Published online May 27, 2014. doi: 10.5496/wjmg.v4.i2.34
Revised: February 10, 2014
Accepted: February 16, 2014
Published online: May 27, 2014
Processing time: 211 Days and 0.7 Hours
Osteopathia striata with cranial sclerosis (OSCS, OMIM#300373) is an X-linked dominant sclerosing bone dysplasia that shows a distinct phenotype in females and males. In 2009, Zandra Jenkins et al found that germline mutations in the FAM123B/WTX/AMER1 gene, mapped to chromosome Xq11.2, cause both the familial and sporadic forms of OSCS. Intriguingly, the WTX gene was already known as a putative tumor suppressor gene, since in 2007 Rivera et al had reported inactivating WTX mutations in Wilms’ tumor (WT), the most frequent renal tumor of childhood. Here we review the heterogeneous clinical presentation of OSCS patients and the involvement of WTX anomalies in OSCS and in WT.
Core tip: Osteopathia striata with cranial sclerosis (OSCS), a condition often benign in females and severe and lethal in males, has a clinically heterogeneous presentation. Germline anomalies affecting the WTX gene, mapped to chromosome X, are causative of OSCS. Despite WTX mutations in Wilms’ tumor (WT) that closely mirror those identified in OSCS patients, individuals with OSCS do not develop WT. This is in contrast with other syndromic conditions, in which germline mutations or epimutations, also found as somatic events in sporadic WTs, predispose to WT development.
-
Citation: Cattaneo E, Ciceri S, Liberati N, Radice P, Tarani L, Selicorni A, Perotti D. Osteopathia striata with cranial sclerosis, Wilms’ tumor and the
WTX gene. World J Med Genet 2014; 4(2): 34-38 - URL: https://www.wjgnet.com/2220-3184/full/v4/i2/34.htm
- DOI: https://dx.doi.org/10.5496/wjmg.v4.i2.34
The X-linked inheritance pattern of osteopathia striata with cranial sclerosis (OSCS, OMIM#300373), previously predicted on clinical grounds, found confirmation when germline mutations involving the WTX gene, mapped to chromosome Xq11.2, were identified as the cause of OSCS[1]. All mutations either deleted the whole gene or resulted in the premature termination of translation[1].
The FAM123B/WTX/AMER1 gene encodes a 1135-amino acid protein with multiple protein-protein interaction domains and N-terminal phosphatidylinositol 4,5-bisphosphate binding domains mediating its localization to the plasma membrane[1-3]. The WTX protein has been demonstrated to regulate the stability of β-catenin[2], a key effector of the WNT/β-catenin signaling pathway (reviewed in Clevers and Nusse 2012[4]). The critical importance of this pathway during embryogenesis is clearly demonstrated by the pleiotropic clinical presentation of OSCS patients. The WTX gene has two splice forms, WTXs1 full length and WTXs2, a shorter form encoding a 858-amino acid protein that lacks residues 50-326 and does not localize to the plasma membrane[1,5]. Only WTXs1 is considered to be important in regulating the WNT signaling in the context of the development since disease-causing WTX mutations that do not affect the integrity of WTXs2 have been reported[1,6-8].
Females affected with OSCS present a great variability of the phenotype and, while previous studies[9-11] suggested, at least in some cases, a nonrandom X-inactivation[9-11] that could explain this phenomenon[6], Jenkins et al[1] demonstrated that in 19 WTX mutation-bearing heterozygous females X-inactivation ratios were not skewed.
Among the features that constitute the OSCS female phenotype, sclerosis of bone (especially the increased thickness and density mostly of the cranial base) and the fine, uniform, linear striations of the tubular bones are considered the hallmarks of the disease.
Cranial sclerosis is the most typical and early feature, being present since birth. It appears before the longitudinal striae that become evident in the first years of life. Fan-like striations of the iliac bones are present in more than 50% of cases. It is worth mentioning that longitudinal striations at the metaphyses and diaphyses of the tubular bones are seen only in females and in males that are mosaic for a WTX mutation[6,7,9,12].
Other skeletal defects reported in the literature, although quite rare, are thoracic (pectus excavatum, broad flat ribs) and vertebral anomalies (2%), digital flexion contractures, phalangeal duplication, syndactyly, short or absent fibula and club feet (3%)[12]. Coronal craniostenosis has been described in one patient only[9].
Facial dysmorphisms are rather frequent and sometimes the only pathological feature in addition to the sclerosis of the skull and the longitudinal striation of the long bones. Macrocephaly is documented in almost half of patients, followed by frontal and occipital bossing, prominent forehead, maxillary hypoplasia, mandible overgrowth with protuberance of the jaw and dental malocclusion. Female patients can also manifest ocular hypertelorism, downslanting palpebral fissures, broad and depressed nasal bridge, narrow high arched palate and low set dysplastic ears[13-22]. Dental anomalies have been reported in 30% of patients[20]. Regarding the neurological manifestations, intellectual disability has been described in a small percentage of patients, mainly associated with central nervous system defects (ventricular dilatation, abnormal gyration, corpus callosum hypoplasia or agenesis, hydrocephalus), as well as developmental and speech delay.
Conductive hearing loss can be considered a distinctive symptom of the disease, occurring in almost 50% of patients. In the remaining half, hearing loss is sensorineural or of mixed type[6,7,12,20,23]. Deafness is the result of bone sclerosis of mastoid cells, narrowing of the middle ear cavity, the mastoid antrum and the Eustachian canal, and of impaired mobility of the ossicles. High resolution computed tomography of the temporal bone has shown the presence in different patients of bilateral thickening and bone sclerosis of the skull base and mastoid cells, and the abnormal ossicular fixation to the bone surface of the middle ear cavity[23]. Sensorineural hearing loss could instead be due to the nerve encroachment.
Other cranial nerve deficiencies (oculomotor and hypoglossal, abducens and maxillary nerves) due to the narrowing of the nerve canals and foramina by the sclerosing process are reported. Unilateral peripheral facial palsy and congenital facial palsy were described in 4 patients[20,24]. The optic nerve may also be involved due to the narrowing of the optic foramina. Nerve palsy might be due to the sclerosing bone process[25] but it is also hypothesized that disruption of the nerve supporting vessels may lead to secondary cranial nerve deficiencies[20]. Lumbar spinal stenosis, defined as narrowing of the lumbar spinal canal, nerve root canal or intervertebral foramina, has been described in one patient only and could be thought of as a neurological complication of the disease[26].
As already mentioned, OSCS manifests only with the hallmarks of the syndrome (cranial sclerosis and longitudinal striations of the long bones) accompanied by minor facial dysmorphisms or in association with internal organ anomalies, growth and mental retardation.
In female patients, the most frequently affected organs are the heart, with congenital defects including ventricular septal defects, patent ductus arteriosus, pulmonary atresia and valve stenosis, the lungs and the respiratory system in general, and the gastrointestinal and urogenital systems. The respiratory system may be affected in many patients. In particular, laryngotracheomalacia, nasal obstruction and recurrent bronchitis are reported in 13.5% of patients[12,20]. Cleft palate (Pierre Robin’s triad) and bifid uvula can also be observed. Gastrointestinal anomalies, including omphalocele, intestinal malrotation and Hirschsprung’s disease have been reported in 12% of patients[9,27,28]. Anal stenosis has been described in two girls only[29,30].
OSCS in males is more severe than in females because it follows an X-linked dominant pattern of inheritance that determines hemizygosity of the mutation and, consequently, a wide spectrum of severe clinical manifestations, such as abortion, stillbirth and post-natal lethality. Despite this, cases with long survival are also described, allowing a clinical distinction in severe and mild forms in males. The male severe phenotype exhibits macrocephaly, facial dysmorphisms (frontal bossing, hypertelorism, low set ears, broad depressed nasal bridge and micrognathia) and bony sclerosis (more marked than in females), with no metaphyseal striations, while other features are less frequent. The latter include genitourinary malformations (18%), bilateral absence of fibula (65%), cardiac defects (patent ductus arteriosus, atrial and ventricular septal defects, left ventricular non-compaction, tricuspid insufficiency and vascular ring) (31%), omphalocele and cleft of lips and palate (50%), ventriculomegaly and duplicated phalanges (30%)[8,31]. Prominent lumbar lordosis, joint luxation, camptodactyly and flexion contractures are also present with a lower incidence. Gastrointestinal anomalies such as omphalocele, duodenal web, malrotation of the gut, inguinal hernia and Hirschsprung’s disease have also been reported[9,10,21,27,32].
In the severe form, the prognosis is related to the severity of visceral malformations and a short survival is often present.
The mild phenotype is qualitatively different from the severe one, being characterized by the presence of mild neurodevelopmental delay (50% of patients), which might be attributable to the relative longevity of these patients and progressive neuromuscular disease, histologically similar to nemaline myopathy[8], and by the absence of some anomalies such as fibular aplasia, duplicated phalanges, syndactyly[1,8], and gastrointestinal[9,10,21,27,32], cardiac[9,21,27,31,33,34] and genitourinary malformations[1,8,21,35,36].
Characteristic features of the mild form are short stature, facial dysmorphisms and macrocephaly with cranial sclerosis, frontal bossing, hearing loss, high arched and cleft palate (75%), bifid uvula (25%), and extensive bony sclerosis with absent metaphyseal striations[8]. Milder bony sclerosis has been detected in males with mosaic mutation of WTX[37]. Striations of the long bones have also been observed in molecularly confirmed or suspected mosaics for WTX mutations[8,24,26,34,37,38].
A possible genotype-phenotype correlation between the position of the WTX mutation and survival in males had been initially proposed[1] but further studies showed that this correlation is not absolute[6,7].
Intriguingly, WTX has been also identified as a putative tumor suppressor gene in Wilms’ tumor (WT). Since this gene, as already mentioned, resides on the X chromosome, it has been speculated that its anti-oncogenic activity can be inactivated by a single “hit” both in hemizygous males and in heterozygous females if the mutation affects the only functional allele on the active X chromosome[39]. WT, the most common renal tumor of childhood, is an embryonal malignancy of the kidney that is thought to arise from metanephric mesenchyme. Histologically, it resembles fetal kidney, with varying proportions of blastemal, epithelial and stromal elements[40]. Approximately 40% of WTs occur in association with nephrogenic rests (NRs), embryonal remnants in the kidney which are known precursor lesions for WT[41]. The genetics of WT is heterogeneous and the WT1 gene at 11p13 and the WT2 locus at 11p15.5 have been associated with WT pathogenesis (reviewed in Huff[42] 2011, Royer-Pokora[43] 2013). Further genes involved in WT development include, in addition to WTX, CTNNB1 and TP53[42,43]. WTX anomalies have been described in approximately 20% of WTs[39,44-47]. While WTX deletions and truncating mutations are somatically acquired, missense mutations of unknown functional relevance can be present in the germline (reviewed in Huff[42] 2011).
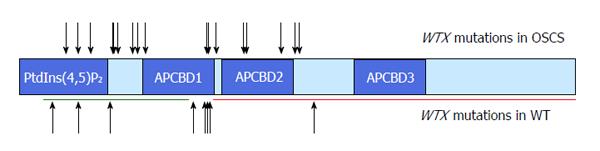
Whole gene deletions represent the majority of WTX mutations in WTs, whereas truncating mutations are more common in OSCS (reviewed in Huff[42] 2011). However, the spectrum of WTX truncating mutations in OSCS patients and in WTs is very similar[1,42] (Figure 1).
Different syndromic conditions associated with susceptibility to WT, such as the WAGR, the Denys-Drash and the Beckwith-Wiedemann syndromes, are due to germline mutations or epimutations affecting genes/loci also found involved in somatic events in sporadic WTs (reviewed in Scott et al[48] 2006). In contrast, individuals with OSCS do not seem to have any predisposition to develop either WT or other malignancies[1,22,35], although in a few of these patients the presence of bilateral multifocal NRs has been reported[8,35]. However, it has to be noted that NRs are found in approximately 1% of infant autopsies and that most of them do not form WT but spontaneously undergo regression or involution[40]. Thus, the detection of NRs in OSCS patients does not allow establishing a link with WT. Consistently, the WTX-knockout mice, despite exhibiting somatic overgrowth and malformation of several organs including kidney, do not appear to be tumor prone[49]. The lack of association between OSCS and WT could be explained assuming that WTX is mainly involved in WT progression rather than in its early phase. This possibility is supported by a study detecting various levels of WTX mutation in different microdissected areas of the same tumor[46].
Overall, current evidence suggests a possible involvement of the WTX gene in kidney development but is not consistent with its role in WT predisposition.
| 1. | Jenkins ZA, van Kogelenberg M, Morgan T, Jeffs A, Fukuzawa R, Pearl E, Thaller C, Hing AV, Porteous ME, Garcia-Miñaur S. Germline mutations in WTX cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat Genet. 2009;41:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Grohmann A, Tanneberger K, Alzner A, Schneikert J, Behrens J. AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J Cell Sci. 2007;120:3738-3747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4516] [Article Influence: 322.6] [Reference Citation Analysis (0)] |
| 5. | Rivera MN, Kim WJ, Wells J, Stone A, Burger A, Coffman EJ, Zhang J, Haber DA. The tumor suppressor WTX shuttles to the nucleus and modulates WT1 activity. Proc Natl Acad Sci USA. 2009;106:8338-8343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Perdu B, de Freitas F, Frints SG, Schouten M, Schrander-Stumpel C, Barbosa M, Pinto-Basto J, Reis-Lima M, de Vernejoul MC, Becker K. Osteopathia striata with cranial sclerosis owing to WTX gene defect. J Bone Miner Res. 2010;25:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Perdu B, Lakeman P, Mortier G, Koenig R, Lachmeijer AM, Van Hul W. Two novel WTX mutations underscore the unpredictability of male survival in osteopathia striata with cranial sclerosis. Clin Genet. 2011;80:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Holman SK, Daniel P, Jenkins ZA, Herron RL, Morgan T, Savarirayan R, Chow CW, Bohring A, Mosel A, Lacombe D. The male phenotype in osteopathia striata congenita with cranial sclerosis. Am J Med Genet A. 2011;155A:2397-2408. [PubMed] |
| 9. | Viot G, Lacombe D, David A, Mathieu M, de Broca A, Faivre L, Gigarel N, Munnich A, Lyonnet S, Le Merrer M. Osteopathia striata cranial sclerosis: non-random X-inactivation suggestive of X-linked dominant inheritance. Am J Med Genet. 2002;107:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Rott HD, Krieg P, Rütschle H, Kraus C. Multiple malformations in a male and maternal osteopathia strata with cranial sclerosis (OSCS). Genet Couns. 2003;14:281-288. [PubMed] |
| 11. | Kraus C, König R, Rott HD. Comments on „osteopathia striata cranial sclerosis: non-random X-inactivation suggestive of X-linked dominant inheritance“. Am J Med Genet A. 2003;119A:400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Zicari AM, Tarani L, Perotti D, Papetti L, Nicita F, Liberati N, Spalice A, Salvatori G, Guaraldi F, Duse M. WTX R353X mutation in a family with osteopathia striata and cranial sclerosis (OS-CS): case report and literature review of the disease clinical, genetic and radiological features. Ital J Pediatr. 2012;38:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Jones MD, Mulcahy ND. Osteopathia striata, osteopetrosis, and impaired hearing. A case report. Arch Otolaryngol. 1968;87:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Franklyn PP, Wilkinson D. Two cases of osteopathia striata, deafness and cranial osteopetrosis. Ann Radiol (Paris). 1978;21:91-93. [PubMed] |
| 15. | Cortina H, Vallcanera A, Vidal J. Familial osteopathia striata with cranial condensation. Pediatr Radiol. 1981;11:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Clément A, Garrigues C, Coursault-Durand R, Ledoux-Lebard G, Bonnin A. [A rare bone disease, but one not to be ignored: osteopathia striata]. J Radiol. 1982;63:673-676. [PubMed] |
| 17. | Robinow M, Unger F. Syndrome of osteopathia striata, macrocephaly, and cranial sclerosis. Am J Dis Child. 1984;138:821-823. [PubMed] |
| 18. | Piechowiak H, Goebel FD, Hirche U, Tyrell R. Cranial sclerosis with striated bone disease (osteopathia striata). Klin Padiatr. 1986;198:418-424. [PubMed] |
| 19. | Kornreich L, Grunebaum M, Ziv N, Shuper A, Mimouni M. Osteopathia striata, cranial sclerosis with cleft palate and facial nerve palsy. Eur J Pediatr. 1988;147:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | König R, Dukiet C, Dörries A, Zabel B, Fuchs S. Osteopathia striata with cranial sclerosis: variable expressivity in a four generation pedigree. Am J Med Genet. 1996;63:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Pellegrino JE, McDonald-McGinn DM, Schneider A, Markowitz RI, Zackai EH. Further clinical delineation and increased morbidity in males with osteopathia striata with cranial sclerosis: an X-linked disorder? Am J Med Genet. 1997;70:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Behninger C, Rott HD. Osteopathia striata with cranial sclerosis: literature reappraisal argues for X-linked inheritance. Genet Couns. 2000;11:157-167. [PubMed] |
| 23. | Magliulo G, Parrotto D, Zicari AM, Zappala D, Lo Mele L, Primicerio P, Marini M. Osteopathia striata-cranial sclerosis: otorhinolaryngologic clinical presentation and radiologic findings. Am J Otolaryngol. 2007;28:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Ciceri S, Cattaneo E, Fossati C, Radice P, Selicorni A, Perotti D. First evidence of vertical paternal transmission of osteopatia striata with cranial sclerosis. Am J Med Genet A. 2013;161A:1173-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | De Keyser J, Bruyland M, De Greve J, Leemans J, Potvliege R, Six R, Ebinger G. Osteopathia striata with cranial sclerosis. Report of a case and review of the literature. Clin Neurol Neurosurg. 1983;85:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Deniz FE, Köseoğlu RD. Osteopathia striata with cranial sclerosis and lumbar spinal stenosis. Acta Neurochir (Wien). 2007;149:811-815; discussion 815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Bar-Oz B, Mogle P, Ben-Neriah Z, Sheffer R, Arad I. Duodenal web in the syndrome of osteopathia striata with cranial sclerosis. Clin Genet. 1996;49:329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Koudstaal MJ, Wolvius EB, Ongkosuwito EM, van der Wal KG. Surgically assisted rapid maxillary expansion in two cases of osteopathia striata with cranial sclerosis. Cleft Palate Craniofac J. 2008;45:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Savarirayan R, Nance J, Morris L, Haan E, Couper R. Osteopathia striata with cranial sclerosis: highly variable phenotypic expression within a family. Clin Genet. 1997;52:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Ward LM, Rauch F, Travers R, Roy M, Montes J, Chabot G, Glorieux FH. Osteopathia striata with cranial sclerosis: clinical, radiological, and bone histological findings in an adolescent girl. Am J Med Genet A. 2004;129A:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Currarino G, Friedman JM. Severe craniofacial sclerosis with multiple anomalies in a boy and his mother. Pediatr Radiol. 1986;16:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Keymolen K, Bonduelle M, De Maeseneer M, Liebaers I. How to counsel in osteopathia striata with cranial sclerosis. Genet Couns. 1997;8:207-211. [PubMed] |
| 33. | Bueno AL, Ramos FJ, Bueno O, Olivares JL, Bello ML, Bueno M. Severe malformations in males from families with osteopathia striata with cranial sclerosis. Clin Genet. 1998;54:400-405. [PubMed] |
| 34. | Lüerssen K, Ptok M. Osteopathia striata with cranial sclerosis and hearing loss. Eur Arch Otorhinolaryngol. 2006;263:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Fukuzawa R, Holman SK, Chow CW, Savarirayan R, Reeve AE, Robertson SP. WTX mutations can occur both early and late in the pathogenesis of Wilms tumour. J Med Genet. 2010;47:791-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Murphy-Ryan M, Kirmani S, Thompson DM, Binkovitz LA, Thomas KB, Babovic-Vuksanovic D. A novel sclerosing skeletal dysplasia with mixed sclerosing bone dysplasia, characteristic syndromic features, and clinical and radiographic evidence of male-male transmission. Am J Med Genet A. 2012;158A:2292-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Chénier S, Noor A, Dupuis L, Stavropoulos DJ, Mendoza-Londono R. Osteopathia striata with cranial sclerosis and developmental delay in a male with a mosaic deletion in chromosome region Xq11.2. Am J Med Genet A. 2012;158A:2946-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Joseph DJ, Ichikawa S, Econs MJ. Mosaicism in osteopathia striata with cranial sclerosis. J Clin Endocrinol Metab. 2010;95:1506-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, Kim JC, Feinberg AP, Gerald WL, Vargas SO. An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science. 2007;315:642-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Beckwith JB. Nephrogenic rests and the pathogenesis of Wilms tumor: developmental and clinical considerations. Am J Med Genet. 1998;79:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 41. | Beckwith JB, Kiviat NB, Bonadio JF. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms’ tumor. Pediatr Pathol. 1990;10:1-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 424] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | Huff V. Wilms’ tumours: about tumour suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer. 2011;11:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Royer-Pokora B. Genetics of pediatric renal tumors. Pediatr Nephrol. 2013;28:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Ruteshouser EC, Robinson SM, Huff V. Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer. 2008;47:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Perotti D, Gamba B, Sardella M, Spreafico F, Terenziani M, Collini P, Pession A, Nantron M, Fossati-Bellani F, Radice P. Functional inactivation of the WTX gene is not a frequent event in Wilms’ tumors. Oncogene. 2008;27:4625-4632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Wegert J, Wittmann S, Leuschner I, Geissinger E, Graf N, Gessler M. WTX inactivation is a frequent, but late event in Wilms tumors without apparent clinical impact. Genes Chromosomes Cancer. 2009;48:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Fukuzawa R, Anaka MR, Weeks RJ, Morison IM, Reeve AE. Canonical WNT signalling determines lineage specificity in Wilms tumour. Oncogene. 2009;28:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43:705-715. [PubMed] |
| 49. | Moisan A, Rivera MN, Lotinun S, Akhavanfard S, Coffman EJ, Cook EB, Stoykova S, Mukherjee S, Schoonmaker JA, Burger A. The WTX tumor suppressor regulates mesenchymal progenitor cell fate specification. Dev Cell. 2011;20:583-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
P- Reviewers: Fukuzawa R, Sangkhathat S S- Editor: Gou SX L- Editor: Roemmele A E- Editor: Liu SQ