Published online Dec 18, 2021. doi: 10.5500/wjt.v11.i12.512
Peer-review started: July 26, 2021
First decision: September 2, 2021
Revised: September 25, 2021
Accepted: November 14, 2021
Article in press: November 14, 2021
Published online: December 18, 2021
Processing time: 140 Days and 16.5 Hours
Patients undergoing solid organ transplantation, particularly those who live or have lived in tuberculosis (TB) endemic areas, are at a high risk of developing TB. The majority of post-transplantation TB cases are associated with reactivation of latent TB infection (LTBI). Brazil is in a single position with overlapping areas of high TB endemicity and high transplant activity. In liver transplant (LT), one should be aware of the potential hepatotoxicity associated with the treatment regimens for LTBI.
To evaluate the frequency of LTBI in LT patients and treatment-related issues.
This was a retrospective analysis of a cohort of cirrhotic patients aged ≥ 18 years, who underwent LT at a high-complexity teaching hospital from January 2005 to December 2012.
Overall, 429 patients underwent LT during the study period. Of these, 213 (49.7%) underwent the tuberculin skin test (TST) during the pre-transplant period, and 35 (16.4%) of them had a positive result. The treatment for LTBI was initiated after LT in 12 (34.3%) of the TST-positive patients; in 3 (25.0%), treatment was main
The prevalence of LTBI was lower than expected. Initiation and completion of LTBI treatment was limited by difficulties in the management of these special patients.
Core Tip: In liver transplant, one should be aware of the potential hepatotoxicity associated with the treatment regimens for latent tuberculosis infection (LTBI). The aim of this study was to evaluate the frequency of LTBI in liver transplant patients and treatment-related issues. The prevalence of LTBI was lower than expected, probably due to low tuberculin skin test sensitivity in patients with impaired liver function. In addition, the initiation and completion of LTBI treatment was limited by difficulties in the management of patients in the presence of elevated liver enzymes and a potential risk of hepatotoxicity.
- Citation: Lauar ID, Faria LC, Romanelli RMC, Clemente WT. Latent tuberculosis: Risk factors, screening and treatment in liver transplantation recipients from an endemic area. World J Transplant 2021; 11(12): 512-522
- URL: https://www.wjgnet.com/2220-3230/full/v11/i12/512.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i12.512
In 2018, the World Health Organization estimated that 1.7 billion people, 23% of the world’s population, were infected with Mycobacterium tuberculosis (MTB). Among these, 5%-20% will develop tuberculosis (TB) during their lifetime[1]. Patients un
The reactivation of latent TB infection (LTBI) is responsible for the majority of post-transplantation TB cases[4,5]. In areas of limited resources, the tuberculin skin test (TST) is commonly used to investigate LTBI, and it is recommended for all SOT candidates regardless of previous Bacillus Calmette-Guérin vaccination[4,5]. Unfortunately, the sensitivity and specificity for the TST in this population is not well defined due to the absence of a gold standard test for LTBI diagnosis.
When available and affordable, interferon-gamma release assays (IGRAs) may be performed to detect interferon-gamma production in response to MTB antigens. The clinical history of the patient must be investigated. Patients diagnosed with TB infection should be questioned about symptoms and undergo chest radiography or computed tomography to rule out an active TB infection[6-8].
Treatment for LTBI is an effective strategy for the prevention of active TB in SOT recipients and is recommended in the following conditions: SOT candidates positive for the TST or IGRA who have not been previously treated; those at high-risk of pre-transplant exposure to MTB, even if their TST or IGRA results are negative; those with a history of active TB infection who were inadequately treated; and previous untreated TB, as suggested by chest imaging reports[9,10]. For the treatment of LTBI, isoniazid (INH) 300 mg daily supplemented with vitamin B6 for 9 mo[3,5,10] is recommended and is usually started before transplantation. However, in patients undergoing liver transplantation (LT), hepatotoxicity may be associated with INH or other anti-TB drug treatment. Therefore, the treatment for LTBI is commonly provided in the post-transplant period[4,5] considering that LTBI treatment may result in worsening of liver function in a patient with an already borderline condition and taking into account the impact on the outcome since transplantation may not be possible at that time. Nevertheless, in patients with compensated liver cirrhosis, preventive therapy could be initiated before LT, with strict monitoring for possible toxicities[10-12].
One should highlight that Brazil presents a particular epidemiological context for the development of TB in transplant recipients, considering the high absolute number of transplants performed in an area of high TB endemicity. Despite the efforts to reduce the incidence rates, the burden of TB continues to remain high. In 2018, there were 33.5 cases per 100000 inhabitants. During the period of this study (2005–2012), the incidence rate of TB in Brazil ranged from 37.0 to 41.5 cases per 100000 habitants per year[13,14].
The identification and treatment of LTBI in patients undergoing LT is a very relevant subject; however, publications on this topic are still scarce, especially in the context of higher endemicity. A better understanding of LTBI is needed in areas with a high risk of infection and limited resources. The aim of this study was to determine the prevalence of LTBI (by using the TST) and to evaluate the frequency of and tolerance to treatment for LTBI in LT recipients. It is worth mentioning that this landscape illustrates the majority of countries endemic for TB with an active and public trans
This is a retrospective analysis of a cohort of cirrhotic patients aged ≥ 18 years who underwent LT at a high-complexity teaching hospital from January 2005 to December 2012. The hospital provides medical care for patients from all regions of Minas Gerais state and has been responsible for the majority of LT performed in the state (81.6%) during the period of the study[15]. It is worth mentioning that Minas Gerais state presented a TB incidence of 15.8 cases per 100000 inhabitants in 2017, without marked difference among different cities[13,14].
A TB screening program began at the beginning of transplant activities in 1994, but it was restructured in 2009/2010 when institutional protocols were reviewed. Scree
The study was approved by the Federal University of Minas Gerais Research Ethics Committee (Approval number: 0614.0.203.000-11).
A TST result was considered positive when the diameter of the indurate area was ≥ 5 mm 48-72 h after intradermal injection of 2 UT of purified protein derivative RT23. Results after a second TST were not analyzed because it was rarely performed, despite the current recommendation for two-step TST.
The patients’ data were collected from electronic medical records and included sex, age at LT, the etiology of cirrhosis, clinical laboratory test results at the nearest date of completion of the TST [albumin, creatinine, sodium (Na+), bilirubin, hemoglobin, international normalized ratio], model for end-stage liver disease (MELD) score, MELD-Na and Child-Turcotte-Pugh scores, information regarding previous TB and LTBI diagnosis and treatment, and close contact with TB patients (positive epide
Treatment of LTBI with INH at a dose of 5–10 mg/kg/d, with a maximum dose of 300 mg/d, was the protocol indicated for LT recipients since the beginning of transplant activities in the 1990s. After July 2010, when a TB protocol following international guidelines was implemented, an effort was made to standardize the approach. INH is currently initiated in the post-transplant period, after liver enzymes stabilization, with intended duration of 6 mo according to Brazilian official protocol recommendation. From the patients who received the treatment for LTBI, we collected data including the start and end date of treatment, dose of INH, the need for treatment discontinuation due to suspected INH-induced hepatotoxicity or other adverse events and serum levels of liver enzymes following the initiation of INH treatment.
Analyses were performed using the SPSS 2009 release (PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.) software package. Descriptive statistics were pre
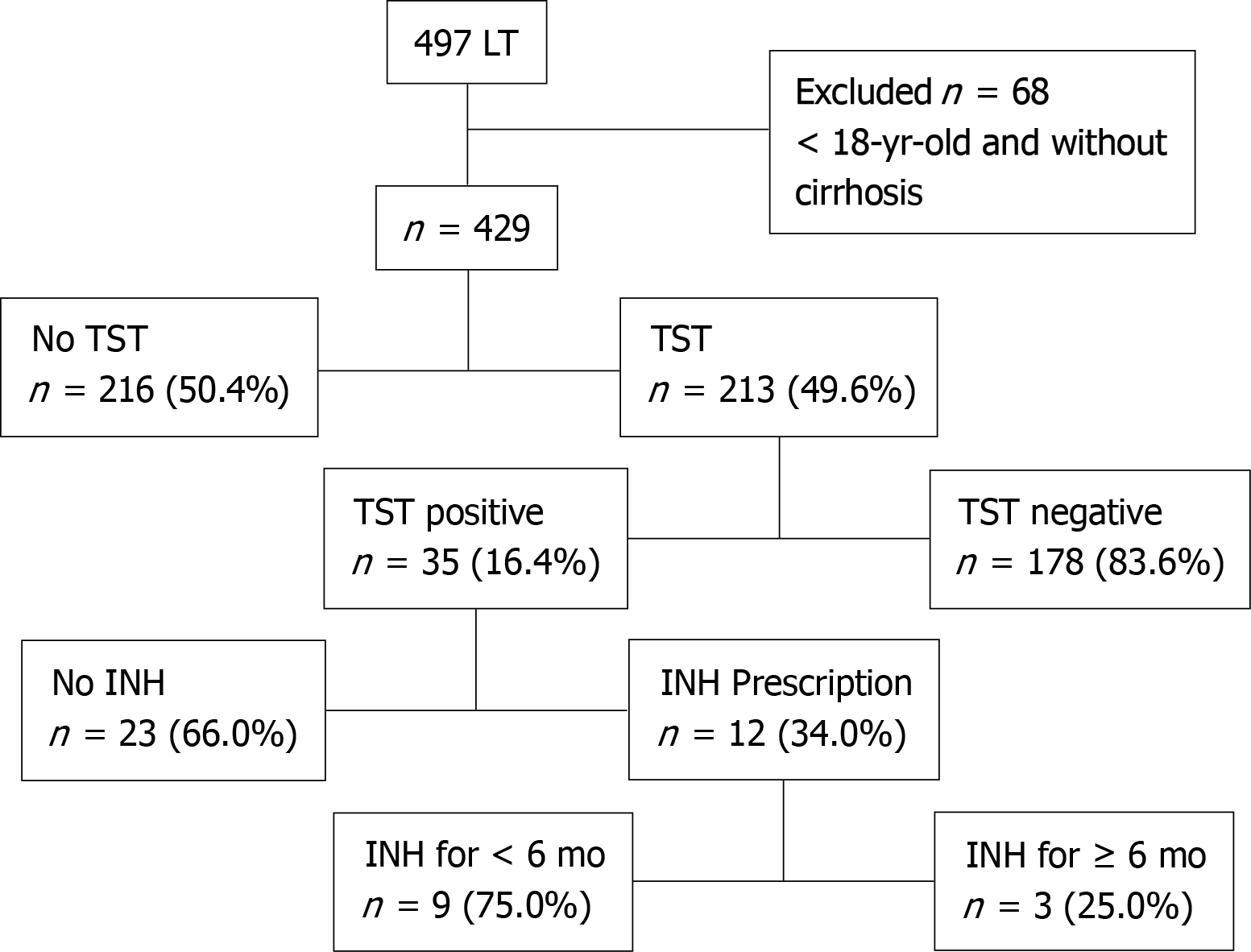
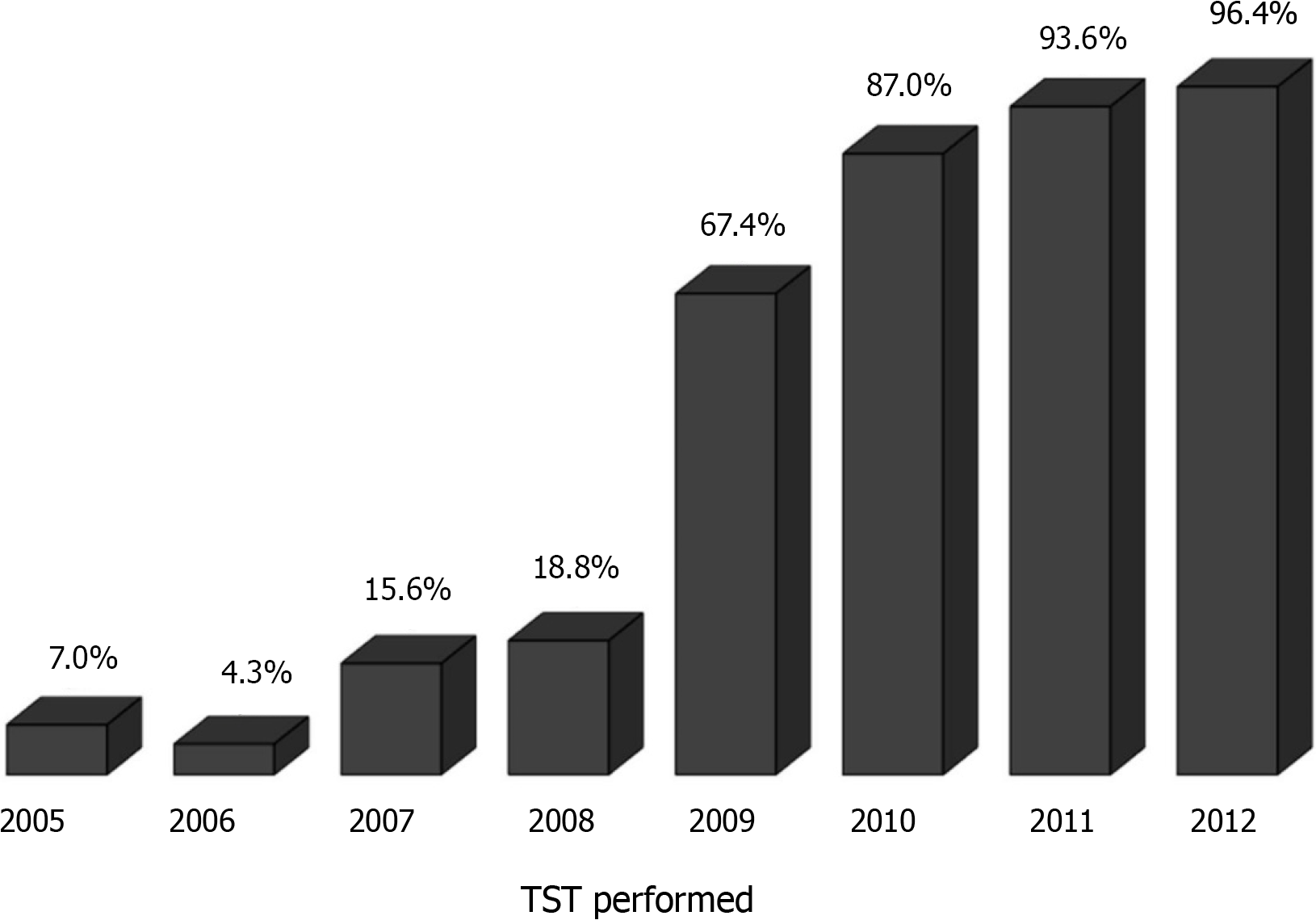
Overall, 497 patients underwent primary LT at our hospital. Of these, the following 68 patients were excluded from the analyses: 48 aged < 18 years and 20 who did not have liver cirrhosis. Among the remaining 429 patients, the TST was performed in 213 (49.7%), and the results were positive in 35 (16.4%) (Figure 1). In a chronological analysis of TST implementation, a progressive increase in LTBI screening from 7.0% in 2005 to 96.4% in 2012 was observed (Figure 2). The average follow-up time after LT was 3.2 ± 1.6 years.
The main clinical and laboratorial characteristics of patients who underwent TST and univariate analysis between TST-positive and -negative patients are shown in Table 1. Previous contact with TB patients was observed in 18 (8.5%) patients, without a significant association with TST positivity in univariate analysis (P = 0.09). The TST results were positive at a significantly lower frequency in patients with autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis than in patients with other diagnoses and at a significantly higher proportion among the patients with hepatocellular carcinoma (HCC) than in those without HCC. Patients with positive TST results had higher serum Na levels and lower MELD-Na score than those with negative TST results. For other laboratory parameters, no significant difference was observed (Table 1).
| Characteristic | General | TST positive | TST negative | P1 |
| (n = 213) | (n = 35) | (n = 178) | ||
| Age (yr) | 53.2 ± 11.0 | 56.1 ± 8.6 | 52.6 ± 11.3 | 0.13 |
| Male | 153 (71.8) | 25 (71.4) | 128 (71.9) | 0.95 |
| Cirrhosis etiology | ||||
| Viral hepatitis | 68 (31.9) | 12 (34.3) | 56 (31.5) | |
| Alcoholic | 64 (30.0) | 13 (37.1) | 51 (28.7) | |
| Cryptogenic | 45 (21.1) | 6 (17.1) | 39 (21.9) | 0.01 |
| AIH, PBC, PSC | 27 (12.7) | 0 (0) | 27 (15.2) | |
| Other etiologies | 9 (4.2) | 4 (11.4) | 5 (2.8) | |
| Previous contact with TB patients | 18 (8.5) | 6 (20.0) | 12 (8.3) | 0.09 |
| Hepatocellular carcinoma | 41 (19.2) | 11 (31.4) | 30 (16.9) | 0.046 |
| MELD score | 16.4 ± 5.0 | 15.4 ± 4.0 | 16.6 ± 5.1 | 0.22 |
| MELD Na | 18.2 ± 5.3 | 16.5 ± 4.5 | 18.5 ± 5.4 | 0.045 |
| Child | ||||
| Child A | 39 (18.3) | 11 (31.4) | 28 (15.7) | |
| Child B | 107 (50.2) | 14 (40.0) | 93 (52.2) | 0.136 |
| Child C | 67 (31.5) | 10 (28.6) | 57 (32.0) | |
| Hemoglobin | 12.2 ± 1.9 | 12.6 ± 2.3 | 12.1 ± 1.7 | 0.257 |
| Creatinine (mg/dL) | 0.99 ± 0.62 | 0.93 ± 0.25 | 1.00 ± 0.67 | 0.471 |
| Albumin (g/dL) | 3.1 ± 0.6 | 3.3 ± 0.8 | 3.1 ± 0.5 | 0.181 |
| Sodium (mEq/L) | 137.7 ± 4.7 | 139.5 ± 4.6 | 137.3 ± 4.7 | 0.043 |
| Bilirubin (mg/dL) | 4.33 ± 5.96 | 2.92 ± 1.76 | 4.60 ± 6.44 | 0.364 |
| INR | 1.62 ± 0.43 | 1.59 ± 0.39 | 1.62 ± 0.44 | 0.795 |
In multivariate logistic regression analysis, seven variables were included (age, cirrhosis etiology, presence of HCC, Child-Turcotte-Pugh and MELD-Na scores, previous contact with TB patients, serum sodium levels and serum albumin levels). There was a significant association between the history of previous contact with TB patients and a positive TST result [odds ratio (OR): 6.66, 95% confidence interval (CI): 3.17–14.08; P < 0.01]. Additionally, patients classified as Child-Pugh class A had a greater chance of a positive TST result then those classified as Child-Pugh class C (OR: 3.18, 95%CI: 1.14–8.89; P = 0.03).
There was no significant difference in post-LT survival between patients with positive and negative TST results (log-rank P = 0.44).
INH was prescribed to 12 (34.3%) of the 35 patients who had a positive TST result before LT in a median of 11 (8-56) d after LT (Figure 1). Among the 23 (65.7%) patients who did not receive INH, 5 died early in the post-transplant period, without oppor
Among the 12 (34.3%) patients who were prescribed INH, 3 (25.0%) used INH for at least 6 mo (180-232 d) and 9 (75.0%) did not complete the 6 mo of INH treatment (Figure 1). Drug withdrawal was prompted by changes in the serum levels of liver enzymes in 2 patients who used INH for 57 d and 80 d, respectively, and due to a polyserositis in 1 patient who used it for 93 d. In 6 patients, drug withdrawal was not justified, with an average usage time of 143 d, ranging from 112–171 d (Table 2). No alternative regimen was tried for patients who had the drug withdrawn.
| Patient | Usage time (d) | Reason for drug withdraw |
| Patient 1 | > 180 | LTBI treatment complete |
| Patient 2 | > 180 | LTBI treatment complete |
| Patient 3 | > 180 | LTBI treatment complete |
| Patient 4 | 57 | Changes in liver enzymes |
| Patient 5 | 80 | Cholestasis |
| Patient 6 | 93 | Clinical worsening - polyserositis |
| Patient 7 | 112 | Not justified |
| Patient 8 | 142 | Not justified |
| Patient 9 | 146 | Not justified |
| Patient 10 | 162 | Not justified |
| Patient 11 | 171 | Not justified |
| Patient 12 | 172 | Not justified |
There were no cases of active TB among patients evaluated and submitted to TST pre-transplant in a median follow-up of 37 mo.
Transplant recipients have a higher risk of developing TB in the post-transplantation period, which is associated with a high lethality rate. Since reactivation of LTBI is the main cause of the illness, development of preventive treatment strategies is recom
Based on our standard transplant protocol, all candidates with a history of in
Considering the possibility of protocol failures regarding adherence to LTBI diagnosis, therapy and hepatoxicity, we decided to study LTBI diagnosis and treatment in LT patients. Although advised by the American Society of Transplan
In our study, only 16.4% of the patients had a positive TST result, which was lower than expected. In countries with disease burden lower or similar to Brazil (Spain, Saudi Arabia and South Korea), a higher rate of TST positivity (between 24% and 38%) has been detected[7,13,20,21]. Studies conducted in Brazil and carried out in the states of Rio de Janeiro and São Paulo showed a positive TST result of 30.0% and 17.2% of the patients, respectively[12,18]. Notably, the incidence of TB in Brazil is not uniform. The incidence coefficient in the states of Rio de Janeiro is higher than São Paulo and Minas Gerais (63.5, 39.4 and 15.8 cases per 100000 inhabitants in 2017, respectively). The lower coefficient observed in Minas Gerais state may explain the low prevalence of LTBI in our study[13,14].
It should be highlighted that LTBI diagnosis using the TST presents several limitations, including false-negative results, especially in patients with end-stage liver disease. In addition, it is worth mentioning that IGRA was thought to be more sensitive and specific than the TST. However, regarding patients awaiting LT, the overall performance of IGRA was similar to TST[17,22-24]. None of our patients underwent IGRA, considering the higher costs and unavailability of the assay in our routine practice.
In the multivariate analysis, Child-Pugh class C cirrhosis was associated with a lower TST positivity rate than Child-Pugh class A cirrhosis, which can probably be explained by a higher grade of immunosuppression associated with more advanced liver disease. Patients with HCC had a higher frequency of positive TST results than those without HCC. Among HCC patients, we observed an absolute predominance of a MELD score < 20 (97.6%). The presence of a less advanced liver disease is a possible explanation for a better response to TST and a greater chance of positivity in HCC patients[17].
In the univariate analysis, a significant association was observed between TST positivity and serum Na levels; this was also reflected in the MELD-Na scores. A low Na level is an unfavorable prognostic factor for patients with liver diseases and therefore a marker of disease severity[25,26].
Patients with autoimmune liver diseases (autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis) showed positive TST results less frequently. Immunosuppressors, which are used to treat autoimmune hepatitis, are a well-established factor responsible for increased false-negative TST results[27].
In the multivariate analysis, there was a positive association between TB epide
Concerning previous studies, even though LTBI treatment is widely recommended[5,10,31], INH prescription is quite variable, varying from 18% to 100%[7,17,32,33]. In the current study, treatment for LTBI was provided to 34.3% of the patients with a positive TST result; all treatments were in the post-transplant period. A total of 23 patients did not receive INH treatment: 5 patients died early in the post-trans
Besides initiation, maintaining the treatment for LTBI was also difficult. Only 25.0% of the patients who were prescribed INH received the medication for at least 6 mo. LT candidates and recipients are more likely to discontinue medication when compared with other SOT patients[34,35]. Usually, treatment interruption is caused by the increased levels of liver enzymes. This was observed in 2 (16.6%) patients in this study. Although medication-related hepatotoxicity was not confirmed (increased liver enzymes were more likely related to viral hepatitis C recurrence and biliary stenosis), the drug was not restarted. A third patient had the medication discontinued because of worsening of the overall condition with polyserositis and was not restarted. However, the small number of patients limit further conclusions.
The efficacy of the treatment for LTBI in preventing TB varies with the duration of treatment[31,36-38]. The American Society of Transplantation and European Society of Clinical Microbiology and Infectious Diseases suggest INH treatment for 9 mo[5,10], whereas the Brazilian Ministry of Health recommends INH use for at least 6 mo[31]. For 6 (50.0%) patients in our study, INH was withdrawn without explanation before completing 6 mo, with average usage time of 143 d, ranging from 112–171 d. It is possible that the date of transplantation was considered as the start of INH instead of the date of prescription. Since there are difficulties in maintaining LTBI therapy due to possible drug interactions with immunosuppressants and hepatotoxicity, especially seen in this group of patients, shorter treatments would be desirable and possibly easier to manage.
Although we observed limitations on protocol adherence, there were no TB cases during this period. In a systematic review evaluating the incidence of TB in patients with a positive TST result, there was no significant difference between patients who received INH and those who did not. However, considering the incidence of TB in LT patients, in the presence of risk factors (TST positivity, clinical history, compatible radiological changes), the use of INH reduced the incidence of TB (P = 0.02)[16].
This study presents limitations that are inherent to retrospective studies, such as the quality of data depending on clinical records. The patient enrollment occurred over a long period of time, with the possible consequences of different protocols and no standardized management across the years. Also, during the time of recruitment, there was an improvement in screening and sometimes a lack of TST. Another limitation in assessing the impact of LTBI screening and treatment in TB cases is the fact that we are evaluating a disease with a relatively low incidence (15.8 per 100000 habitants per year in our state). Even though LT increases this incidence, we would still need a much larger number of patients to assess the impact of screening and treatment strategies. Multicentric studies could contribute to this assessment.
Despite the limitations, this study presents some important information regarding the approach and management of LTBI in liver transplant candidates and recipients in a middle income country. Therefore, we understand that since diagnostic methods available (TST and IGRA) for LTBI diagnosis have limitations, especially in patients with end-stage liver disease as observed in the present study, and ahead of the recent reduction in availability of TST, it is necessary to adopt other criteria to indicate the treatment of LTBI for patients submitted to LT. LTBI treatment is essential for patients with positive TST and for patients with a history of incompletely treated TB, history of direct contact with patients with TB and presence of residual lesions on imaging tests[5,9,10]. Patients with recent TST conversion, recent direct contact with MTB and more intense immunosuppression are at a greater risk of acquiring the infection[4,9,10]. The present study also demonstrated the difficulty to initiate and complete INH treatment due to the associated hepatotoxicity and the complex management of these patients. Further research is necessary to develop an effective and well-tolerated alternative therapeutic strategy for LTBI.
In solid organ transplants, one should be aware of the potential risk for tuberculosis, usually because reactivation of latent tuberculosis infection (LTBI).
Dealing with tuberculosis risk is especially difficult in countries with high endemic rates. In liver transplant recipients, we also have to deal with hepatotoxicity associated with the treatment regimens for LTBI.
The aim of this study was to evaluate the frequency of LTBI in liver transplant patients and treatment-related issues.
This is a retrospective analysis of a cohort of cirrhotic patients aged ≥ 18 years who underwent liver transplantation at a high-complexity teaching hospital from January 2005 to December 2012. LTBI diagnosis and treatment were analyzed.
The prevalence of LTBI was lower than expected, probably due to low TST sensitivity in patients with impaired liver function. In addition, the initiation and completion of LTBI was limited by difficulties in the management of patients in the presence of elevated liver enzymes and a potential risk of hepatotoxicity.
The prevalence of LTBI was lower than expected, and the initiation and completion of LTBI treatment was limited by difficulties in the management of these special patients.
It is necessary to search for other criteria to indicate the treatment of LTBI for patients submitted to liver transplantation, and further research is necessary to develop an effective and well-tolerated alternative therapeutic strategy for LTBI.
| 1. | World Health Organization. Global tuberculosis report 2018. [cited 17 March 2021]. Available from: https://www.who.int/tb/publications/global_report/en/. |
| 2. | Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27:1266-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 414] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Aguado JM, Torre-Cisneros J, Fortún J, Benito N, Meije Y, Doblas A, Muñoz P. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 5. | Subramanian AK, Theodoropoulos NM; Infectious Diseases Community of Practice of the American Society of Transplantation. Mycobacterium tuberculosis infections in solid organ transplantation: Guidelines from the infectious diseases community of practice of the American Society of Transplantation. Clin Transplant. 2019;33:e13513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Aguado JM, Silva JT, Samanta P, Singh N. Tuberculosis and Transplantation. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Benito N, Sued O, Moreno A, Horcajada JP, González J, Navasa M, Rimola A. Diagnosis and treatment of latent tuberculosis infection in liver transplant recipients in an endemic area. Transplantation. 2002;74:1381-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Lyu J, Lee SG, Hwang S, Lee SO, Cho OH, Chae EJ, Lee SD, Kim WS, Kim DS, Shim TS. Chest computed tomography is more likely to show latent tuberculosis foci than simple chest radiography in liver transplant candidates. Liver Transpl. 2011;17:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Santoro-Lopes G, Subramanian AK, Molina I, Aguado JM, Rabagliatti R, Len O. Tuberculosis Recommendations for Solid Organ Transplant Recipients and Donors. Transplantation. 2018;102:S60-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Meije Y, Piersimoni C, Torre-Cisneros J, Dilektasli AG, Aguado JM; ESCMID Study Group of Infection in Compromised Hosts. Mycobacterial infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20 Suppl 7:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Yehia BR, Blumberg EA. Mycobacterium tuberculosis infection in liver transplantation. Liver Transpl. 2010;16:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Stucchi RS, Boin IF, Angerami RN, Zanaga L, Ataide EC, Udo EY. Is isoniazid safe for liver transplant candidates with latent tuberculosis? Transplant Proc. 2012;44:2406-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Secretaria de Vigilância em Saúde – Ministério da Saúde (BR). Boletim Epidemiológico. Implantação do Plano Nacional pelo Fim da Tuberculose como Problema de Saúde Pública no Brasil: primeiros passos rumo ao alcance das metas. [cited 17 March 2021]. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2018/marco/26/2018-009.pdf. |
| 14. | Secretaria de Vigilância em Saúde – Ministério da Saúde (BR). Boletim Epidemiológico. O controle da tuberculose no Brasil: avanços, inovações e desafios. [cited 17 March 2021]. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2014/maio/29/BE-2014-45--2--tb.pdf. |
| 15. | Governo do estado de Minas Gerais - MG transplantes. Epidemiologia e estaística de notificação, distribuição e transplantes de órgãos e tecidos em Minas Gerais. Janeiro a dezembro de 2012. [cited 17 March 2021]. Available from: http://www.fhemig.mg.gov.br/pt/mg-transplantes/consulte-numeros. |
| 16. | Holty JE, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant recipients: a systematic review and meta-analysis of individual patient data. Liver Transpl. 2009;15:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Jafri SM, Singal AG, Kaul D, Fontana RJ. Detection and management of latent tuberculosis in liver transplant patients. Liver Transpl. 2011;17:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 18. | Agoglia L, Balbi E, Halpern M, Roma J, Carius L, Martinho JM, Moreira LP. Tuberculosis in liver transplant recipients: prophylaxis in an endemic area. Transplant Proc. 2011;43:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Chaparro SV, Montoya JG, Keeffe EB, Rhee JT, Small PM. Risk of tuberculosis in tuberculin skin test-positive liver transplant patients. Clin Infect Dis. 1999;29:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Al-Moamary MS, Al-Baz S, Alothman A, Memish Z, Al-Jahdali H, Al-Abdulkareem A. Does tuberculin skin test predict tuberculosis in patients with end-stage liver disease? Saudi Med J. 2003;24:1269-1270. [PubMed] |
| 21. | Moon HH, Park SY, Kim JM, Park JB, Kwon CHD, Peck KR, Kim SJ, Lee SK, Joh JW. Isoniazid Prophylaxis for Latent Tuberculosis Infections in Liver Transplant Recipients in a Tuberculosis-Endemic Area. Ann Transplant. 2017;22:338-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Casas S, Muñoz L, Moure R, Castellote J, Guerra MR, Gonzalez L, Andreu A, Rafecas AG, Alcaide F, Santin M. Comparison of the 2-step tuberculin skin test and the quantiFERON-TB Gold In-Tube Test for the screening of tuberculosis infection before liver transplantation. Liver Transpl. 2011;17:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Márquez M, Fernández-Gutiérrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodríguez-Ramos C, Girón-González JA. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol. 2009;158:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, Peleg AY, Cobos I, Kumar D. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant. 2007;7:2797-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 293] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Gianotti RJ, Cardenas A. Hyponatraemia and cirrhosis. Gastroenterol Rep (Oxf). 2014;2:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, Jones CE, Kampmann B, Kotton CN, Lange C, Ljungman P, Milburn H, Morris MI, Muller E, Muñoz P, Nellore A, Rieder HL, Sester U, Theodoropoulos N, Wagner D, Sester M. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40:990-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Gavaldà J, Vidal E, Lumbreras C. Infection prevention in solid organ transplantation. Enferm Infecc Microbiol Clin. 2012;30 Suppl 2:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Horne DJ, Narita M, Spitters CL, Parimi S, Dodson S, Limaye AP. Challenging issues in tuberculosis in solid organ transplantation. Clin Infect Dis. 2013;57:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Chee CB, Sester M, Zhang W, Lange C. Diagnosis and treatment of latent infection with Mycobacterium tuberculosis. Respirology. 2013;18:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Ministério da Saúde (BR). Manual de Recomendações para o Controle da Tuberculose no Brasil. [cited 17 March 2021]. Available from: http://portal.saude.gov.br/portal/saude/profissional/area.cfm?id_area=1527. |
| 32. | Fábrega E, Sampedro B, Cabezas J, Casafont F, Mieses MÁ, Moraleja I, Crespo J, Pons-Romero F. Chemoprophylaxis with isoniazid in liver transplant recipients. Liver Transpl. 2012;18:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Torre-Cisneros J, Doblas A, Aguado JM, San Juan R, Blanes M, Montejo M, Cervera C, Len O, Carratala J, Cisneros JM, Bou G, Muñoz P, Ramos A, Gurgui M, Borrell N, Fortún J, Moreno A, Gavalda J; Spanish Network for Research in Infectious Diseases. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 34. | Sidhu A, Verma G, Humar A, Kumar D. Outcome of latent tuberculosis infection in solid organ transplant recipients over a 10-year period. Transplantation. 2014;98:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Grim SA, Layden JE, Roth P, Gallitano S, Adams W, Clark NM. Latent tuberculosis in kidney and liver transplant patients: a review of treatment practices and outcomes. Transpl Infect Dis. 2015;17:768-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Lobue P, Menzies D. Treatment of latent tuberculosis infection: An update. Respirology. 2010;15:603-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow up in the IUAT trial. Bull World Health Organ. 1982;60:555-564. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martin-Onraet A, Sahin TT S-Editor: Liu M L-Editor: A P-Editor: Liu M