Published online Dec 15, 2019. doi: 10.5495/wjcid.v9.i3.23
Peer-review started: May 23, 2019
First decision: August 7, 2019
Revised: September 3, 2019
Accepted: November 26, 2019
Article in press: November 26, 2019
Published online: December 15, 2019
Processing time: 202 Days and 23.6 Hours
Non-Aggregatibacter aphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella spp. (non-AACEK) gram-negative bacilli (GNBs) are an infrequent and challenging cause of endocarditis associated previously with mainly intravenous drug use. Currently, this pathology has increasingly become a healthcare-associated issue. Current guidelines do not clearly define the management of non-AACEK GNB endocarditis due to a lack of prospective trials. We review characteristics, outcomes and treatment of non-AACEK GNB endocarditis, in particular Serratia marcescens endocarditis.
We describe the case report of a 46-year-old man who presented to the emergency department with high-grade fever and a purulent exudate on an intracardiac device site. Serratia marcescens mitral valve endocarditis as a consequence of complicated generator pocket infection was diagnosed. The patient was treated with complete device removal and a long course of broad-spectrum antibiotics for 6 wk after surgery with intravenous piperacillin-tazobactam and ciprofloxacin, which was later switched to oral ciprofloxacin and sulfamethoxazole-trimethoprim. The patient had complete resolution of symptoms and inflammatory parameters at the end of the treatment and at follow-up.
Long-term dual-antibiotic therapy containing a beta-lactam is indicated for most non-AACEK GNB endocarditis, whereas valve surgery may not be necessary in all patients.
Core tip: While gram-negative bacillus (GNB) Non-Aggregatibacter aphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella spp. (non-AACEK) endocarditis has been associated with mainly intravenous drug use, the role of healthcare-associated contact has been highlighted in two prospective observational studies. Our aim was to review the characteristics and management of non-AACEK GNB endocarditis, especially in the case of Serratia marcescens. This bacterium has become a rare cause of endocarditis, but community acquisition still has an important role in this disease. We discuss treatment options, supporting long-term dual-antibiotic treatment as the preferred option for most patients with non-AACEK GNB endocarditis, whereas valve surgery does not seem to be necessary in all patients.
- Citation: Mertes H, Morissens M, Mahadeb B, Maillart E, Moreau A, Clevenbergh P. Serratia marcescens and other non-AACEK GNB endocarditis: A case report and review of literature. World J Clin Infect Dis 2019; 9(3): 23-30
- URL: https://www.wjgnet.com/2220-3176/full/v9/i3/23.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v9.i3.23
Gram-negative bacillus (GNB) non-Aggregatibacter aphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella spp. (non-AACEK) bacteria are a rare cause of infectious endocarditis. The International Collaboration on Endocarditis (ICE) prospective study from Morpeth et al[1]. showed that among 2761 patients with definite endocarditis, only 49 (1.8%) had endocarditis due to non-AACEK GNB[1]. The recent Italian Endocarditis Study (SEI) from Falcone et al[2] reported a slightly higher incidence of 3.3% (58 patients) among 1722 patients studied. The most prevalent bacteria found in these observational prospective studies about GNB non-AACEK endocarditis were Escherichia coli (29 and 31%), Pseudomonas aeruginosa (19 and 22%) and Klebsiella pneumoniae (10%). In contrast, Serratia marcescens is less common and represents between 3.5% and 8% of endocarditis pathogens[1,2].
Historically, in the 1970s and 1980s, GNB non-AACEK endocarditis was associated with nosocomial exposure and IVDU[3-7]. Serratia marcescens was a predominant cause of community-acquired endocarditis, as highlighted by Mills et al[3] and Cooper et al[4]. In their reviews, individuals with a history of IVDU accounted for 88 and 89%, respectively, of patients with Serratia marcescens endocarditis. Currently, GNB non-AACEK endocarditis has become a healthcare-associated (HCA) issue[1,2]. Morpeth et al[1] were first to describe that association in a prospective observational study: 57% of non-AACEK GNB endocarditis cases were HCA (nosocomial and non-nosocomial), predominantly in individuals undergoing non-dental invasive procedures and in patients with intracardiac devices (ICDs). Falcone et al[2] reported that 44.7% of GNB non-AACEK endocarditis cases were HCA (nosocomial and non-nosocomial), but for most patients, the acquisition was community acquired (55.2%), which was again associated with the presence of an ICD (OR = 3.6) but also with immunosuppression (OR = 5.16). In both studies, the most important source of infection was the genitourinary tract[1,2].
Currently, mortality related to GNB non-AACEK endocarditis has decreased from between 30% and 68% in the 1980s[3-7] to between 13% and 24%[1,2]. Cardiac valve surgery is not associated with better outcomes except for in those individuals presenting with complications (heart failure, cardiac abscess, fistula, dehiscence and valve perforation). The multidrug resistance patterns of the bacteria seem to play an important role in the in-hospital mortality rate (HR = 21.89)[2].
European[8], American[9] and British[10] guidelines do not specifically define treatment of GNB non-AACEK endocarditis due to a lack of prospective studies. They recommend long-term (6 wk) combined bactericidal antibiotic treatment associated with surgery[8-10]: Beta-lactams should be the cornerstone of the antibiotic regimen and combined with aminoglycosides (AGs). Sulfamethoxazole-trimethoprim or fluoroquinolones (FQs) could also be added to beta-lactams or reinforce the combined regimen with a BL and an AG[8,9]. In the ICE study[1], most patients (57%) were treated equally with a combination of a BL and either an AG or FQ. Eight percent of the patients were treated with a triple antibiotic therapy, whereas 14% received a monotherapy regimen consisting of a BL[1]. In the more recent Italian Endocarditis Study (SEI)[2], 30% of the patients were treated with BL monotherapy, while 61% received combination therapy consisting of a BL and an AG in 34% and BL with FQ in 18%. Triple therapy and regimens containing sulfamethoxazole-trimethoprim were administered in only 4% of cases[2]. In both studies, there was no comparison of mortality with regard to each antibiotic regimen chosen, but combination therapy did not show superiority compared to monotherapy[2].
Serratia marcescens is a gram-negative rod belonging to the family Enterobacteriaceae. This ubiquitous bacterium is a human opportunistic pathogen and has been implicated in septicaemia, ventilator-associated pneumonia, meningitis, endocarditis, and urinary tract and HCA wound infections[1,2,11-13]. This bacterium has the ability to proliferate in moist environments (such as disinfectants, intravenous solutions and different medical materials) and has therefore been responsible for nosocomial outbreaks[14]. Serratia marcescens has multiple pathogenicity and virulence factors: adhesins, lipopolysaccharides, fimbriae and siderophores, which facilitate host penetration, adherence to solid surfaces and resistance to serum killing[13,14]. Serratia marcescens carries a chromosomally encoded AmpC-type beta-lactamase that confers inducible resistance to beta-lactams when exposed to them[12]. It then readily hydrolyses penicillins and cephalosporins, including those of the third generation, and is responsible for the reduced activity of other beta-lactams[12]. This pathogen is also capable of acquiring mobile genetic elements encoding resistance determinants, such as extended-spectrum beta-lactamases or metallo beta-lactamases[14].
Since the 1990s, the English literature has only reported 15 cases of Serratia marcescens endocarditis in adults: In addition to 6 cases reported in the prospective studies discussed earlier[1,2], 9 case reports, including our case, have been published (Table 1). The mean age of the patients in the case reports is 53 years. In 4 of the 9 patients (44.4%), endocarditis was HCA[15-17]. It was community-acquired in the remaining 5 patients (55.6%), mainly due to IVD use[12,18-21]. Other risk factors identified were immunosuppression (44.4%), venous catheter presence (33.3%), recent cardiac surgery in one case and ICD presence in 2 patients. All patients were treated with combination beta-lactam-containing antibiotic regimens except for 3 patients treated with monotherapy: One patient received ceftriaxone, another meropenem, and the last was treated with ciprofloxacin. Only 2 patients benefited from valve replacement surgery. The patient with ICD-related endocarditis and our patient underwent percutaneous pacemaker extraction. Mortality in this series of case reports was 22%, with death attributed to massive heart failure with surgery contraindicated due to the presence of cerebral abscesses in one case and the second due to cerebral haemorrhage.
| Ref. | Age (yr) | Risk factors | Acquisition | Treatment and duration (wk) | Valve surgery | Outcome (follow-up) |
| Ena et al[15], 1991 | 29 | IVDU | CA | Ciprofloxacin (4 IV + 1 po) | Y | Survived (13 mo) |
| Körner et al[18], 1994 | 50 | Lymphoma, chemotherapy, CVC | HCA | Azlocillin/gentamycin | N7 | Survived (5 mo) |
| (6 IV) | ||||||
| Baggish et al[19], 2007 | 43 | Splenectomy | CA | Cefepime (6 IV) and gentamycin (2 IV) | Y | Survived (1 yr) |
| De Silva et al[20], 2009 | 67 | ICD | CA | Meropenem and gentamycin (NM), then ciprofloxacin (2 po) | N (ICD extraction) | Survived (6 mo) |
| Hadano et al[16], 2012 | 85 | Diabetes, corticosteroids | HCA | Ceftazidime (6 IV) and gentamycin (5 d) | N | Died |
| Lyall et al[17], 2013 | 65 | Post-Bentall + coronary bypass surgery | HCA | Meropenem and ciprofloxacin and gentamycin (NM) | N | Survived |
| Phadke et al[12], 2016 | 46 | IVDU, HIV | CA | Meropenem (NM) | N | Died |
| Meyer et al[21], 2018 | 42 | IVDU | CA | Ceftriaxone (6 IV) | Y | Survived |
| Current case, 2018 | 46 | ICD | HCA | Piperacillin-tazobactam IV and ciprofloxacin po (5), then ciprofloxacin and trimethoprim/sulfamethoxazole (3 po) | N (ICD extraction) | Survived (6 mo) |
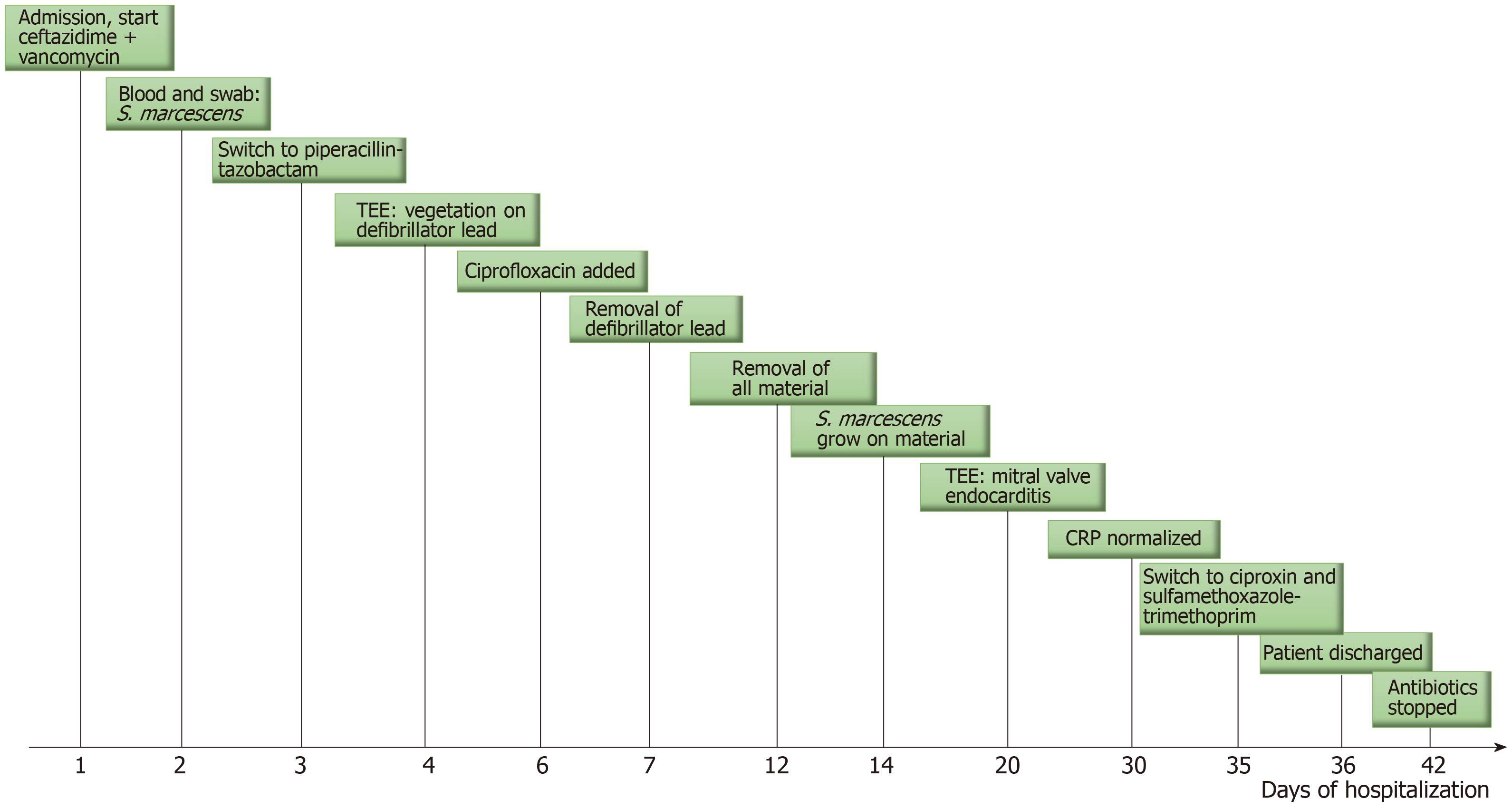
A 46-year-old man was admitted to the cardiology unit for suspicion of an intracardiac device (ICD) infection 4 mo after implantation. He suffered from congenitally corrected transposition of the great arteries with complete atrioventricular bloc, for which a bicavitary pacemaker had been placed in 1994. The patient developed cardiac insufficiency a few months before admission with non-sustained ventricular tachycardia. For these reasons, the pacemaker was upgraded into a defibrillator with resynchronisation. One month after that surgery, an incision site infection was diagnosed. Microbiological culture of the sample was positive for Serratia marcescens, and he was treated with local wound care.
Four days prior to his admission in our hospital, the patient presented to the emergency department of another hospital with high-grade fever (39°C), chills and a purulent exudate on the ICD site. Further clinical examination was without peculiarity. Chest X-ray and electrocardiogram evaluation revealed no abnormalities. Remarkable laboratory findings included a white blood cell count of 11900/μg (normal between 4000 and 10000/μg), a C-reactive protein level of 203 mg/L (normal < 10 mg/L), and normal renal and hepatic function. Empiric antimicrobial therapy was started with vancomycin and ceftazidime. On the second day of hospitalization, two pairs of blood cultures performed in the emergency department both yielded Serratia marcescens, as did a culture of the purulent exudate swab. Antimicrobial susceptibility testing showed the following results: Resistance to amoxicillin-clavulanate, cefuroxime and colistin and sensitivity to temocillin [minimal inhibitory concentration (MIC) ≤ 8 μg/mL], piperacillin-tazobactam (MIC ≤ 4 μg/mL), ceftazidime (MIC ≤ 0.012 μg/mL), cefotaxime (MIC ≤ 0.25 μg/mL), meropenem (MIC ≤ 0.25 μg/mL), gentamycin (MIC ≤ 1 μg/mL), amikacin (MIC ≤ 2 μg/mL), ciprofloxacin (MIC ≤ 0.25 μg/mL), and sulfamethoxazole-trimethoprim (MIC ≤ 20 μg/mL). According to these results, the antibiotic treatment was changed to piperacillin-tazobactam (4 g every 6 h) on day 3 of hospitalization.
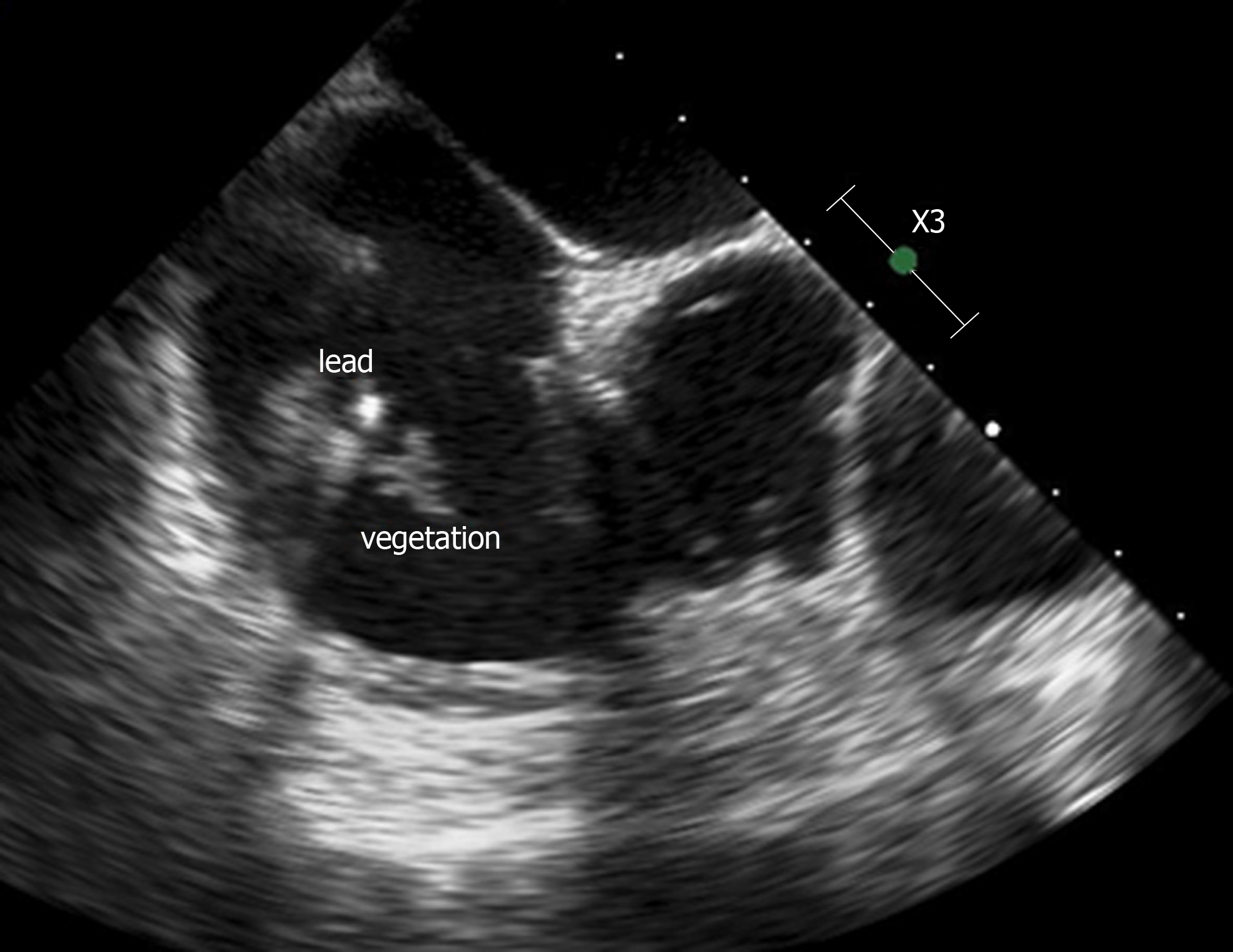
On admission in our hospital, on day 4, the physical examination revealed a temperature of 38.5°C, blood pressure of 112/75 mmHg, pulse of 95 beats/min, surgical site infection with pain, purulent exudate at the ICD site and no abnormal heart murmur. While blood culture performed at that time of admission revealed no more bacterial growth, a transoesophageal echocardiography (TEE) exam showed a vegetation of 16 mm x 8 mm on the defibrillator lead (Figure 1). Although the cardiac valves seemed undamaged, oral ciprofloxacin (500 mg every 12 h) was added to piperacillin-tazobactam on day 6. Furthermore, on day 7, wound debridement was performed, and the defibrillator lead was removed. Because TEE showed persistent images of vegetation on the old pacemaker leads on day 12, a decision was made to finally remove all material (ICD and pacemaker leads), with the exception of the epicardial lead, which was carefully cleaned and connected to a new provisional single chamber pacemaker. All perioperative samples and leads cultured were positive for Serratia marcescens. Despite the removal of all intracardiac material, a TEE carried out on day 20 revealed the presence of vegetation appended to the mitral valve, albeit without valve dysfunction. Finally, the diagnosis of ICD-related mitral valve endocarditis was made.
The dual antibiotic treatment was continued intravenously until 23 d after whole ICD removal. Blood analysis showed normalization of the CRP level and white blood count, and the patient was discharged with oral ciprofloxacin (750 mg every 12 h) and sulfamethoxazole-trimethoprim (800/160 mg every 12 h). Antibiotics were stopped on day 42 after ICD extraction, while TEE showed disappearance of the valvular vegetation (Figure 2). The patient is asymptomatic 10 mo after the completion of treatment and repeated TEE has not shown recurrence of endocarditis.
Serratia marcescens ICD-related mitral valve endocarditis.
Complete device removal associated with dual broad-spectrum antibiotic treatment with piperacillin-tazobactam and ciprofloxacin switched to oral sulfamethoxazole-trimethoprim and ciprofloxacin for 6 wk after complete device removal.
Cure, no recurrence at 1, 3 and 10 mo.
Although it has been shown that GNB non-AACEK endocarditis has increasingly become a concern of healthcare contact, community acquisition of Serratia marcescens endocarditis still remains an important issue with regard to previous and recent data[1,3,4,22]. IVD use and immunosuppression seem to be the most important risk factors for Serratia marcescens endocarditis. It is noteworthy that our case is the second case report of ICD-related endocarditis due to Serratia marcescens, but the prospective ICE and SEI studies do not detail risk factors for each bacterium[1,2]. In accordance with actual guidelines[8-10], we support a long-term dual-antibiotic treatment regimen containing a broad-spectrum beta-lactam in Serratia marcescens endocarditis, considering the presence of inducible AmpC-type beta-lactamase in these bacteria. As there is a risk of clinical failure, cephalosporins, including third-generation cephalosporins, must be avoided. Piperacillin-tazobactam given in our patient was shown to be as effective as meropenem or cefepime in AmpC-type beta-lactamase-producing Enterobacteriaceae[23]. Monotherapy with a beta-lactam should not be the first choice in GNB non-AACEK endocarditis; even if Morpeth et al[1] showed no difference in comparison to a combined antibiotic regimen with regard to mortality, the long-term outcome was not reported in this study. In a series of case reports, monotherapy was effective in two of three patients, but the long-term outcome of one patient who received ceftriaxone is unknown[12,15,21]. Even if most guidelines suggest combining a beta-lactam with aminoglycosides, its use must be outweighed because of potential nephro- and audiotoxicity, and the duration should be limited to 2 wk[10]. We chose to combine piperacillin-tazobactam with ciprofloxacin, a drug with good tissue penetration. Our patient was discharged with oral ciprofloxacin and sulfamethoxazole-trimethoprim after a total duration of 5 wk of IV combination antibiotic treatment (3 wk after ICD removal). This aspect of management could be debated. However, a recent study from Iversen et al[24] showed non-inferiority in patients suffering from endocarditis with a stable condition in which IV antibiotics were switched to oral treatment after 2 wk. As GNB endocarditis patients tend to have more cardiac complications (abscesses, larger vegetation size) than patients with endocarditis due to other pathogens[1,2], guidelines suggest that surgical management be considered early in the course of the disease[8-10]. While complete and early removal of an ICD is highly recommended in ICD-associated endocarditis[8,25], valve repair or replacement surgery in non-AACEK GNB endocarditis did not show better outcomes than medical treatment alone, except for in those patients who presented with cardiac complications[1]. The case series also showed good clinical outcomes even in the absence of valve surgery in 71% of cases. We therefore propose that valve surgery be discussed on an individual basis.
Globally, healthcare contact, immunosuppression and ICD presence are the major risk factors for GNB non-AACEK endocarditis[1,2]. This condition is of growing concern since people survive longer (at home) with more comorbidity than previously. Serratia marcescens, even if it has become an infrequent cause of GNB non-AACEK endocarditis, still should be suspected in the case of community-acquired endocarditis, especially in cases of IVDU. GNB non-AACEK endocarditis represents a challenging issue for clinicians since there are no clear guidelines[8-10]. The increase in multidrug resistant bacteria will certainly complicate treatment as well as outcomes even more. Awaiting further studies and in accordance with actual guidelines, we support treatment with a dual-antibiotic regimen containing broad spectrum beta-lactams in Serratia marcescens endocarditis, while valve surgery should be discussed early in the course of the disease on a case-by-case basis within a multidisciplinary team. Additional insights through prospective randomized trials about treatment in GNB non-AACEK endocarditis are urgently needed.
| 1. | Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, Nacinovich F, Tattevin P, Fernández-Hidalgo N, Dickerman S, Bouza E, del Río A, Lejko-Zupanc T, de Oliveira Ramos A, Iarussi D, Klein J, Chirouze C, Bedimo R, Corey GR, Fowler VG; International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) Investigators. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Falcone M, Tiseo G, Durante-Mangoni E, Ravasio V, Barbaro F, Ursi MP, Pasticci MB, Bassetti M, Grossi P, Venditti M, Rizzi M. Risk Factors and Outcomes of Endocarditis Due to Non-HACEK Gram-Negative Bacilli: Data from the Prospective Multicenter Italian Endocarditis Study Cohort. Antimicrob Agents Chemother. 2018;62:e02208-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Mills J, Drew D. Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse. Ann Intern Med. 1976;84:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Cooper R, Mills J. Serratia endocarditis. A follow-up report. Arch Intern Med. 1980;140:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Komshian SV, Tablan OC, Palutke W, Reyes MP. Characteristics of left-sided endocarditis due to Pseudomonas aeruginosa in the Detroit Medical Center. Rev Infect Dis. 1990;12:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Levine DP, Crane LR, Zervos MJ. Bacteremia in narcotic addicts at the Detroit Medical Center. II. Infectious endocarditis: a prospective comparative study. Rev Infect Dis. 1986;8:374-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 124] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Wieland M, Lederman MM, Kline-King C, Keys TF, Lerner PI, Bass SN, Chmielewski R, Banks VD, Ellner JJ. Left-sided endocarditis due to Pseudomonas aeruginosa. A report of 10 cases and review of the literature. Medicine (Baltimore). 1986;65:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL; Task Force per il Trattamento dell'Endocardite Infettiva della Società Europea di Cardiologia (ESC). [2015 ESC Guidelines for the management of infective endocarditis. The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)]. G Ital Cardiol (Rome). 2016;17:277-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 9. | Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 2234] [Article Influence: 203.1] [Reference Citation Analysis (1)] |
| 10. | Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherap. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2012;67:269-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 11. | Yu VL. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979;300:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 186] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Phadke VK, Jacob JT. Marvelous but Morbid: Infective endocarditis due to Serratia marcescens. Infect Dis Clin Pract (Baltim Md). 2016;24:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 394] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Iguchi A, Nagaya Y, Pradel E, Ooka T, Ogura Y, Katsura K, Kurokawa K, Oshima K, Hattori M, Parkhill J, Sebaihia M, Coulthurst SJ, Gotoh N, Thomson NR, Ewbank JJ, Hayashi T. Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genome Biol Evol. 2014;6:2096-2110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Ena J, Amador C, Parras F, Bouza E. Ciprofloxacin as an effective antibacterial agent in serratia endocarditis. J Infect. 1991;22:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Hadano Y, Kamiya T, Uenishi N. A fatal case of infective endocarditis caused by an unusual suspect: Serratia marcescens. Intern Med. 2012;51:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lyall DA, Gregory ME, McDonnell J, De Villiers F, Tejwani D. Bilateral endogenous Serratia marcescens endophthalmitis secondary to endocarditis following cardiac surgery. Scott Med J. 2013;58:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Körner RJ, Nicol A, Reeves DS, MacGowan AP, Hows J. Ciprofloxacin resistant Serratia marcescens endocarditis as a complication of non-Hodgkin's lymphoma. J Infect. 1994;29:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Baggish AL, Nadiminti H. Intracranial abscess from embolic Serratia marcescens endocarditis. Lancet Infect Dis. 2007;7:630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | De Silva K, Fife A, Murgatroyd F, Gall N. Pacemaker endocarditis: an important clinical entity. BMJ Case Rep. 2009;2009:bcr02.2009.1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Meyer CG, Vacek TP, Bansal A, Gurujal R, Parikh A. Dynamic Course of Serratia marcescens Pulmonic Valve Endocarditis Resulting in Submassive PE and Valve Replacement. J Investig Med High Impact Case Rep. 2018;6:2324709618759128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Laupland KB, Parkins MD, Gregson DB, Church DL, Ross T, Pitout JD. Population-based laboratory surveillance for Serratia species isolates in a large Canadian health region. Eur J Clin Microbiol Infect Dis. 2008;27:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Cheng L, Nelson BC, Mehta M, Seval N, Park S, Giddins MJ, Shi Q, Whittier S, Gomez-Simmonds A, Uhlemann AC. Piperacillin-Tazobactam versus Other Antibacterial Agents for Treatment of Bloodstream Infections Due to AmpC β-Lactamase-Producing Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61:e00276-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Iversen K, Ihlemann N, Gill SU, Madsen T, Elming H, Jensen KT, Bruun NE, Høfsten DE, Fursted K, Christensen JJ, Schultz M, Klein CF, Fosbøll EL, Rosenvinge F, Schønheyder HC, Køber L, Torp-Pedersen C, Helweg-Larsen J, Tønder N, Moser C, Bundgaard H. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N Engl J Med. 2019;380:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 564] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 25. | Sandoe JA, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, Olson E, Perry JD, Prendergast BD, Spry MJ, Steeds RP, Tayebjee MH, Watkin R; British Society for Antimicrobial Chemotherapy; British Heart Rhythm Society; British Cardiovascular Society; British Heart Valve Society; British Society for Echocardiography. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70:325-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Schwan WR S-Editor: Ma YJ L-Editor: A E-Editor: Qi LL