Published online Jul 20, 2023. doi: 10.5496/wjmg.v11.i3.28
Peer-review started: April 13, 2023
First decision: June 1, 2023
Revised: June 10, 2023
Accepted: June 30, 2023
Article in press: June 30, 2023
Published online: July 20, 2023
Processing time: 97 Days and 17.7 Hours
Interleukin-2 (IL-2) is an important cytokine that plays a key role in the immune response. The IL-2 receptor (IL-2R) is composed of three subunits, alpha, beta, and gamma, with the alpha subunit having the highest affinity for IL-2. Several studies reported that immune dysregulation of IL-2 may cause tissue injury as well as damage leading to the pathogenesis of various autoimmune diseases such as acute necrotizing vasculitis in systemic lupus erythematosus (SLE), inflammatory synovitis in rheumatoid arthritis (RA), salivary and lacrimal gland dys-function in Sjogren syndrome (SS), obliterative vasculopathy fibrosis in systemic sclerosis (SSc), and inflammatory demyelination in multiple sclerosis (MS). The aim of this review paper was to examine the role of IL-2/IL-2R in various autoimmune disorders, taking into account recent advancements and discoveries, gaps in the current literature, ongoing debates, and potential avenues for future research. The focus of this review is on systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, sjogren syndrome, and multiple sclerosis, which are all linked to the malfunctioning of IL-2/IL-2R. In genetic studies, gene polymorphisms of IL-2 such as IL-2 330/T, IL-2 330/G, and rs2069763 are involved in increasing the risk of SLE. Furthermore, genetic associations of IL-2/IL-2R such as rs791588, rs2281089, rs2104286, rs11594656, and rs35285258 are significantly associated with RA susceptibility. The IL-2 polymorphism including rs2069762A, rs6822844T, rs6835457G, and rs907715T are significant connections with systemic sclerosis. In addition, rs2104286 (IL-2), rs11594656 (IL-2RA), rs35285258 (IL-2RB) gene polymorphism significant increases the risk of multiple sclerosis. In therapeutic approaches, low-dose IL-2 therapy could regulate Tfr and Tfh cells, resulting in a reduction in disease activity in the SLE patients. In addition, elevated sIL-2R levels in the peripheral blood of SLE patients could be linked to an immunoregulatory imbalance, which may contribute to the onset and progression of SLE. Consequently, sIL-2R could potentially be a target for future SLE therapy. Moreover, Low dose-IL2 was well-tolerated, and low levels of Treg and high levels of IL-21 were associated with positive responses to Ld-IL2 suggested to be a safe and effective treatment for RA. Additionally, low-dose IL-2 treatment improves the exocrine glands' ability to secrete saliva in SS-affected mice. Whereas, Basiliximab targets the alpha chain of the IL-2 receptor suggested as a potential treatment for SSc. Also, pre-and post-treatment with Tregs, MDSCs, and IL-2 may have the potential to prevent EAE induction in patients with MS. It is suggested that further studies should be conducted on IL-2 polymorphism in Sjogren syndrome.
Core Tip: Immune dysregulation of Interleukin-2 (IL-2) may cause tissue injury and damage leads to pathogenesis of various autoimmune diseases The aim of this review paper was to examine the role of IL-2/IL-2R in various autoimmune disorders, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), multiple sclerosis (MS) and Sjogren Syndrome (SS), which are all linked to malfunctioning of IL-2/IL-2R. IL-2 gene polymorphism is involved in increasing the risk of SLE, RA, SSc, and MS but no data has been available about the genetic role of IL-2 in the pathogenesis of Sjogren syndrome. In therapeutic approaches, a low dose of IL-2 therapy is found to be safe, effective, and well-tolerated in the treatment of SLE, RA, SSc, MS, and SS patients.
- Citation: Rafaqat S, Rafaqat S. Role of IL-2/IL-2 receptor in pathogenesis of autoimmune disorders: Genetic and therapeutic aspects. World J Med Genet 2023; 11(3): 28-38
- URL: https://www.wjgnet.com/2220-3184/full/v11/i3/28.htm
- DOI: https://dx.doi.org/10.5496/wjmg.v11.i3.28
Autoimmune diseases are a significant clinical problem that affects a large number of people worldwide. These diseases arise when the immune system mistakenly attacks healthy tissues and organs in the body, leading to chronic inflammation, tissue damage, and organ dysfunction. It can affect any part of the body, including the joints, skin, blood vessels, nerves, and endocrine glands. Some common examples of autoimmune diseases include rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, systemic sclerosis, and Sjogren syndrome.
The pathogenesis of autoimmune diseases is complex and multifactorial, involving both genetic and environmental factors. While the exact mechanisms underlying autoimmune diseases are not fully understood, it is believed that they occur due to a failure of the body's immune regulatory mechanisms. This failure allows self-reactive immune cells to escape tolerance mechanisms and attack healthy tissues, leading to the development of autoimmunity[1]. Autoimmune diseases progress through three stages: Initiation, propagation, and resolution. The initiation phase involves the activation of self-reactive immune cells and their migration to target tissues. The propagation phase is characterized by the activation and expansion of these self-reactive immune cells, leading to chronic inflammation and tissue damage. The resolution phase is defined by the partial and short-term ability to restore the balance of effector and regulatory responses and the reduction of inflammation[1].
Interleukin-2 (IL-2) is an important cytokine that plays a key role in the immune response. The IL-2 receptor is composed of three subunits, alpha, beta, and gamma, with the alpha subunit (IL2RA) having the highest affinity for IL-2. The IL-2 receptor is expressed in a variety of immune cells, including T cells, B cells, and natural killer (NK) cells[2]. The most abundant type of cell that is influenced by IL-2 is T lymphocytes. IL-2 primarily functions to prompt the production of other cytokines and to increase the growth and proliferation of T lymphocytes that are specific to particular antigens, including CD4+ and CD8+ T lymphocytes. This cytokine also promotes the death of activated T lymphocytes, promotes differentiation into Th1 and Th2 subsets in CD4+ T cells, and plays a critical role in the maturation of CD4+ CD25+ Tregs. Additionally, IL-2 increases the cytotoxic activity of CD8+ cells and stimulates the growth of memory CD8+ cells[3].
Genetic variants in IL2RA and IL2RB may be associated with the development of autoimmune diseases and inflammatory diseases. For example, studies have reported that variants in these genes are associated with the risk of multiple sclerosis. However, there is limited research on the association between genetic variants in IL2RA and IL2RB and the risk of rheumatoid arthritis (RA). Further studies are needed to investigate this potential association and to better understand the role of IL-2 in the pathogenesis of RA[2].
Advancements in genotyping technology and the utilization of single nucleotide polymorphism (SNP) assays have facilitated the application of whole genome association approaches. These approaches involve identifying the correlation between genetic variants and susceptibility to various autoimmune diseases[2].
Despite advances in diagnosis and treatment, autoimmune diseases remain a significant clinical challenge due to their chronic nature, associated healthcare cost, and impact on young populations during their prime working and repro-ductive years. Therefore, it is essential to continue to study and understand the mechanisms underlying autoimmune diseases to develop effective treatments and improve the quality of life of affected individuals[1].
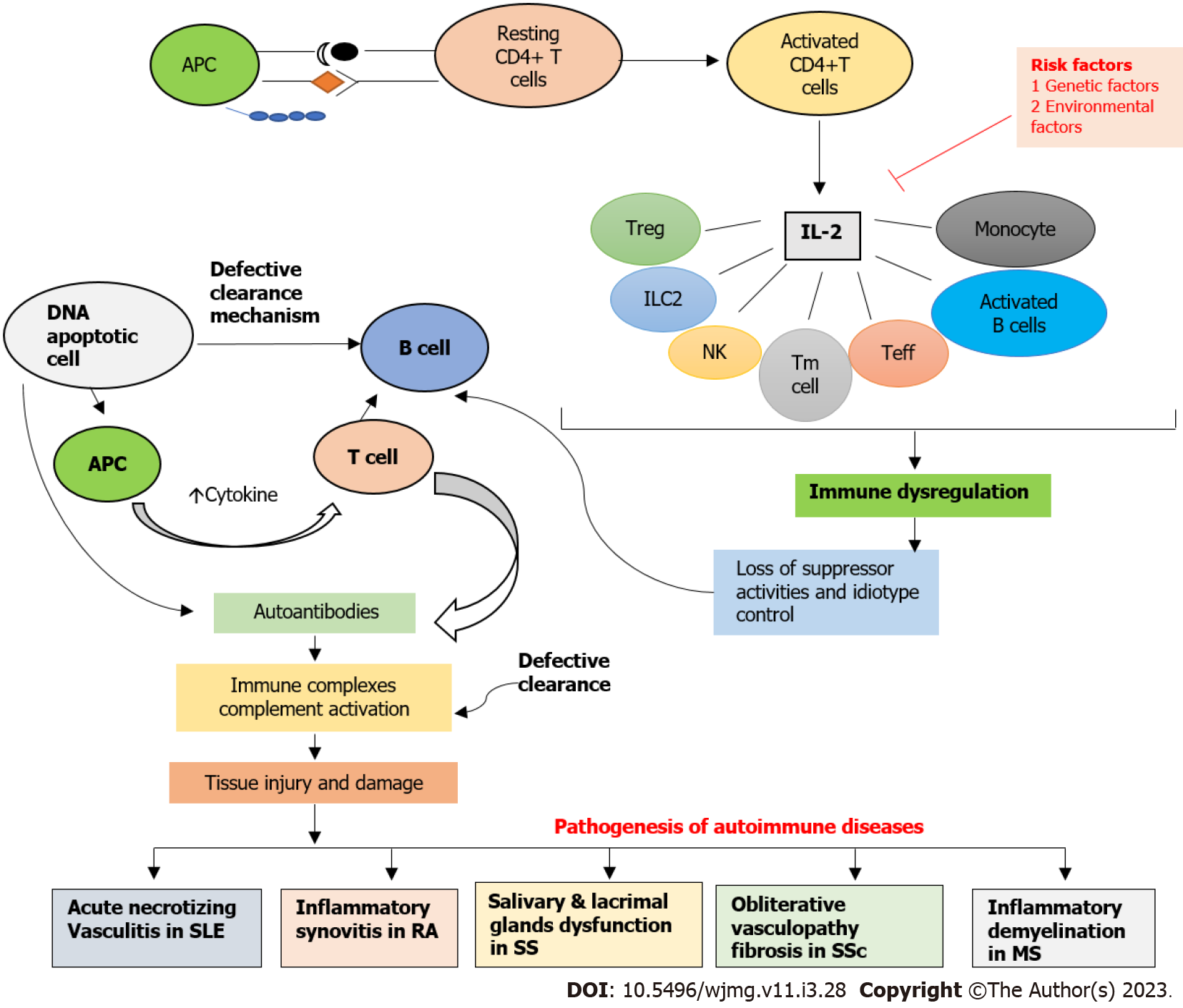
The aim of this review paper was to examine the role of IL-2/IL-2R in the pathogenesis of various autoimmune disorders, taking into account recent advancements and discoveries, gaps in the current literature, ongoing debates, and potential avenues for future research. The focus of this review is on systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, Sjogren syndrome, and multiple sclerosis, which are all linked to the malfunctioning of IL-2/IL-2R, as depicted in Figure 1 and Table 1.
| Autoimmune diseases | Pathogenesis | Genetics (SNP) | Therapy |
| Systemic lupus erythematosus | Acute necrotizing vasculitis | IL-2 330/T (IL-2); IL-2 330/G (IL-2); rs2069763 (IL-2); rs6822844 G/T; (IL2-IL21) | Low dose IL-2, Rituximab |
| Rheumatoid arthritis | Inflammatory synovitis | rs791588 (IL2RA); rs2281089 (IL2RA); rs2104286 (IL2RA) | Low dose IL-2, Infliximab |
| Systemic sclerosis | Obliterative vasculopathy fibrosis | rs2069762A (IL-2); rs6822844T (IL2-IL21); rs6835457G (IL-2); rs907715T (IL-2); IL2-384-G (IL-2); rs11594656 (IL-2RA); rs2104286(IL-2); rs12722495 (IL-2) | Low dose IL-2, Basiliximab |
| Multiple sclerosis | Inflammatory demyelination | rs2104286 (IL-2); rs11594656 (IL-2RA); rs35285258 (IL-2RB) | Tregs, MDSCs, IL-2 |
| Sjogren syndrome | Salivary and lacrimal glands dysfunction | No data | Low dose IL-2 |
The authors utilized various databases, including Google Scholar, PubMed, and Science Direct, to conduct a thorough review of the relevant literature, which was completed on March 30, 2023. The search terms used included patho-physiology, systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, Sjogren syndrome, multiple sclerosis, autoimmune disorder, IL-2, and IL-2R. In addition to these search terms, the authors also searched through the references of relevant articles to identify comparable studies. Only clinical investigations published in English were included, and no time restrictions were imposed, although preference was given to more recent studies.
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects the entire body, causes persistent and severe pain, and is characterized by the production of autoantibodies and inflammation in multiple organs. SLE is a complex and multifactorial disease that has an unpredictable relapsing-remitting course, resulting from various interactions between susceptibility genes, epigenetics, environmental factors, hormones, and immuno-regulatory variables. The disease can manifest with a broad range of clinical symptoms[4]. In SLE, autoantibodies that are directed against nucleic acids and their binding proteins are produced, indicating a general lack of self-tolerance. Recent research has identified over 30 genetic loci that are associated with the pathogenesis of the disease. The loss of tolerance and subsequent immunological dysregulation in SLE is caused by genetic factors in conjunction with environmental triggers and stochastic events[5].
The IL-2 gene has been linked to autoimmune diseases such as multiple sclerosis and rheumatoid arthritis. The genetic association of rs2069763 in IL-2 was investigated in Han Chinese SLE patients and a healthy control group in Taiwan. The findings suggest that the G allele of the mutation is significantly more prevalent in SLE patients and may be linked to the genetic origin of SLE in Taiwanese individuals. The study also found that the G allele is significantly higher in SLE patients with antinuclear antibodies and significantly lower in those with discoid rash[6]. Another study investigated the impact of the IL2/IL21 region, a genetic locus associated with global autoimmunity, on the response to rituximab in individuals with SLE and other autoimmune disorders. The study found that the rs6822844 G/T polymorphism at the IL2-IL21 region was significantly associated with response to rituximab in SLE patients, but not in individuals with other autoimmune disorders. The study suggests that the IL2-IL21 region may influence the response to rituximab in SLE patients specifically[7]. Furthermore, variations in the KIAA1109-interleukin 2 (IL2)-IL21 block on chromosome 4q27 have been associated with various autoimmune disorders. The research findings confirm the link between autoimmune disorders and the KIAA1109/IL2/IL21 gene region, indicating that the 4q27 Locus may contribute to the genetic predisposition of autoimmune disorders in the Tunisian population[8]. A study investigated the association between certain SNPs in the IL-2 and interferon (IFN)-γ genes and the development of juvenile systemic lupus erythematosus (JSLE), a severe autoimmune disease with an unknown cause. The study found a negative allelic association for JSLE in IL-2 330/T and a positive allelic association for IL-2 330/G. The study suggests that IL-2 cytokine gene polymorphisms could serve as genetic risk indicators for JSLE[9].
Studies have investigated the cytokine profiles of SLE patients and found that peripheral blood mononuclear cells (PBMCs) from SLE patients grow less than controls when activated with antigens and mitogens. Lupus T cells release less IL-2 compared to normal T cells and are less sensitive to IL-2 activation. However, the expression of IL-2 is higher in freshly produced SLE PBMCs compared to normal PBMCs. When stimulated optimally, lupus T cells are capable of producing normal levels of IL-2. The reduced in vitro IL-2 production from lupus T cells is likely due to several mechanisms, including the downregulating effects of Th2 cytokines[10]. SLE patients have notably increased levels of sIL-2R in their serum, which are strongly associated with disease activity. Additionally, a research study has revealed for the first time that sIL-2R present in the peripheral blood of SLE patients can combine with IL-2 to form a complex that reduces IL-2's biological effectiveness and detection level. This complex also hinders the development of Treg cells and disrupts peripheral tolerance. The research finding suggests that elevated sIL-2R levels in the peripheral blood of SLE patients could be linked to an immunoregulatory imbalance, which may contribute to the onset and progression of SLE. Consequently, sIL-2R could potentially be a target for future SLE therapy[11].
SLE is characterized by an imbalance between effector and regulatory CD4+ T cells. T cell subsets are regulated by IL-2, and SLE patients have lower levels of IL-2. In this study, researchers found that low doses of recombinant human IL-2 therapy reduced disease activity in SLE patients by selectively affecting the number of regulatory T (Treg), follicular helper T (TFH), and IL-17-producing helper T (TH17) cells, but not Th1 or Th2 cells[12]. In a clinical trial, researchers investigated the effect of low-dose IL-2 on T follicular regulatory (Tfr) and T follicular (Tfh) cells in patients with SLE. They found that low-dose IL-2 therapy could regulate Tfr and Tfh cells, resulting in a reduction in disease activity[13]. In addition, open-label clinical trials have suggested that low-dose IL-2 may be a useful treatment for SLE, but a double-blind, placebo-controlled study is necessary to properly evaluate its safety and effectiveness. Overall, low-dose IL-2 therapy may be beneficial and well-tolerated in the treatment of SLE[14].
The inadequate production of IL-2 is one of the factors that contributes to the disruption of the immune system leading to SLE. Multiple SLE models in mice have revealed a shortage of IL-2, providing valuable insights into the link between SLE and IL-2. Furthermore, research has shown that IL-15 and IL-21, which are members of the IL-2 family, are also dysregulated in SLE. Imbalanced transcriptional regulators in T cells of SLE patients lead to reduced IL-2 Levels as they are responsible for the synthesis or suppression of IL-2 production[15]. It has been recently found that the lymphoproliferation observed in IL-2/IL-2R deficient mice is associated with a significant decrease in the number of Treg in the periphery. Similarly, individuals with SLE have a lower count of regulatory T cells. Although SLE patients do not exhibit significant lymphoproliferation as observed in knockout mice, they do have lower IL-2 levels. Nonetheless, knockout mice with IL-2/IL-2R deficiency have additional features that are present in SLE patients, such as production of autoantibodies, lymphadenopathy, and decreased Treg cells[15].
Rheumatoid Arthritis (RA) is a medical condition characterized by long-term inflammation of the joints that eventually leads to the deterioration of bone and cartilage, causing systemic inflammation, abnormal antibody production, and persistent synovitis leading to severe disability and early death[4]. The development of RA is an imbalance in the subsets of CD4 + T lymphocytes, including T helper (Th) 17 cells, regulatory T (Treg) cells, T follicular helper (Tfh) cells, and T follicular regulatory (Tfr) cells. These cells contribute to autoimmune inflammatory responses by promoting the excessive production of cytokines and abnormal autoantibodies including anti-cyclic citrullinated peptide and rheumatoid factor[16].
The use of SNP assays and advances in genotyping technology has facilitated the application of whole genome association approaches to link genetic variants with disease susceptibility. Heritability studies have shown that genetic susceptibility plays a critical role in the progression of joint destruction in RA, estimated at 45% to 58%. Various SNPs have been identified to be associated with RA predisposition. In addition, large-scale genome-wide association studies have identified over 30 Loci that play a role in RA pathogenesis. Several genetic factors, including the human leukocyte antigen gene (HLA), interleukin genes (IL23R, IL6, IL17, and IL12B), autoimmune regulator gene (AIRE), protein tyrosine phosphatase 22 (PTPN22) gene, and solute carrier family 22 member 4 (SLC22A4), have been implicated in the pathogenesis of RA.
The cytokine IL-2, which is produced by Th1 Lymphocytes, may play a significant role in the development of RA. The research examined changes in the levels of IL-2 in the bloodstream and determined if there was a relationship between these changes and glucose metabolism abnormalities, including insulin resistance in RA patients. The study revealed that individuals with RA exhibit changes in IL-2 regulation and that there is a substantial association between IL-2 levels in the bloodstream and insulin sensitivity[17]. In another study, the connection between serum levels of IL-2 and disease activity, absolute numbers of peripheral lymphocyte subsets, autoantibodies, and cytokines in individuals with RA patients were examined. The findings demonstrated that serum levels of IL-2 were related not only to disease activity and autoantibody levels but also to the imbalance of Th17/Treg immunity in RA patients. Moreover, in individuals with active RA, there was an abnormal increase in NK cell levels, which could be due to high serum IL-2 levels[18]. The production of IL-2 and the proliferative responses of peripheral blood mononuclear cells in patients with RA and normal controls was investigated. Kitas et al[19] study revealed a positive relationship between proliferative responses and IL2-levels with the proportion of CD4+ cells, and an inverse relationship with Tac+ lymphocytes suggested that depressed IL-2 production in RA patients may relate to monocyte effects, but this cannot completely explain the defects observed.
IL-2, a growth factor for T lymphocytes, exerts its effects through the activation of cell surface receptors called IL-2R. These receptors are found on activated T cells, and the protein can be shed from cell membranes resulting in the detection of a soluble form of IL-2R using an enzyme-linked immunosorbent assay. This study analyzed the levels of sIL-2R in the sera of patients with RA over time. The findings indicate that the sIL-2R level in the serum of RA patients reflects the activation of underlying immunopathogenic mechanisms, making it an excellent indicator of clinical disease activity. Moreover, an increasing level of sIL-2 R may predict the exacerbation of disease activity[20].
The genetic risk factors were identified in RA patients. The results showed that a genetic variant (rs791588) in the IL2RA gene was associated with a decreased risk of RA, and two SNPs (rs791588 and rs2281089) were associated with a reduced risk of RA when stratified by gender or age. However, two haplotypes of IL2RA were associated with an increased risk of RA. These findings suggest that inherited genetic alterations at IL2RA may play a role in the development of RA[2]. This study aimed to investigate whether SNPs within the IL2RA/CD25 gene are associated with juvenile idiopathic arthritis (JIA). The IL2RA/CD25 gene is a susceptibility gene for autoimmune diseases due to its role in regulatory T cells. The study found strong evidence that the IL2RA/CD25 gene is a JIA susceptibility locus, and further investigation is necessary using both genetic and functional approaches[21]. Moreover, IL2RA-rs2104286 and sIL2Rα levels have been found to be associated with persistent RA. IL2RA gene variants have been previously shown to have protective effects against multiple sclerosis, diabetes mellitus, and RA. In addition to HLA-SE, IL2RA-rs2104286 is currently the only known genetic variant that is associated with both joint destruction and RA persistence, highlighting the importance of IL2RA in the development and progression of RA[22].
The study investigated various aspects of the production and function of IL-2 using mononuclear cells from synovial fluid (SF), synovial tissue, and peripheral blood (PB) of patients with RA. The findings indicated that both PB and SF rheumatoid lymphocytes produced less IFN-gamma when treated with IL-2 overnight than normal PB lymphocytes. This was inconsistent with the notable improvement in natural killer activity caused by IL-2. Removing adherent cells in synovial fluid did not correct this deficit. These abnormal behaviors of IL-2 and IFN-gamma suggest that impaired T-cell function could be a contributing factor in the immunopathogenesis of RA[23]. However, previous studies in patients with RA have produced conflicting findings regarding the relationship between sIL-2R levels and disease activity scores or other laboratory markers of inflammation. A recent study found that low sIL-2R levels might indicate a rapid response to treatment with infliximab in RA patients. However, this marker did not accurately identify patients in remission after 22 wk in their study group. Additionally, van Steenbergen et al[22] reported that lower sIL-2R levels were associated with sustained remission without the use of disease-modifying antirheumatic drugs in RA patients[4]. The status of regulatory T cells (Tregs) in refractory RA and the potential of low-dose IL-2 therapy in restoring the decreased CD4 Tregs were investigated. Results showed that a decrease in peripheral blood CD4 Tregs in patients with refractory RA was associated with continuing disease activation, but not an increase in Th17 cells. Low-dose IL-2 therapy was found to restore decreased CD4 Tregs and promote rapid remission of patients with refractory RA without overtreatment or observed side effects, making it a potential therapeutic candidate[24]. The potential use of low-dose interleukin-2 (Ld-IL2) as a therapeutic approach to enhance the treatment of RA was investigated through a randomized, double-blind, placebo-controlled trial. The effectiveness and safety of Ld-IL2 in patients with active RA was considered. The findings showed that Ld-IL2 was well-tolerated, and low levels of Treg and high levels of IL-21 were associated with positive responses to Ld-IL2. Therefore, it can be concluded that Ld-IL2 is a safe and effective treatment for RA[25].
Sjögren's syndrome (SS) is a chronic autoimmune disease that is characterized by the loss of function of exocrine glands. The infiltration of exocrine glands by T and B lymphocytes is responsible for the inflammation observed in SS patients. In addition to the loss of exocrine gland function, SS patients may also experience a range of extra-glandular symptoms such as arthralgia, kidney disease, lung disease, and fatigue[26]. SS is characterized by dry mouth (xerostomia), dry eyes (xerophthalmia), and other immune-mediated symptoms. The condition can also cause dryness in the mucosal surfaces of the digestive tract, lungs, and vagina, which is referred to as "Sicca syndrome" or "Sicca Complex." Since it is a systemic illness, SS can impact various organ systems and result in a range of symptoms. These symptoms, which include fatigue, depression, anxiety, and impaired physical performance, can significantly affect a patient's quality of life and daily activities[27].
The etiology of SS, like other autoimmune diseases, is not yet fully understood. However, it is widely recognized that exposure to specific environmental factors may contribute to the dysregulation of the immune system and the onset of the disease in susceptible individuals. The interferon pathway is critical in the early stages of SS by disrupting the innate immune system's barriers[28]. Nonetheless, the adaptive immune system's role is significant in SS development, as persistent B-cell activation and the growth of Th1 and Th17 cells contribute to the disease's progression[29].
Genetic susceptibility plays a significant role in the pathophysiology of SS. Previous studies have identified several genes, including IRF5-TNPO3, STAT4, IL12A, FAM167A-BLK, DDX6-CXCR5, and TNIP1, may be involved as risk factors for SS. IRF5 and STAT4 have been recognized as susceptibility genes based on genome-wide association analysis[30]. IRF5 activates the expression of several proinflammatory factors downstream of the Toll-like receptor and type I IFN receptor, while STAT4 plays a role in autoimmune abnormalities through IFN production[31]. Women are more likely than men to develop SS, and studies have identified GTF2I and RBMS3 as the most significant susceptibility genes for pSS in women[32].
Long non-coding RNA (lncRNA) plays a role in gene expression by modifying chromosomes, DNA, transcription, and post-transcriptional modification, as well as the generation of inflammatory mediators, immune cell growth, and differentiation, which contribute to the development of autoimmunity[33]. Dysregulated lncRNAs have been linked to an increased risk of SS[34]. MicroRNAs (miRNAs) also play a role in the development of autoimmune diseases, including SS. pSS patients have decreased expression of miRNA-181a and miRNA-16 in their salivary glands[35], while the levels of miRNA-146a and miRNA-155 in their peripheral blood mononuclear cells are linked to their clinical symptoms. Epigenetic processes, such as DNA methylation and histone modification, may also contribute to the onset of SS[36].
SS is an autoimmune disorder where immune cells and IL-2 signaling are not working correctly. IL-2 is essential for the growth and maintenance of Treg cells. There is potential for the use of low doses of IL-2 to treat autoimmune diseases, but it is uncertain if it can be effective in treating SS or how it works. Research provides evidence that low-dose interleukin-2 is a promising treatment for SS in NOD mice by restoring immunological balance through promoting Treg cells and suppressing germinal center B cells and effector T cells[37]. The effects of low-dose interleukin-2 on exocrine glands in SS were not previously known. This study investigated the impact of low doses of IL-2 on the development and function of salivary glands in a mouse model of SS. Results indicate that low-dose IL-2 treatment improves the exocrine glands' ability to secrete saliva in SS-affected mice, but it does not restore the damaged structure of the glands[38].
Primary Sjögren syndrome (pSS) is a systemic autoimmune disease characterized by immune cell dysregulation, and it currently lacks effective treatment options. As a result, investigating potential therapeutic strategies is essential. One such strategy is the use of low-dose interleukin 2 (LD-IL-2), which can be studied for its safety, immunological response, and potential to treat pSS. In a randomized clinical trial, pSS patients responded positively to LD-IL-2 treatment, leading to restored immunological balance through the promotion of regulatory T cells and CD24 high CD27+ B cells[39]. A study was designed to understand the mechanism behind the increased Th17 differentiation in pSS patients and the role of IL-2 in balancing Th17 and Tregs. The findings showed that the increased Th17 generation in pSS was not dependent on Tregs, but rather due to the lack of IL-2-mediated suppression of Th17 differentiation. Therefore, this study revealed a new mechanism of immune suppression mediated by IL-2 in pSS[40].
Systemic sclerosis (SSc) is an autoimmune disease that affects multiple organs, including the skin, joints, tendons, gastrointestinal tract, lungs, heart, blood vessels, and kidneys, due to inflammation and fibrosis. It primarily affects women, with a female-to-male ratio of 4:1-10:1, depending on age and ethnicity. The disease has two clinical subtypes based on the extent of skin involvement: Diffuse cutaneous SSc (dcSSc), which affects the skin proximal to elbows and/or knees, or that affects the thorax and/or abdomen at any given time during the disease, and limited cutaneous SSc (lcSSc), which affects the skin distal to elbows and knees without the involvement of the thorax or abdomen[41].
The disease can lead to major disabilities, malnutrition, and decreased quality of life due to vascular complications, cardiopulmonary involvement, inflammatory myopathy, arthritis, and gastrointestinal tract involvement[41]. The immune response cells, inflammatory mediators, fibroblasts, and other components of the extracellular matrix, as well as endothelial damage, play a central role in the disease's numerous pathogenic pathways. This has changed the previous paradigm that the disease was predominantly fibrotic and is now viewed as a complex syndrome that requires simultaneous treatment of its various pathogenic pathways[41].
The chromosome 4q27 region that contains the IL-2 and IL-21 genes has been linked to various autoimmune diseases, as both genes are involved in immune system functions. This study aimed to investigate the role of the IL-2/IL-21 Locus in SSc. The research found that the allelic combination of rs2069762A-rs6822844T-rs6835457G-rs907715T showed a significant association with SSc and the limited cutaneous SSc subtype. Therefore, the study concluded that the IL-2/IL-21 Locus is involved in the genetic susceptibility to SSc. These findings also add to the evidence that the IL-2/IL-21 Locus is a common genetic factor in autoimmune diseases[42].
The connections were investigated between 9 SNPs in the IL10, IL1B, IL1A, IL1RN, IL2, LTA, and IL6 genes and the development of SSc, as well as the clinical subtype of SSc in patients. A total of 78 SSc patients (31 with diffuse SSc and 47 with limited SSc) and 692 healthy blood donors were genotyped for 9 SNPs: IL10 T-3575A, IL10 A-1082G, IL1B C-31T, IL1B C-511T, IL1A C-889T, IL1RN A9589T, IL2 T-384G, LTA T-91G, and IL6 G-174C. The study found that the IL1B and IL2 gene SNPs may contribute to the susceptibility to SSc. Additionally, the IL2-384-G allele may serve as an indicator for the limited phenotype of SSc[43].
IL-2A and IL-12R gene variants have been linked to SSc. IL-2 helps maintain the immune system's balance and tolerance to self, promotes B-cell immunoglobulin production, and stimulates natural killer cell proliferation and differentiation. Two studies explored the role of IL-2 in SSc. The studies found that certain IL-2 receptor alpha gene variants (rs11594656, rs2104286, and rs12722495) were associated with SSc, limited cutaneous SSc, and anticentromere antibody (ACA) positivity. However, the associations were highly dependent on ACA positivity, as removing ACA from the analysis resulted in the loss of association. Among the IL-2 RA gene variants, rs2104286 showed the strongest association with ACA positivity, and the associations with the other variants disappeared after accounting for rs2104286[44]. The minor allele of rs907715 and rs682284 are linked to SSc. The variant rs6822844 specifically affects lcSSc and ACA positivity. An allelic combination, is rs2069762*A, rs6822844*T-rs6835457G-rs907715* T is linked to both dcSSc and lcSSc. Protective for lcSSc and ACA positive is the T allele for rs6822844[42].
Basiliximab, a monoclonal antibody, targets the alpha chain (CD25) of the IL-2 receptor and has been suggested as a potential treatment for SSc. This is due to recent findings that suggest a significant role of T effector cells, specifically T-17 and T regulatory subsets, in the pathogenesis of SSc[45]. Schmidt et al[45] study suggested that treatment with this medication could improve skin involvement, lung fibrosis, disease progression, and mortality in systemic sclerosis. They based this on the correlation between these factors and serum levels of soluble IL-2 receptor, which could be reduced by the drug[46]. In an accessible SSc research, basiliximab medication was generally well tolerated, and the side effects that were reported were primarily modest[47]. In an open-label study on SSc, minor side-effects were mostly reported, and in general, basiliximab therapy was well tolerated[47].
Relationship between immune system and lung fibrosis in Fra-2-transgenic mice was investigated, which can serve as a model for SSc. The study examined whether an imbalance between effector and regulatory CD4+ T cells may be responsible for the lung phenotype observed in these mice. The results suggest that immunotherapies targeting the restoration of Treg cell homeostasis may be beneficial for SSc. Low-dose IL-2 injection has been suggested as a potential therapy to achieve this, which may be explored further in future studies. SSc is an autoimmune disease with significant pulmonary complications that can decrease life expectancy[48].
Multiple sclerosis (MS) is a disease of the nervous system that causes chronic, progressive, and degenerative symptoms. It occurs when the immune system is not functioning properly, leading to damage of the central nervous system, including demyelination and axonal damage. In most cases, the first sign of MS is a demyelinating disease referred to as a clinically isolated syndrome. This involves focal or multifocal areas of the central nervous system, commonly affecting the optic nerve, brainstem, or spinal cord. It is caused by a disruption in the regulation of the immune system, which may be due to genetic factors and/or environmental triggers such as viral infections The prevalence of MS is highest among individuals of Caucasian ethnicity and those of northern European descent. The disease is widespread, with approximately 2 million individuals diagnosed worldwide, and the number of cases is projected to increase due to population growth[49]. MS is prevalent in young adults and is the primary cause of non-traumatic disability in this population. MS is more common in females than males, with a ratio of 2-3:1, although there is no gender imbalance in primary progressive MS[49].
IL-2 and soluble IL-2 receptor (sIL-2R) levels in serum and CSF could serve as markers of disease activity in MS. A research study involved 63 MS patients, and the results showed that the levels of IL-2 and sIL-2R in the CSF were significantly higher in patients experiencing relapses than those in remission or the control group. Additionally, these levels correlated with disease severity scores, the number of relapses per year, and the total duration of the disease. The findings suggested that there is an association between an activated immune system and MS pathology, and measuring IL-2 and sIL-2R levels could provide a reliable indicator of disease activity in MS patients[50].
The role of CD8+ T cells in the development of MS has been established. IL-2Rα is crucial for the function of CD8+ T cells, and IL2RA gene SNPs increase the risk of MS. In this study, the researchers investigated the methylation and gene expression of the IL2RA gene in isolated CD8+ T cells in relation to the MS-associated SNP rs2104286 and soluble IL-2Rα (sIL-2Rα). The results showed that rs2104286 SNP has a minor effect on CD8+ T cells and sIL-2Rα might negatively regulate the CD8+ T cell response[51].
The IL-2/IL-2R pathway is crucial for T cell proliferation, survival, and regulatory T cell production. Genome-wide studies have identified polymorphisms in the IL2/IL21 genes (located in 4q27), as well as in genes encoding the IL2RA and IL2RB subunits (located in 10p15 and 22q13, respectively), that are associated with various autoimmune diseases. In a combined Spanish cohort, regression analyses indicated that two independent IL2RA association signals, namely rs2104286 and rs11594656/rs35285258, are relevant for MS susceptibility and support their common effect on autoimmune risk. Additionally, the association of IL2/IL21 requires further investigation[52].
The ability to predict the severity of MS in a newly diagnosed patient would allow for more personalized treatment strategies and improved clinical outcomes. Therefore, there is a need for prognostic biomarkers. A study was conducted to evaluate the prognostic value of intrathecal IgM synthesis, as well as cerebrospinal fluid and serum levels of IL-2, IL-6, IL-10, chitinase 3-like 2, and neurofilament heavy chains obtained shortly after the onset of the disease. The study found that the IL-2:IL-6 ratio, IL-2, and chitinase 3-like 2 (all in cerebrospinal fluid) may serve as valuable prognostic biomarkers in the early stages of multiple sclerosis[53].
This research explores the potential therapeutic and protective effects of Tregs, myeloid-derived suppressor cells (MDSCs), and IL-2 on a MS disease model. The study involved immunizing C57BL/6 mice to develop an experimental autoimmune encephalomyelitis (EAE) model, followed by investigating the effects of pre-and post-treatment of Tregs, MDSCs, and IL-2 on inflammation, demyelination in brain tissue, and the number of Treg, granulocytic-MDSC, and Th-17 cells in the spleen. The results showed that pre-and post-treatment with Tregs, MDSCs, and IL-2 prevented EAE induction, with reduced Th-17 cells and suppression of pathological properties, and no weight change in the mice. Therefore, the study concludes that pre-and post-treatment with Tregs, MDSCs, and IL-2 may have the potential to prevent EAE induction[53].
According to the genetic studies of IL-2, gene polymorphisms of IL-2 such as IL-2 330/T, IL-2 330/G, and rs2069763 are involved in triggering increased risk of SLE. Furthermore, genetic associations of IL-2/IL-2R such as rs791588, rs2281089, rs2104286, rs11594656, and rs35285258 are significantly associated with RA susceptibility. The IL-2 polymorphism including rs2069762A, rs6822844T, rs6835457G, and rs907715T are significant connections with systemic sclerosis. In addition, rs2104286 (IL-2), rs11594656 (IL-2RA), rs35285258 (IL-2RB) gene polymorphism is significantly increases the risk of multiple sclerosis. In the case of therapeutic approaches, low-dose IL-2 therapy could regulate Tfr and Tfh cells, resulting in a reduction in disease activity in SLE patients. In addition, elevated sIL-2R levels in the peripheral blood of SLE patients could be linked to an immunoregulatory imbalance, which may contribute to the onset and progression of SLE. Consequently, sIL-2R could potentially be a target for future SLE therapy. Moreover, Ld-IL2 was well-tolerated, and low levels of Treg and high levels of IL-21 were associated with positive responses to Ld-IL2 suggested to be a safe and effective treatment for RA. Additionally, low-dose IL-2 treatment improves the exocrine glands' ability to secrete saliva in SS-affected mice. Whereas, Basiliximab targets the alpha chain of the IL-2 receptor suggested as a potential treatment for SSc. Also, pre-and post-treatment with Tregs, MDSCs, and IL-2 may have the potential to prevent EAE induction in patients with MS. Further studies should be conducted on IL-2 gene polymorphisms in Sjogren syndrome.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jia J, China; Yildiz K, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest. 2015;125:2228-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 2. | Yang Y, Yuan S, Che M, Jing H, Yuan L, Dong K, Jin T. Genetic analysis of the relation between IL2RA/IL2RB and rheumatoid arthritis risk. Mol Genet Genomic Med. 2019;7:e00754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Chistiakov DA, Voronova NV, Chistiakov PA. The crucial role of IL-2/IL-2RA-mediated immune regulation in the pathogenesis of type 1 diabetes, an evidence coming from genetic and animal model studies. Immunol Lett. 2008;118:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Dik WA, Heron M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth J Med. 2020;78:220-231. [PubMed] |
| 5. | Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus-an update. Curr Opin Immunol. 2012;24:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Lin YJ, Wan L, Sheu JJ, Huang CM, Lin CW, Lan YC, Lai CH, Hung CH, Tsai Y, Tsai CH, Lin WY, Liu HP, Lin TH, Huang YM, Tsai FJ. G/T polymorphism in the interleukin-2 exon 1 region among Han Chinese systemic lupus erythematosus patients in Taiwan. Clin Immunol. 2008;129:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Márquez A, Dávila-Fajardo CL, Robledo G, Rubio JL, de Ramón Garrido E, García-Hernández FJ, González-León R, Ríos-Fernández R, Barrera JC, González-Escribano MF, García MT, Palma MJ, del Mar Ayala M, Ortego-Centeno N, Martín J. IL2/IL21 region polymorphism influences response to rituximab in systemic lupus erythematosus patients. Mol Biol Rep. 2013;40:4851-4856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 8. | Bouzid D, Fourati H, Amouri A, Marques I, Abida O, Tahri N, Penha-Gonçalves C, Masmoudi H. Autoimmune diseases association study with the KIAA1109-IL2-IL21 region in a Tunisian population. Mol Biol Rep. 2014;41:7133-7139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Harsini S, Ziaee V, Tahghighi F, Mahmoudi M, Rezaei A, Soltani S, Sadr M, Moradinejad MH, Aghighi Y, Rezaei N. Association of interleukin-2 and interferon-γ single nucleotide polymorphisms with Juvenile systemic lupus erythematosus. Allergol Immunopathol (Madr). 2016;44:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 567] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 11. | Long D, Yu S, Zhang L, Guo Y, Xu S, Rao Y, Huang Z, Luo Q, Li J. Increased sIL-2Rα leads to obstruction of IL-2 biological function and Treg cells differentiation in SLE patients via binding to IL-2. Front Immunol. 2022;13:938556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Su Y, Guo J, Shen N, Morand EF, Yu D, Li Z. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 13. | Miao M, Xiao X, Tian J, Zhufeng Y, Feng R, Zhang R, Chen J, Zhang X, Huang B, Jin Y, Sun X, He J, Li Z. Therapeutic potential of targeting Tfr/Tfh cell balance by low-dose-IL-2 in active SLE: a post hoc analysis from a double-blind RCT study. Arthritis Res Ther. 2021;23:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | He J, Zhang R, Shao M, Zhao X, Miao M, Chen J, Liu J, Zhang X, Jin Y, Wang Y, Zhang S, Zhu L, Jacob A, Jia R, You X, Li X, Li C, Zhou Y, Yang Y, Ye H, Liu Y, Su Y, Shen N, Alexander J, Guo J, Ambrus J, Lin X, Yu D, Sun X, Li Z. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79:141-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 15. | Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J Biomed Biotechnol. 2010;2010:740619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Wu R, Li N, Zhao X, Ding T, Xue H, Gao C, Li X, Wang C. Low-dose Interleukin-2: Biology and therapeutic prospects in rheumatoid arthritis. Autoimmun Rev. 2020;19:102645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Oncül O, Top C, Ozkan S, Cavuşlu S, Danaci M. Serum interleukin 2 levels in patients with rheumatoid arthritis and correlation with insulin sensitivity. J Int Med Res. 2002;30:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Li B, Guo Q, Wang Y, Su R, Gao C, Zhao J, Li X, Wang C. Increased Serum Interleukin-2 Levels Are Associated with Abnormal Peripheral Blood Natural Killer Cell Levels in Patients with Active Rheumatoid Arthritis. Mediators Inflamm. 2020;2020:6108342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Kitas GD, Salmon M, Farr M, Gaston JS, Bacon PA. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin Exp Immunol. 1988;73:242-249. [PubMed] |
| 20. | Wood NC, Symons JA, Duff GW. Serum interleukin-2-receptor in rheumatoid arthritis: a prognostic indicator of disease activity? J Autoimmun. 1988;1:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Hinks A, Ke X, Barton A, Eyre S, Bowes J, Worthington J, Thompson SD, Langefeld CD, Glass DN, Thomson W; UK Rheumatoid Arthritis Genetics Consortium; British Society of Paediatric and Adolescent Rheumatology Study Group. Association of the IL2RA/CD25 gene with juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:251-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | van Steenbergen HW, van Nies JA, Ruyssen-Witrand A, Huizinga TW, Cantagrel A, Berenbaum F, van der Helm-van Mil AH. IL2RA is associated with persistence of rheumatoid arthritis. Arthritis Res Ther. 2015;17:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Combe B, Pope RM, Fischbach M, Darnell B, Baron S, Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985;59:520-528. [PubMed] |
| 24. | Wang J, Zhang SX, Chang JS, Cheng T, Jiang XJ, Su QY, Zhang JQ, Luo J, Li XF. Low-dose IL-2 improved clinical symptoms by restoring reduced regulatory T cells in patients with refractory rheumatoid arthritis: A randomized controlled trial. Front Immunol. 2022;13:947341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Miao M, Zhang R, Liu X, Zhao X, Shao M, Liu T, Jin Y, Chen J, Liu H, Zhang X, Li Y, Zhou Y, Yang Y, Li R, Yao H, Liu Y, Li C, Ren L, Su Y, Sun X, He J, Li Z. Efficacy and safety of low-dose interleukin-2 in combination with methotrexate in patients with active rheumatoid arthritis: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2022;7:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Zhang G, Zhang L, Zhao M, Huang H. Decreased microRNA-181a and -16 expression levels in the labial salivary glands of Sjögren syndrome patients. Exp Ther Med. 2018;15:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Negrini S, Emmi G, Greco M, Borro M, Sardanelli F, Murdaca G, Indiveri F, Puppo F. Sjögren's syndrome: a systemic autoimmune disease. Clin Exp Med. 2022;22:9-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 294] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 28. | Shimizu T, Nakamura H, Kawakami A. Role of the Innate Immunity Signaling Pathway in the Pathogenesis of Sjögren's Syndrome. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Chivasso C, Sarrand J, Perret J, Delporte C, Soyfoo MS. The Involvement of Innate and Adaptive Immunity in the Initiation and Perpetuation of Sjögren's Syndrome. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 30. | Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, Kelly JA, Dozmorov MG, Miceli-Richard C, Bowman S, Lester S, Eriksson P, Eloranta ML, Brun JG, Gøransson LG, Harboe E, Guthridge JM, Kaufman KM, Kvarnström M, Jazebi H, Cunninghame Graham DS, Grandits ME, Nazmul-Hossain AN, Patel K, Adler AJ, Maier-Moore JS, Farris AD, Brennan MT, Lessard JA, Chodosh J, Gopalakrishnan R, Hefner KS, Houston GD, Huang AJ, Hughes PJ, Lewis DM, Radfar L, Rohrer MD, Stone DU, Wren JD, Vyse TJ, Gaffney PM, James JA, Omdal R, Wahren-Herlenius M, Illei GG, Witte T, Jonsson R, Rischmueller M, Rönnblom L, Nordmark G, Ng WF; UK Primary Sjögren's Syndrome Registry, Mariette X, Anaya JM, Rhodus NL, Segal BM, Scofield RH, Montgomery CG, Harley JB, Sivils KL. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat Genet. 2013;45:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 414] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 31. | Nakamura H, Tanaka T, Pranzatelli T, Ji Y, Yin H, Perez P, Afione SA, Jang SI, Goldsmith C, Zheng CY, Swaim WD, Warner BM, Hirata N, Noguchi M, Atsumi T, Chiorini JA. Lysosome-associated membrane protein 3 misexpression in salivary glands induces a Sjögren's syndrome-like phenotype in mice. Ann Rheum Dis. 2021;80:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Song IW, Chen HC, Lin YF, Yang JH, Chang CC, Chou CT, Lee MM, Chou YC, Chen CH, Chen YT, Wu JY. Identification of susceptibility gene associated with female primary Sjögren's syndrome in Han Chinese by genome-wide association study. Hum Genet. 2016;135:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Cao X. Long noncoding RNAs in innate immunity. Cell Mol Immunol. 2016;13:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 34. | Shi H, Cao N, Pu Y, Xie L, Zheng L, Yu C. Long non-coding RNA expression profile in minor salivary gland of primary Sjögren's syndrome. Arthritis Res Ther. 2016;18:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Imgenberg-Kreuz J, Sandling JK, Almlöf JC, Nordlund J, Signér L, Norheim KB, Omdal R, Rönnblom L, Eloranta ML, Syvänen AC, Nordmark G. Genome-wide DNA methylation analysis in multiple tissues in primary Sjögren's syndrome reveals regulatory effects at interferon-induced genes. Ann Rheum Dis. 2016;75:2029-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Feng R, Cheng G, Huang B, Tian J, Gan Y, Jin Y, Miao M, Zhang X, Sun X, He J, Li Z. Low Dose Interleukin-2 Ameliorates Sjögren's Syndrome in a Murine Model. Front Med (Lausanne). 2022;9:887354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Wen J, Zhu F, Yu X, Xie H, Li C. Low-dose interleukin-2 can improve salivary secretion but not lymphocyte infiltration of salivary glands in a murine model of Sjögren's syndrome. BMC Immunol. 2022;23:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | He J, Chen J, Miao M, Zhang R, Cheng G, Wang Y, Feng R, Huang B, Luan H, Jia Y, Jin Y, Zhang X, Shao M, Li J, Zhao X, Wang H, Liu T, Xiao X, Su Y, Mu R, Ye H, Li R, Liu X, Liu Y, Li C, Liu H, Hu F, Guo J, Liu W, Zhang WB, Jacob A, Ambrus JL Jr, Ding C, Yu D, Sun X, Li Z. Efficacy and Safety of Low-Dose Interleukin 2 for Primary Sjögren Syndrome: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2241451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Luo J, Ming B, Zhang C, Deng X, Li P, Wei Z, Xia Y, Jiang K, Ye H, Ma W, Liu Z, Li H, Yang XP, Dong L. IL-2 Inhibition of Th17 Generation Rather Than Induction of Treg Cells Is Impaired in Primary Sjögren's Syndrome Patients. Front Immunol. 2018;9:1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Sierra-Sepúlveda A, Esquinca-González A, Benavides-Suárez SA, Sordo-Lima DE, Caballero-Islas AE, Cabral-Castañeda AR, Rodríguez-Reyna TS. Systemic Sclerosis Pathogenesis and Emerging Therapies, beyond the Fibroblast. Biomed Res Int. 2019;2019:4569826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Diaz-Gallo LM, Simeon CP, Broen JC, Ortego-Centeno N, Beretta L, Vonk MC, Carreira PE, Vargas S, Román-Ivorra JA, González-Gay MA, Tolosa C, López-Longo FJ, Espinosa G, Vicente EF, Hesselstrand R, Riemekasten G, Witte T, Distler JH, Voskuyl AE, Schuerwegh AJ, Shiels PG, Nordin A, Padyukov L, Hoffmann-Vold AM, Scorza R, Lunardi C, Airo P, van Laar JM, Hunzelmann N, Gathof BS, Kreuter A, Herrick A, Worthington J, Denton CP, Zhou X, Arnett FC, Fonseca C, Koeleman BP, Assasi S, Radstake TR, Mayes MD, Martín J; Spanish Scleroderma Group. Implication of IL-2/IL-21 region in systemic sclerosis genetic susceptibility. Ann Rheum Dis. 2013;72:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Mattuzzi S, Barbi S, Carletto A, Ravagnani V, Moore PS, Bambara LM, Scarpa A. Association of polymorphisms in the IL1B and IL2 genes with susceptibility and severity of systemic sclerosis. J Rheumatol. 2007;34:997-1004. [PubMed] |
| 43. | Martin JE, Carmona FD, Broen JC, Simeón CP, Vonk MC, Carreira P, Ríos-Fernández R, Espinosa G, Vicente-Rabaneda E, Tolosa C, García-Hernández FJ, Castellví I, Fonollosa V, González-Gay MA, Sáez-Comet L, Portales RG, de la Peña PG, Fernández-Castro M, Díaz B, Martínez-Estupiñán L, Coenen M, Voskuyl AE, Schuerwegh AJ, Vanthuyne M, Houssiau F, Smith V, de Keyser F, De Langhe E, Riemekasten G, Witte T, Hunzelmann N, Kreuter A, Palm Ø, Chee MM, van Laar JM, Denton C, Herrick A, Worthington J, Koeleman BP, Radstake TR, Fonseca C, Martín J; Spanish Scleroderma Group. The autoimmune disease-associated IL2RA locus is involved in the clinical manifestations of systemic sclerosis. Genes Immun. 2012;13:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Ong VH, Denton CP. Innovative therapies for systemic sclerosis. Curr Opin Rheumatol. 2010;22:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Schmidt K, Martinez-Gamboa L, Meier S, Witt C, Meisel C, Hanitsch LG, Becker MO, Huscher D, Burmester GR, Riemekasten G. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther. 2009;11:R111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Becker MO, Brückner C, Scherer HU, Wassermann N, Humrich JY, Hanitsch LG, Schneider U, Kawald A, Hanke K, Burmester GR, Riemekasten G. The monoclonal anti-CD25 antibody basiliximab for the treatment of progressive systemic sclerosis: an open-label study. Ann Rheum Dis. 2011;70:1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Frantz C, Cauvet A, Durand A, Gonzalez V, Pierre R, Do Cruzeiro M, Bailly K, Andrieu M, Orvain C, Avouac J, Ottaviani M, Thuillet R, Tu L, Guignabert C, Lucas B, Auffray C, Allanore Y. Driving Role of Interleukin-2-Related Regulatory CD4+ T Cell Deficiency in the Development of Lung Fibrosis and Vascular Remodeling in a Mouse Model of Systemic Sclerosis. Arthritis Rheumatol. 2022;74:1387-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Gold R, Wolinsky JS. Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand. 2011;124:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Sharief MK, Thompson EJ. Correlation of interleukin-2 and soluble interleukin-2 receptor with clinical activity of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Buhelt S, Laigaard HM, von Essen MR, Ullum H, Oturai A, Sellebjerg F, Søndergaard HB. IL2RA Methylation and Gene Expression in Relation to the Multiple Sclerosis-Associated Gene Variant rs2104286 and Soluble IL-2Rα in CD8(+) T Cells. Front Immunol. 2021;12:676141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Cavanillas ML, Alcina A, Núñez C, de las Heras V, Fernández-Arquero M, Bartolomé M, de la Concha EG, Fernández O, Arroyo R, Matesanz F, Urcelay E. Polymorphisms in the IL2, IL2RA and IL2RB genes in multiple sclerosis risk. Eur J Hum Genet. 2010;18:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Petržalka M, Meluzínová E, Libertínová J, Mojžišová H, Hanzalová J, Ročková P, Elišák M, Kmetonyová S, Šanda J, Sobek O, Marusič P. IL-2, IL-6 and chitinase 3-like 2 might predict early relapse activity in multiple sclerosis. PLoS One. 2022;17:e0270607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 53. | Ghorbani MM, Farazmandfar T, Abediankenari S, Hassannia H, Maleki Z, Shahbazi M. Treatment of EAE mice with Treg, G-MDSC and IL-2: a new insight into cell therapy for multiple sclerosis. Immunotherapy. 2022;14:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 54. | Mitra S, Leonard WJ. Biology of IL‐2 and its therapeutic modulation: Mechanisms and strategies. J Leukoc Biol. 2018;103:643-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |