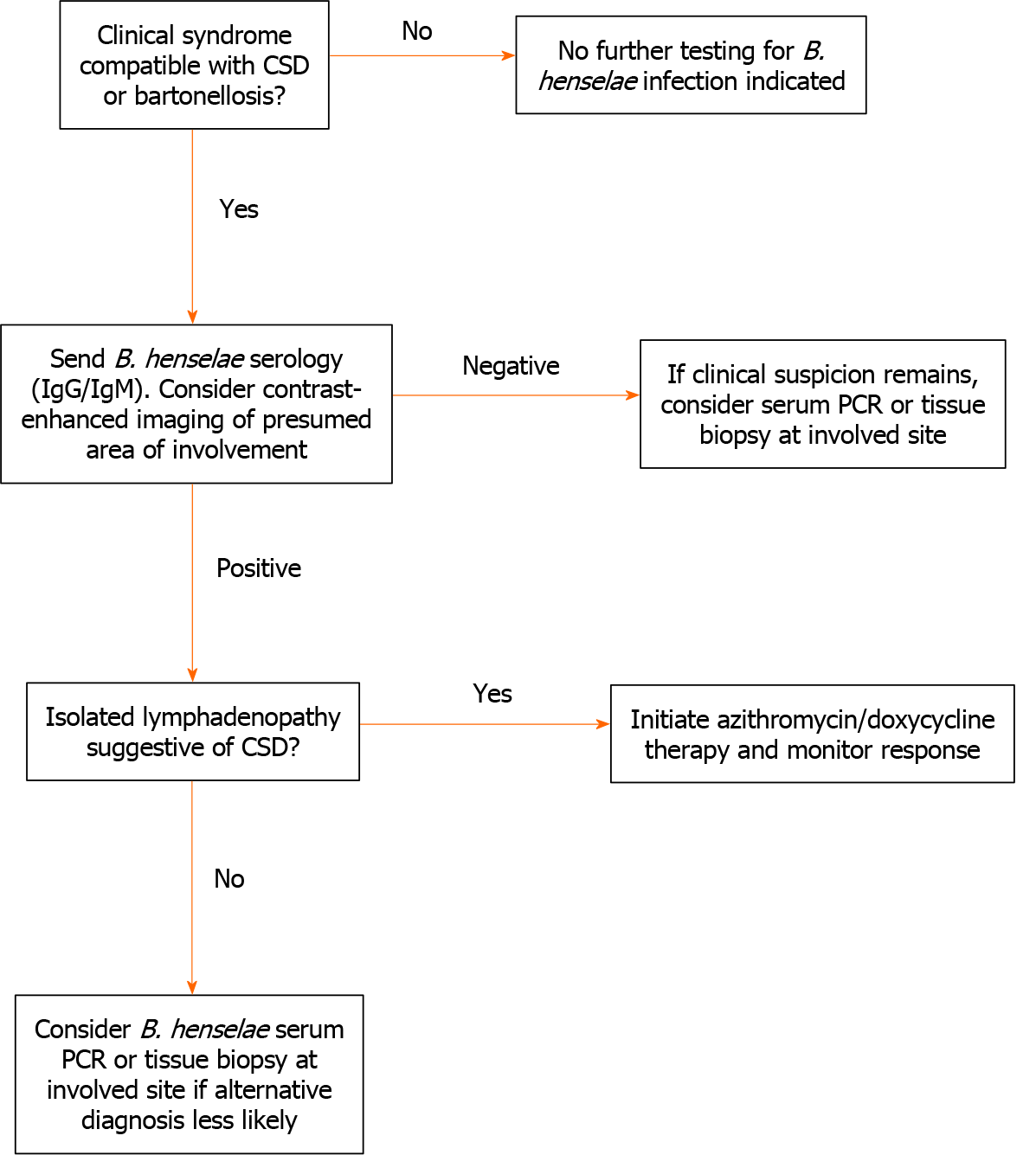
In our series, the diagnosis of bartonellosis proved to be difficult. In general, proposed diagnostic criteria for typical CSD center on epidemiological risk factors (e.g. cat or kitten exposure), laboratory or radiographic findings, positive Bartonella spp. PCR, pathological criteria, and other clinical data[12]. Three criteria may be sufficient to diagnose typical CSD; however, four may be required for atypical or disseminated disease. Figure 5 offers a basic approach on when to consider further diagnostic approaches. In general, imaging can often be helpful. Patients with hepatosplenic involvement often have ultrasound studies showing hypoechoic lesions or CT scans showing hypoattenuated lesions[13,14]. For our cases, PET-CT was informative, and its utility has been previously reported[15].
In general, fever and lymphadenopathy in a transplant recipient can stem from a broad range of diagnoses which include miliary tuberculosis, tularemia, invasive fungal infections, toxoplasmosis, brucellosis, PTLD, and malignancy[16,17]. In two of the cases discussed above, both patients had EBV viremia and PTLD remained high on the differential diagnosis. One patient even received rituximab due to concern for concurrent PTLD. Several other reports from this population also demonstrated diagnostic uncertainty between non-CSD manifestations of bartonellosis vs PTLD, and patients have been treated for both conditions in some reports[4,7,18,19]. As PTLD has a significant impact on mortality, distinguishing these two conditions is paramount and can often be accomplished via histopathology. Treating both conditions may be considered with caution if a solitary diagnosis cannot be established[20].
In the immunocompetent host, conservative management is usually recommended for CSD, and short-term azithromycin may offer minimal benefit[21]. However, immunocompromised hosts require treatment. The optimal treatment duration of SOT and HSCT recipients is not known and has been extrapolated from HIV/acquired immunodeficiency syndrome treatment guidelines[22]. Treatment duration has ranged from 5 d to 12 mo in transplant recipients, and most reports lack relapses despite shorter treatment durations[23]. In absence of ample evidence to guide tapering of immunosuppressive regimens, we recommend multidisciplinary discussions between provider teams when approaching the possibility of reducing immunosuppression in transplant recipients undergoing treatment for bartonellosis.
Regarding testing, B. henselae is a fastidious organism, and tissue or blood cultures have low sensitivity. In endocarditis, only 20% of blood cultures return positive, and growth can take up to 2-6 wk[14,24,25]. PCR is rapid, specific (as high as 100%)[26], and able to be performed on blood or tissue samples, although there is no standardization across laboratories[14]. PCR sensitivity for lymph nodes in CSD ranges from 61 to 84%, and tissue PCR is thought to be more sensitive than other modalities when diagnosing disseminated disease[26].
Alongside PCR, serology has two common methods: enzyme linked immunosor
Pathology is often key to the diagnosis of bartonellosis. A positive Warthin-Starry stain may show clumps of extracellular gram-negative rods or L-shaped bacteria within areas of necrotic debris[7,31]. For bacillary angiomatosis, lesions closely resemble a pyogenic granuloma with vascular proliferation. In reported cases of disseminated disease, 9 of 15 biopsies were positive on Warthin-Starry staining[1,4,5]; however, immunohistochemistry (IHC) staining is more sensitive than Warthin-starry staining. This was exemplified in Case 1 above. Staining methods can be highly variable with one pathological case series (n = 24) on confirmed lymphadenitis cases with clinical or histologic concern for CSD finding 46% positive by silver stain, 38% by PCR, and 25% by IHC. Only 2 of 24 cases were positive by all three tests[32]. In a more recent study (n = 46) on paraffin-embedded lymphadenitis specimens with clinical or histologic concern for CSD, 80% were positive by IHC while Warthin-Starry was positive in 52% cases[33]. In transplant recipients, obtaining the diagnosis can be more challenging as pathologic findings are highly variable. One study reported 53% of cases with granulomatous inflammation, 27% with bacillary angiomatosis-peliosis, and 13% with mixed pathology[1].
Overall, bartonellosis should be considered in the differential diagnosis of SOT and HSCT recipients with persistent fever, known cat exposures, lymphadenopathy, and/or hepatosplenomegaly. Diagnostic approaches include serology, PCR, Warthin-Starry staining, and specific immunohistochemical staining for B. henselae. PET-CT can be useful to determine which region would be amenable to tissue biopsy. Serologic testing for B. henselae appears to be accurate for the diagnosis of infection in the transplanted host, although serology may not be positive until convalescence. We report three cases of bartonellosis treated at our institution which reinforce the notion that B. henselae infection should be considered when transplant recipients present with symptoms overlapping those of PTLD.
Bartonellosis is a rare but challenging condition to diagnose with a spectrum of clinical presentations in the immunocompromised host.
In order to better understand this rare syndrome, we reviewed our center’s experience with bartonellosis in transplant recipients.
We aimed to characterize the presenting symptoms and detail the diagnostic methods required to establish the diagnosis of bartonellosis in this population.
We conducted a retrospective study of all Bartonella henselae (B. henselae) testing for 5012 transplant recipients who received their care at our institution between 2011 and 2018.
We identified 38 patients who underwent testing for B. henselae, and three of 38 were found to have bartonellosis. Two of the patients were renal transplant recipients and presented with symptoms concerning for post-transplant lymphoproliferative disorder (PTLD). One autologous stem cell transplant recipient presented with cat scratch disease.
We report three cases of bartonellosis treated at our institution which reinforce the notion that B. henselae infection should be considered when transplant recipients present with symptoms overlapping those of PTLD.
Overall, bartonellosis should be considered in the differential diagnosis of solid organ transplant or hematopoietic stem cell transplant recipients with persistent fever, known cat exposures, lymphadenopathy, and/or hepatosplenomegaly.
| 1. | Psarros G, Riddell J 4th, Gandhi T, Kauffman CA, Cinti SK. Bartonella henselae infections in solid organ transplant recipients: report of 5 cases and review of the literature. Medicine (Baltimore). 2012;91:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Perkocha LA, Geaghan SM, Yen TS, Nishimura SL, Chan SP, Garcia-Kennedy R, Honda G, Stoloff AC, Klein HZ, Goldman RL. Clinical and pathological features of bacillary peliosis hepatis in association with human immunodeficiency virus infection. N Engl J Med. 1990;323:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 170] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Brzewski P, Kwiecińska M, Sułowicz J, Podolec K, Obtułowicz A, Dyduch G, Wojas-Pelc A. Bacillary Angiomatosis in Renal Transplant Recipient: A Case Report. Transplant Proc. 2020;52:2524-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Rostad CA, McElroy AK, Hilinski JA, Thompson MP, Drew CP, Denison AM, Zaki SR, Mahle WT, Rogers J, Abramowsky CR, Shehata B. Bartonella henselae-mediated disease in solid organ transplant recipients: two pediatric cases and a literature review. Transpl Infect Dis. 2012;14:E71-E81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Antar AAR, Goyal A, Murphy K, Schimmel M, Gilotra NA, Martin I, Crane GM, Sciortino C, Avery RK, Houston BA. Disseminated cat-scratch disease presenting as nausea, diarrhea, and weight loss without fever in a heart transplant recipient. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:2058-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Rheault MN, van Burik JA, Mauer M, Ingulli E, Ferrieri P, Jessurun J, Chavers BM. Cat-scratch disease relapse in a kidney transplant recipient. Pediatr Transplant. 2007;11:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Barka NE, Hadfield T, Patnaik M, Schwartzman WA, Peter JB. EIA for detection of Rochalimaea henselae-reactive IgG, IgM, and IgA antibodies in patients with suspected cat-scratch disease. J Infect Dis. 1993;167:1503-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 597] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Vermeulen MJ, Diederen BMW, Verbakel H, Peeters MF. Low sensitivity of Bartonella henselae PCR in serum samples of patients with cat-scratch disease lymphadenitis. J Med Microbiol. 2008;57:1049-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore). 1961;40:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 722] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 12. | Margileth AM. Recent Advances in Diagnosis and Treatment of Cat Scratch Disease. Curr Infect Dis Rep. 2000;2:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Rohr A, Saettele MR, Patel SA, Lawrence CA, Lowe LH. Spectrum of radiological manifestations of paediatric cat-scratch disease. Pediatr Radiol. 2012;42:1380-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics. 2008;121:e1413-e1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Norredam M, Knudsen A, Thomsen C, Wiese L. Disseminated Bartonella henselae Infection Visualized by [18F]FDG-PET/CT and MRI. Diagnostics (Basel). 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Apsemidou A, Rauwolf K, Tragiannidis A, Brentrup A, Schiborr M, Becker K, Ahlmann M, Groll AH. Disseminated Bartonella henselae disease mimicking Langerhans' cell histiocytosis. Pediatr Blood Cancer. 2019;66:e27573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Mazur-Melewska K, Jończyk-Potoczna K, Mania A, Kemnitz P, Szydłowski J, Służewski W, Figlerowicz M. The significance of Bartonella henselae bacterias for oncological diagnosis in children. Infect Agent Cancer. 2015;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Dharnidharka VR, Richard GA, Neiberger RE, Fennell RS 3rd. Cat scratch disease and acute rejection after pediatric renal transplantation. Pediatr Transplant. 2002;6:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gai M, d'Onofrio G, di Vico MC, Ranghino A, Nappo A, Diena D, Novero D, Limerutti G, Messina M, Biancone L. Cat-Scratch Disease: Case Report and Review of the Literature. Transplant Proc. 2015;47:2245-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Trofe J, Buell JF, Beebe TM, Hanaway MJ, First MR, Alloway RR, Gross TG, Succop P, Woodle ES. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn International Transplant Tumor Registry experience. Am J Transplant. 2005;5:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Bass JW, Freitas BC, Freitas AD, Sisler CL, Chan DS, Vincent JM, Person DA, Claybaugh JR, Wittler RR, Weisse ME, Regnery RL, Slater LN. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1-207; quiz CE1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Lopez SMC, Davis A, Zinn M, Feingold B, Green M, Michaels MG. Bartonella henselae infection in the pediatric solid organ transplant recipient. Pediatr Transplant. 2020;e13823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Schutze GE. Diagnosis and treatment of Bartonella henselae infections. Pediatr Infect Dis J. 2000;19:1185-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84:162-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Hansmann Y, DeMartino S, Piémont Y, Meyer N, Mariet P, Heller R, Christmann D, Jaulhac B. Diagnosis of cat scratch disease with detection of Bartonella henselae by PCR: a study of patients with lymph node enlargement. J Clin Microbiol. 2005;43:3800-3806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Sander A, Posselt M, Oberle K, Bredt W. Seroprevalence of antibodies to Bartonella henselae in patients with cat scratch disease and in healthy controls: evaluation and comparison of two commercial serological tests. Clin Diagn Lab Immunol. 1998;5:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Metzkor-Cotter E, Kletter Y, Avidor B, Varon M, Golan Y, Ephros M, Giladi M. Long-term serological analysis and clinical follow-up of patients with cat scratch disease. Clin Infect Dis. 2003;37:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Koehler JE, Duncan LM. Case records of the Massachusetts General Hospital. Case 30-2005. A 56-year-old man with fever and axillary lymphadenopathy. N Engl J Med. 2005;353:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Moulin C, Kanitakis J, Ranchin B, Chauvet C, Gillet Y, Morelon E, Euvrard S. Cutaneous bacillary angiomatosis in renal transplant recipients: report of three new cases and literature review. Transpl Infect Dis. 2012;14:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Caniza MA, Granger DL, Wilson KH, Washington MK, Kordick DL, Frush DP, Blitchington RB. Bartonella henselae: etiology of pulmonary nodules in a patient with depressed cell-mediated immunity. Clin Infect Dis. 1995;20:1505-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Caponetti GC, Pantanowitz L, Marconi S, Havens JM, Lamps LW, Otis CN. Evaluation of immunohistochemistry in identifying Bartonella henselae in cat-scratch disease. Am J Clin Pathol. 2009;131:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Peng J, Fan Z, Zheng H, Lu J, Zhan Y. Combined Application of Immunohistochemistry and Warthin-Starry Silver Stain on the Pathologic Diagnosis of Cat Scratch Disease. Appl Immunohistochem Mol Morphol. 2020;28:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bellini MI, Bozkurt HS, de Araujo H S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY