©The Author(s) 2018.
World J Transplantation. Sep 10, 2018; 8(5): 122-141
Published online Sep 10, 2018. doi: 10.5500/wjt.v8.i5.122
Published online Sep 10, 2018. doi: 10.5500/wjt.v8.i5.122
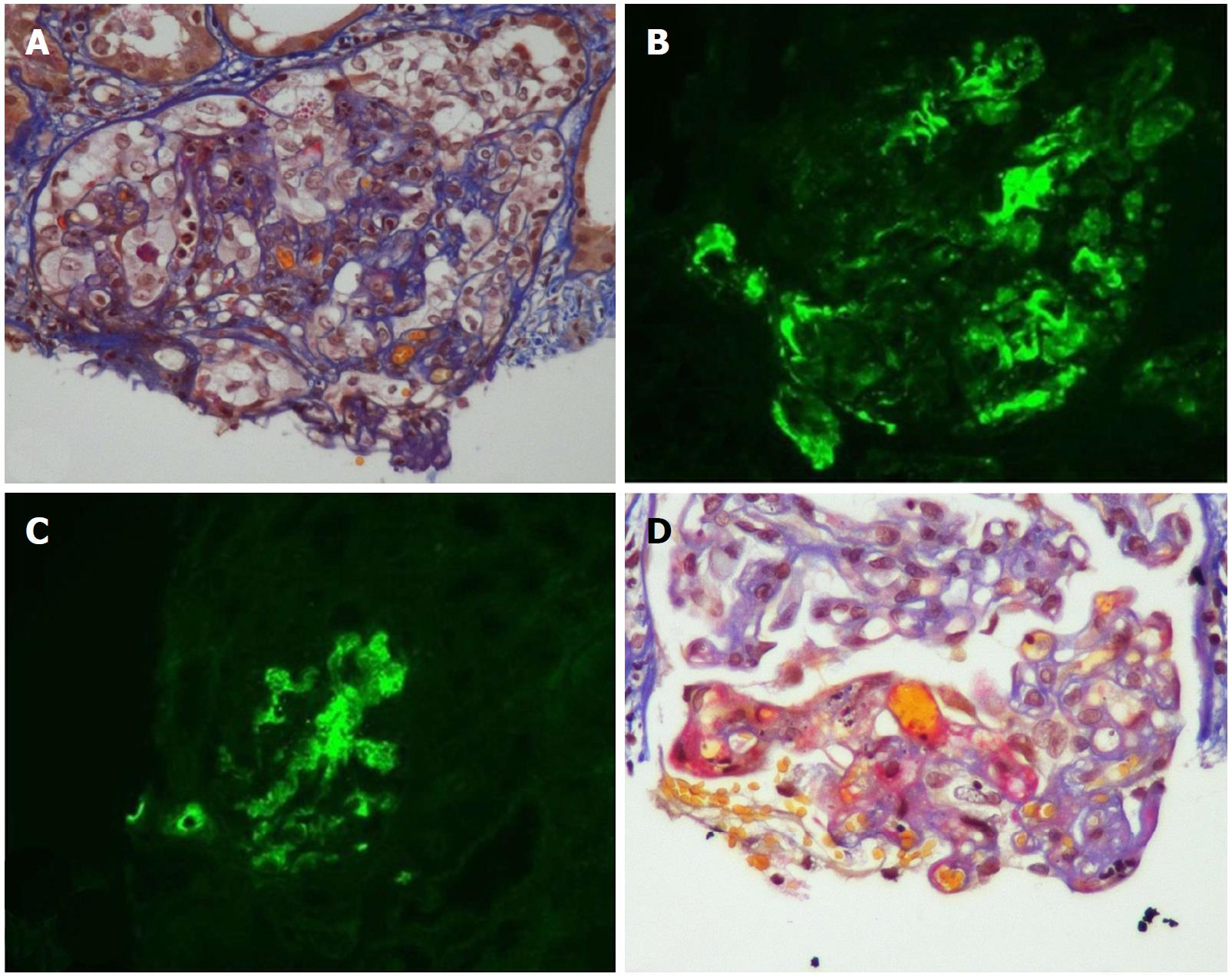
Figure 1 Acute and chronic thrombotic microangiopathy and calcineurin inhibitors-associated arteriolopathy with severe acute ischemic tubular lesions.
A: Advanced interstitial inflammatory fibrosis (Masson trichrome stain); B: Immunofluorescence, diffuse and segmental C3; C: C1q deposits within glomerular capillary walls; D: Diffuse acute and chronic arteriolar and glomerular thrombotic microangiopathy lesions on light microscopy (LM). (Adapted from: Yassine et al[45]).
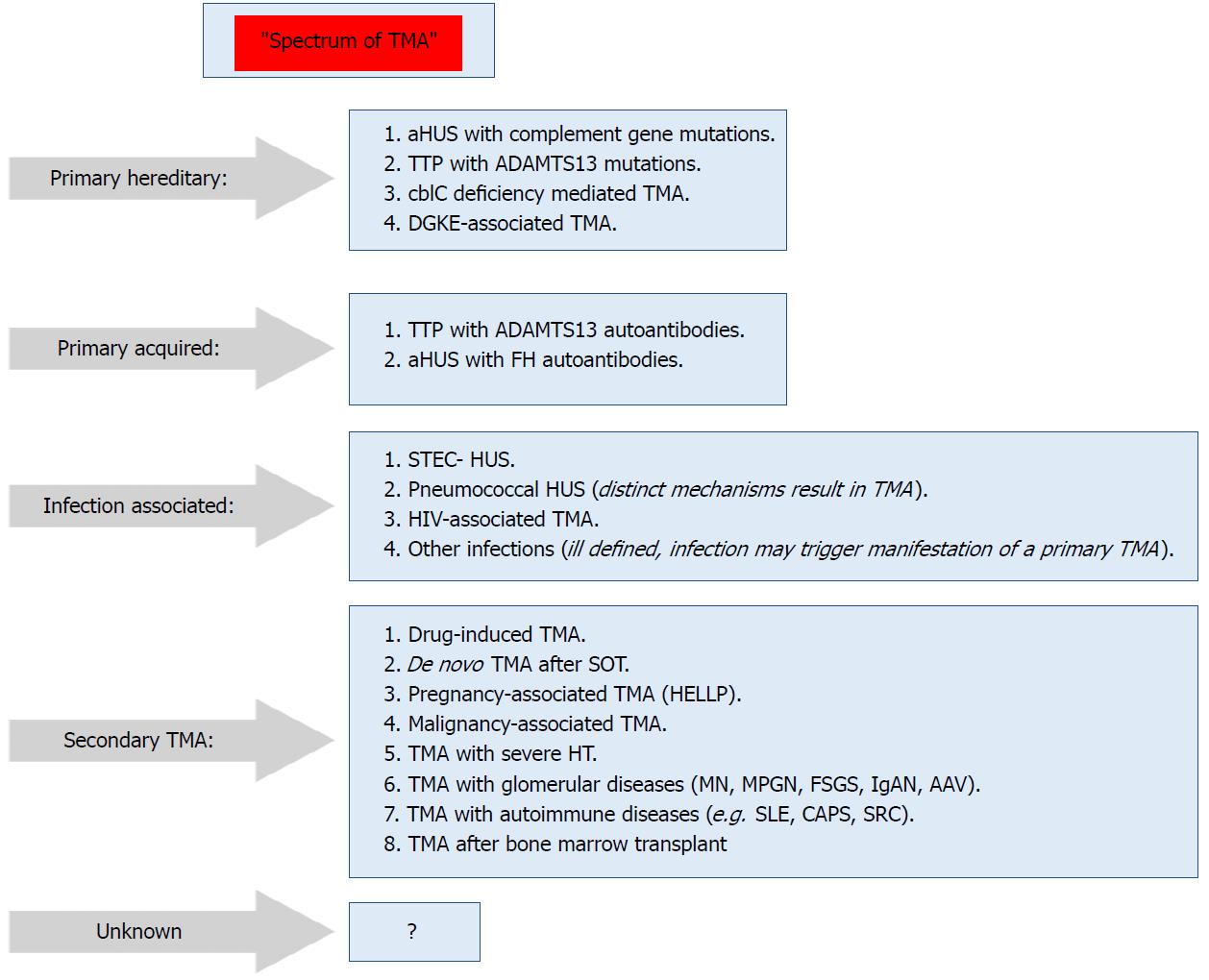
Figure 2 Spectrum of thrombotic microangiopathy[64].
AAV: ANCA-associated vasculitis; ADAMTS13: A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aHUS: Atypical hemolytic uremic syndrome; C3G: C3 glomerulopathy; CAPS: Catastrophic antiphospholipid syndrome; cblC: Cobalamin C type; DGKE: Gene encoding diacylglycerol kinase ε; FH: Factor H; HELLP: Syndrome of hemolysis, elevated liver enzymes, and low platelets; HUS: Hemolytic uremic syndrome; IgAN: IgA nephropathy; MN: Membranous nephropathy; MPGN: Membranoproliferative GN; SRC: Scleroderma renal crisis; STEC: Shiga toxin–producing Escherichia coli; TMA: Thrombotic microangiopathy; TTP: Thrombotic thrombocytopenic purpura.
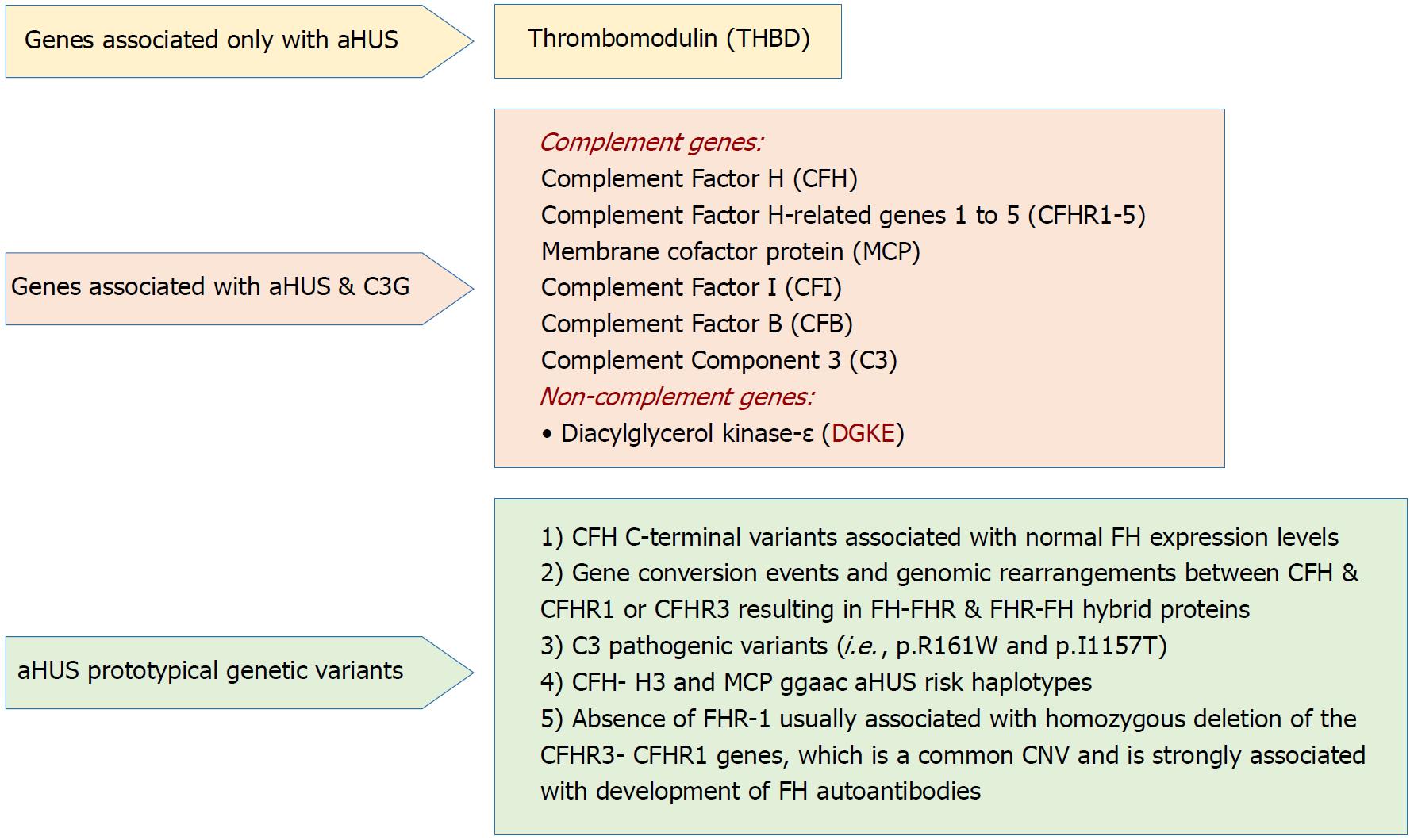
Figure 3 Genetic drivers in atypical hemolytic uremic syndrome (Adapted from: Goodship et al[58]).
aHUS: Atypical hemolytic uremic syndrome; C3G: C3 glomerulopathy; CNV: Copy number variation; SCR: Short consensus repeat.
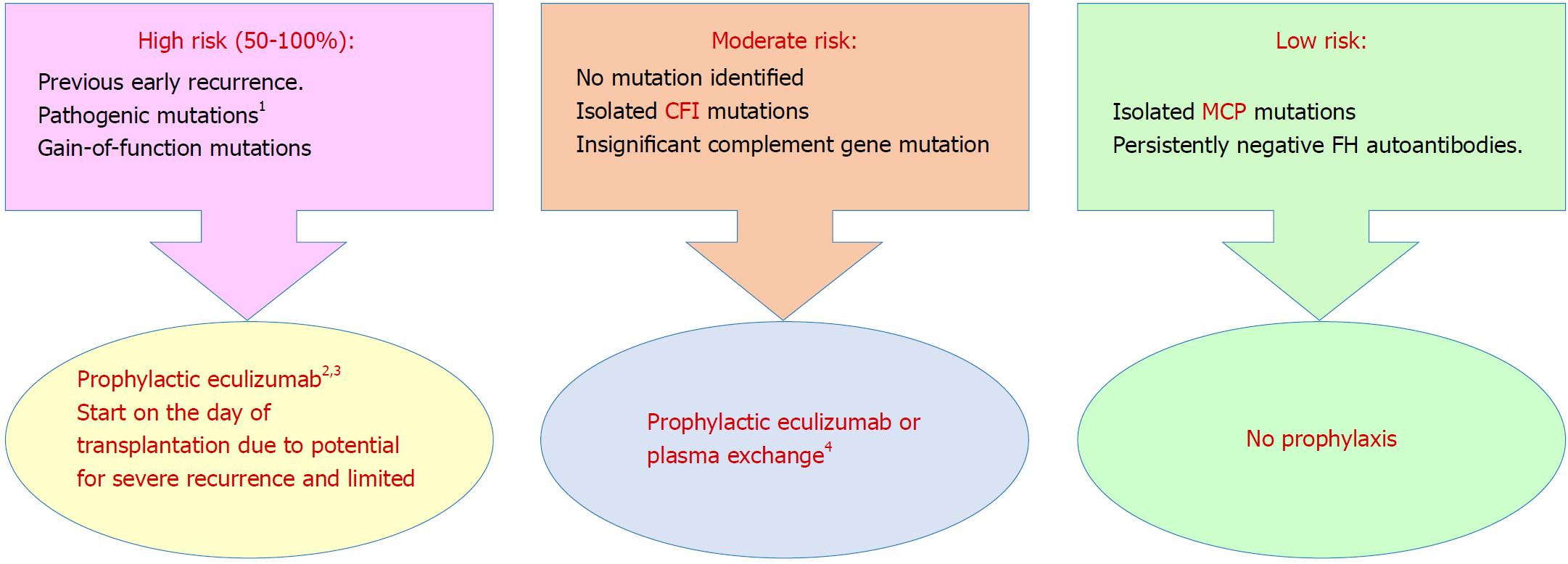
Figure 4 Prophylaxis against atypical hemolytic uremic syndrome recurrence in allograft based on a risk-assessment strategy[96] (Adapted from: Goodship et al[58]).
1Requires complete screening of all genes implicated in atypical hemolytic uremic syndrome; 2Prophylactic regimens are based on local center protocols; no trial data exist to support superiority of one protocol over another; 3Liver transplantation can be considered for renal transplant recipients with liver-derived complement protein abnormalities, uncontrolled disease activity despite eculizumab therapy or financial considerations regarding cost of long-term eculizumab therapy; 4Decision to perform or not to perform prophylactic plasma exchange or complement inhibition is left to the discretion of the clinician. aHUS: Atypical hemolytic uremic syndrome; CFI: Complement factor I gene; FH: Complement factor H protein; MCP: Membrane cofactor protein gene.
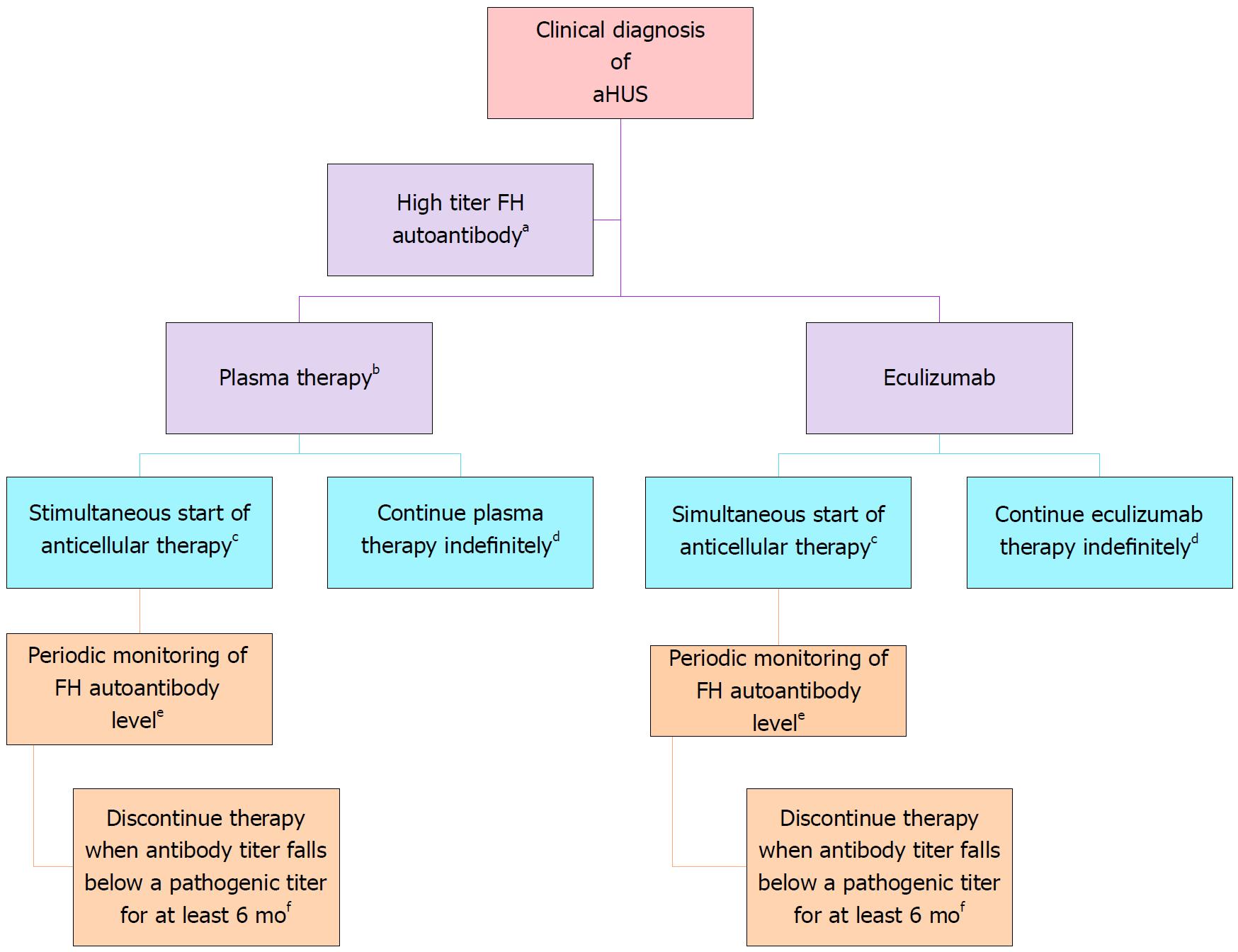
Figure 5 Treatment of complement factor H autoantibody-mediated atypical hemolytic uremic syndrome.
There are no prospective controlled studies in patients with atypical hemolytic uremic syndrome (aHUS) due to anti–factor H protein (FH) antibodies, and thus the proposed management is based on a pediatric consensus[84] (Adapted from: Goodship et al[58]). aAbnormal titer depends on the testing laboratory; bThe decision to use plasma therapy versus eculizumab will be based on patient age and local resource availability; cCyclophosphamide, rituximab, or mycophenolate mofetil; dThe decision to continue anticomplement therapy indefinitely is not informed by data; eThe interval may be monthly or quarterly and is based on local resources; fThis recommendation is based on limited retrospective case reviews[172-174].
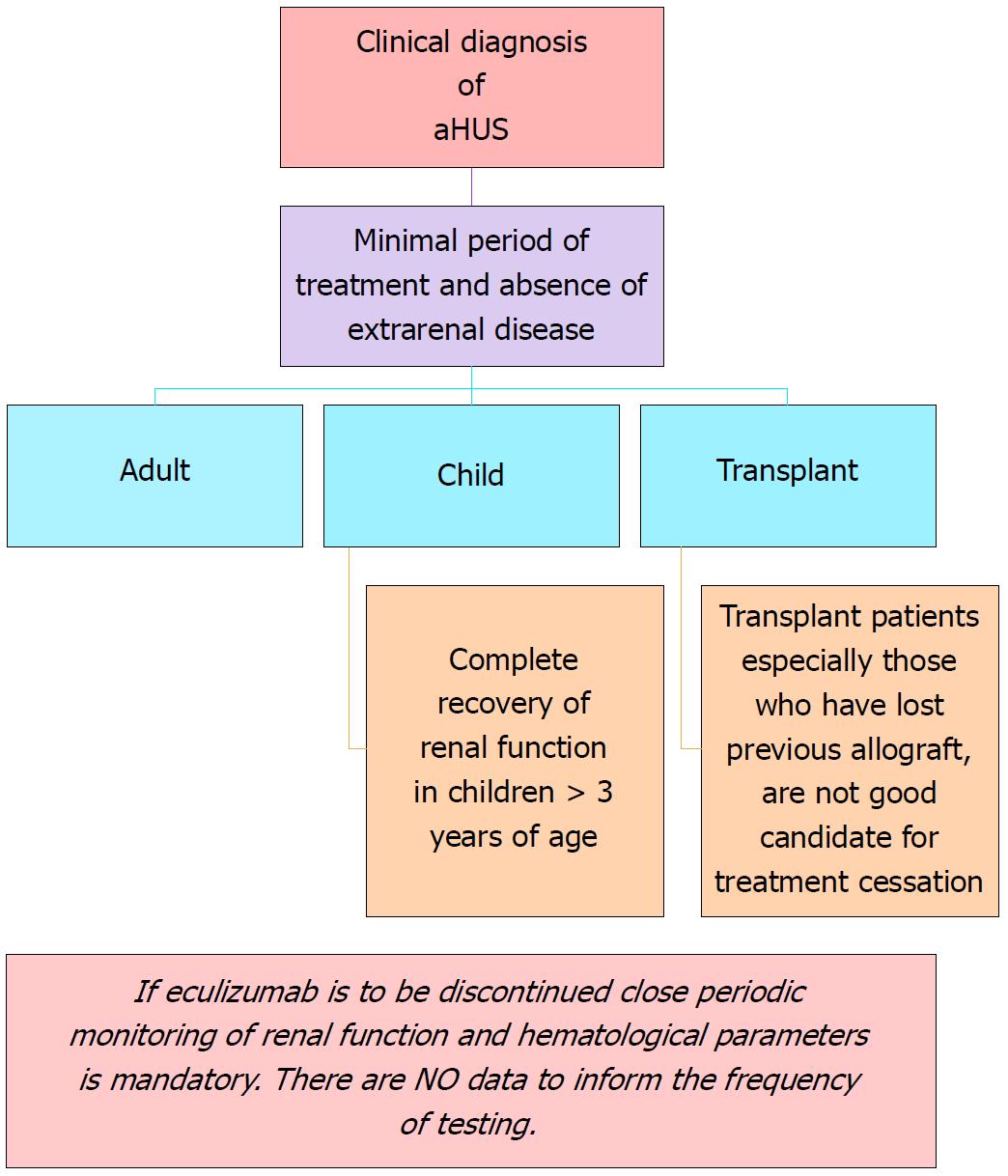
Figure 6 Recommendations for cessation of treatment with complement inhibitors.
There are no prospective controlled studies in patients with atypical hemolytic uremic syndrome (aHUS) to define criteria for discontinuation of eculizumab therapy. This flow diagram is based on expert opinion[176-178]. Discontinuation can be considered on a case-by-case basis in patients after at least 6-12 mo of treatment and at least 3 mo of normalization (or stabilization in the case of residual CKD) of kidney function. Earlier cessation (at 3 mo) may be considered in patients (especially children) with pathogenic variants in MCP if there has been rapid remission and recovery of renal function. Patients on dialysis, eculizumab should be maintained for at least 4 to 6 mo before discontinuation. In this setting, assessment of fibrotic changes in kidney biopsy may be helpful. In transplant patients, especially patients who have lost previous allografts, discontinuation is not recommended. Adapted from: Goodship et al[58]. aHUS: Atypical hemolytic uremic syndrome.
- Citation: Abbas F, El Kossi M, Kim JJ, Sharma A, Halawa A. Thrombotic microangiopathy after renal transplantation: Current insights in de novo and recurrent disease. World J Transplantation 2018; 8(5): 122-141
- URL: https://www.wjgnet.com/2220-3230/full/v8/i5/122.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i5.122