Published online May 25, 2017. doi: 10.5495/wjcid.v7.i2.11
Peer-review started: August 29, 2016
First decision: November 21, 2016
Revised: December 6, 2016
Accepted: January 20, 2017
Article in press: January 22, 2017
Published online: May 25, 2017
Processing time: 269 Days and 15 Hours
Periprosthetic joint infection (PJI) is considered one of the most challenging complications compromising patient health and is considered an economic burden. Despite all strategies PJI prevalence is between 1%-2%. Considerable efforts have been investigated in the past decade to diminish or erradicate PJI prevalence. This article manages the definition of PJI and the new major and minor criteria from Parvizi et al Then a scientific analysis of every minor and major criteria. Multidisciplinary management is reccommended according to guidelines. A numerous of surgical options exist each and everyone with its indications, contraindications and specific antibiotic therapy regimen. Surgical options are: (1) irrigation and cleaning with retention of the prosthesis with a success rate 0%-89%; (2) single-stage revision surgery with a succes rate of > 80%; and (3) two-stage revision surgery (authors preferred method) with a succes rate of 87%. Radical treatment options like arthrodesis and amputation are reserved for specific group of patients, with a succes rate varying from 60%-100%. The future of PJI is focused on improving the diagnostic tools and to combat biofilm. The cornerstone of management consists in a rapid diagnosis and specific therapy. This article presents the most current diagnostic and treatment criteria as well as the different surgical treatment options depending on the type of infection, bacterial virulence and patient comorbidities.
Core tip: The total replacement surgery is a highly effective surgery that improves the quality of life of patients. The periprosthetic infection is considered a devastating complication that increases patients morbidity, mortality and an economic burden. The cornerstone of management consists in a rapid diagnosis and specific therapy. This article presents the most current diagnostic and treatment criteria, as well as the different surgical treatment options depending on the type of infection, bacterial virulence and patient comorbidities.
- Citation: Vilchez-Cavazos F, Villarreal-Villarreal G, Peña-Martinez V, Acosta-Olivo C. Management of periprosthetic infections. World J Clin Infect Dis 2017; 7(2): 11-20
- URL: https://www.wjgnet.com/2220-3176/full/v7/i2/11.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v7.i2.11
Total joint replacement is a highly effective surgery that provides relief of pain, improves the range of motion, independence, and lastly, quality of life in the patient[1]. It is estimated that in 2030 a total of 4 million total hip and/or knee replacements will be done every year in the United States[2]. Prosthetic infections are considered a serious and devastating complication of total replacement; in general, the incidence of this complication is 1%-2%[3,4]. Nonetheless, there are reports ranging from 0.3% by the British Medical Research Council[5] up until 7%-16% in hip revision surgeries according to the Scandinavian Arthroplasty Report[6].
The key for the management of a prosthetic infection is based on an early diagnosis, which will allow adequate and fast treatment[7]. However, this represents a clinical burden, since the majority of the cases we are up against are complex, immunocompromised patients and antibiotic-resistant bacteria[8]. It also represents an economical burden since a prosthetic infection increments costs by 76% and 52% in total hip replacement and total knee replacement surgeries, respectively[9].
The objective of the present article is to update and summarize the diagnostic and therapeutic methods in periprosthetic joint infections (PJIs) in both knee and hip arthroplasty.
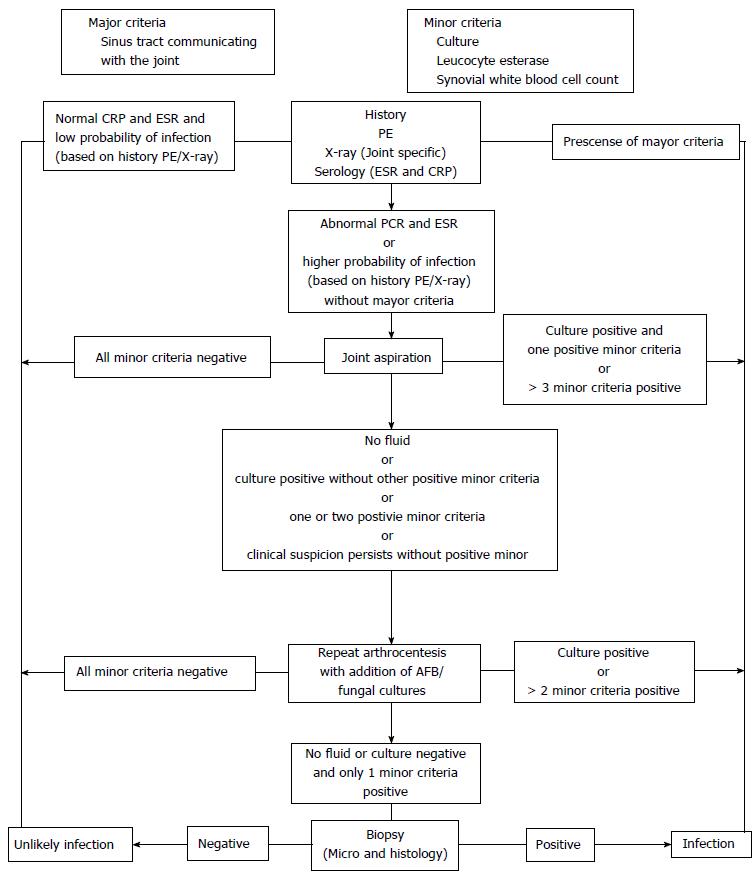
For the diagnosis of prosthetic infection a high suspicion and laboratory studies are needed. There is no gold standard for the diagnosis of prosthetic infections, rather a series of clinical findings, laboratory and imaging studies guide the diagnosis[8]. In 2011, the Musculoskeletal Infection Society proposed a series of major and minor criteria[10], the latter then modified by the International Consensus Meeting on PJIs to give a numeric value to the serological markers[11].
To consider the diagnosis a prosthetic infection, one of the following criteria must be met: (1) two positive periprosthetic cultures (fluid or tissue) for the same microorganism; (2) the presence of sinus tract that communicates with the joint; and (3) three of the following criteria exist: Increase of 100 mg/L of C-reactive protein (CRP) in an acute infection; > 10 mg/L in a chronic infection and a rise in the erythrocyte sedimentation rate (ESR) > 30 mm/h in a chronic infection (not applicable in acute infections); elevated synovial leukocyte count (> 10000 cells/μL in acute and > 3000 cells/μL in chronic infections) and/or ++ or more in Leukocyte esterase dipstick test; elevated synovial neutrophil percentage (PMN%); > 90% in acute and > 80% in chronic infections; positive preriprosthetic histological analysis (> 5 neutrophils per field); a single positive culture (fluid or tissue).
To achieve a systematic approach to diagnosing periprosthetic infections, in 2010 Della Valle et al[12] proposed an algorithm in the American Academy of Orthopedic Surgeons (Figure 1); changes have been made to this algorithm, such as the proposal of Parvizi et al[13] in 2016. However, in all cases this algorithm is only a tool and should never be considered a diagnosis. Any case of high clinical suspicion of infection should be subjected to this algorithm[11].
There are predisposing factors such as systemic malignancy, diabetes mellitus, rheumatoid arthritis, immunocompromised host, obesity, malnutrition, intravenous drug use, steroid therapy, systemic skin diseases, history of prior total replacement, and previous history of septic arthritis; intraoperative factors such as low body temperature, hypoxemia, duration of surgery, contaminated implants, and flow and configuration of the operating room; postoperative factors such as hematoma formation, transfusions, Foley catheter > 24 h as well as surgical site infection[14-16]. Clinically, patients with prosthetic infection usually present with pain, wound dehiscence and wound output[8]. However this varies significantly according to the evolution time and the pathogen involved[13]. Patients with less than 3 mo of evolution present with pain and rapidly progressive stiffness. On physical examination edema, erythema, warmth, increased sensibility and/or fever, effusion, surgical site infection and wound edge necrosis are usually present. Patients with 3-12 mo evolution usually present pain and slow but progressive stiffness. They are usually indistinguishable from aseptic loosening or present with an active fistula into the joint. In infections of > 12 mo of evolution the patient can present symptoms in two ways: (1) acute onset of pain and stiffness with a history of trauma or bacteremia (acute hematogenous infection); and (2) chronic pain and stiffness. The patients with acute hematogenous infections, clinically presents with more severe symptoms of pain, redness, warmth, increased tenderness and/or fever compared with patients with acute infections[13,17,18]. An important sign is fever, although it is considered a cardinal symptom of infection, it is reported that there may be an increase in the temperature of the postoperative patient of a total replacement surgery for up to five days and is considered as a physiological postsurgical process[19].
Because of their ease, fast delivery, and low cost, plain radiographs are the study of choice even if they have low sensitivity and specificity for the diagnosis of a prosthetic infection[13,20]. In regards to other studies, the evidence doesn’t show a routine use. For example magnetic resonance imaging, produces visual artifacts, is difficult to interpret and has a high cost. Ultrasound is limited to the acquisition of collections and is operator-dependent[20-22]. Except for plain radiographs, none of the aforementioned studies are part of the current recommendations for the management of prosthetic infection. The radiographic findings are easy to interpret and amongst them are: (1) focal osteolysis (radiolucency > 2 mm in the bone-metal interface or cement-bone); (2) loosening of the components; (3) cement fractures; and (4) subperiosteal reaction[20,23]. Regarding gammagraphy, there is no consensus for the use in diagnosis of periprosthetic infection; even the American Academy guidelines do not recommend its routine use[12].
In the current diagnostic criteria for infection, CRP and ESR are part of the minor criteria for prosthetic infection diagnosis and are studies every patient with high suspicion for prosthetic infection should undergo[11]. These markers can be elevated in patients with rheumatic or chronic inflammatory diseases. It is reported that an ESR > 30 mm per hour has a sensitivity of 82% and 85% specificity, a positive predictive value of 58% and a 95% negative predictive value. Meanwhile, CRP > 10 mg/L is associated with 96% and 92% sensitivity and specificity respectively; with a positive predictive value of 74% and a negative predictive value of 99%[24]. Another advantage offered by the CRP over the ESR is the return to normal values in 3 wk compared with ESR which can take up to a year[25,26]. The current recommendation is that all patients with suspected prosthetic infection undergo both serological studies, as the combination of these normal parameters is an excellent predictor of absence of infection and the combination of both positive tests approaches a 98% of diagnosis of prosthetic infection[24,27]. Finally it should be emphasized that the ESR has no diagnostic value in acute infections (< 6 wk) because it normally remains elevated after surgery for several weeks. Positive minor criteria are CRP > 100 mg/L in acute infections, and CRP > 10 mg/L and ESR > 30 mm in chronic infections[11,13].
After the initial approach to the suspected diagnosis of prosthetic infection, including clinical history, physical examination, and initial laboratory and imaging studies, the next step is a diagnostic arthrocentesis, specifically a cell count to determine percentage of polymorphonuclear leukocytes (PMN), leukocyte esterase levels and a synovial fluid culture[13,21]. According Parvizi et al[13] a percentage of PMN above 65% has 97% sensitivity and 98% specificity for the diagnosis of prosthetic infections. As for the leukocyte count in the synovial fluid, figures above 4200/μL have sensitivity of 84% and a specificity of 93% for the diagnosis of infection.
The leukocyte esterase dipstick test is a fast, cheap and reproducible test. It consists of dipping a urinary test strip in the previously collected synovial fluid, leaving it submerged for two minutes and then interpreting the result according to color change. Leukocyte esterase is an enzyme released by neutrophils in response to infection[10,28]. It is reported that a leukocyte esterase value of ++ has a sensitivity of 81%, specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 93% for the diagnosis of prosthetic infection.
The synovial fluid culture is a routine test within the studies in diagnostic arthrocentesis, performed to ensure specific antibiotic treatment for the infecting pathogen. This study has a sensibility of 86%-92% and a specificity of 82%-97%[29,30]. The use of a Petri dish is preferred because of its sensibility (90.92%) over intraoperative cultures in swabs or sterile containers (77%-82% sensibility)[31]. For optimal results, the following recommendations are made: (1) withhold antimicrobial therapy 2 wk prior sampling; and (2) prolong incubation period cultures at least two weeks for a definitive result[32,33]. However, it should be emphasized that the preoperative dose of antibiotic prophylaxis should not be suspended because it does not affect the sensitivity of intraoperative culture, in case the necessary diagnostic arthrocentesis sample was not obtained[34]. The full analysis of synovial fluid: Leukocyte count, PMN percentage, leukocyte esterase, and synovial culture, are part of the minor criteria for diagnosis of prosthetic infection and should be taken routinely in every patient[1,11,13].
Another minor criterion for the diagnosis of periprosthetic infection is tissue biopsy[1,11,13]. As definition, a biopsy is considered positive when: It contains 5-10 PMN per high-power field in at least 5 different fields[21]. There are low-virulence bacteria that may be present in the simple and be reported as an inflammatory reaction or fibrosis. These bacteria are Propionibacterium acnes and coagulase-negative staphylococci, and may not be reported as positive findings. For this study, it is recommended to: (1) send 3-6 samples; and (2) take the sample with dissection techniques without the use of cautery (risk of false positives)[11].
The management of prosthetic infection requires surgical intervention and prolonged periods of intravenous or oral antibiotics[1,35]. There is a lot of basic science and clinical research dedicated to the treatment of prosthetic infections; nonetheless, there are still many doubts as to how to treat them. Multidisciplinary management (orthopedist, infectious disease specialists, plastic surgeons) is of vital importance in these cases, as is following the consensus of therapeutic guidelines to diminish costs and morbidity and mortality in the patient[1]. There are several surgical options for treating prosthetic infections depending on the type of infection, virulence of the pathogen, and health status of the patient: (1) debridement, irrigation and cleaning with retention of the prosthesis; (2) single-stage revision surgery; (3) two-stage revision surgery; (4) arthrodesis; and (5) amputation[1]. So far, there are no randomized clinical trials where these surgical techniques are evaluated; most studies include patients from only one hospital, are non-comparative and decisions are based on cohort studies or case-control studies[1,36]. No matter the method of treatment, a prosthetic infection is not considered an emergency procedure (except in patient with sepsis). The patient must be in optimal condition for surgery, have normal glycaemia, hemoglobin > 10 mg/dL, and should be in optimal conditions for surgery[37].
This technique has specific indications: (1) infection < 30 d in duration; (2) implants without evidence of loosening; (3) acute hematogenous infection; and (4) that the prosthesis was placed < 3 mo prior[1,38]. Contraindications include: (1) wound not closing on first intention; (2) presence of a fistula; and (3) evidence of prosthesis loosening. Relative contraindications are: (1) infection with highly virulent organisms (Methicillin-resistant Staphylococcus aureus MRSA); (2) polymicrobial infection; and (3) immunocompromised patients[17,39,40]. In a systematic review by Romanò et al[41] it was estimated that the success rate with this method varies between 0%-89%. There are factors that increase the success rate of the procedure such as infection by organisms of low virulence, rapid surgical treatment of patient with acute symptoms (less than 72 h and antibiotic treatment administered in the first month post-debridement[17,38,42,43].
During surgery, the same approach that was used for the placement of the prosthesis is performed[37,44]. By incising the deep dissection plane a better visualization of the structures is achieved[45]; the mobile components of the prosthesis are removed. When the modular components are removed, access to the surfaces underneath is achieved[17,38,46,47], 3-6 samples for culture and histology studies are taken[1,13], then the surgical site is irrigated with 6-9 L to avoid trauma to adjacent structures[37,48].
Medical treatment with antibiotic therapy is critical after surgery[17,43]. Various authors recommend rifampin combination with the antibiotic of choice. This is due to the action of rifampicin against biofilm, although there is no consensus as to when is the best time to start this treatment; several authors recommend initiating use in conjunction with intravenous antibiotic therapy in order to reduce the risk of selecting resistant mutants, others recommend to start rifampicin when oral antibiotics are started[49-51]. In a double-blind study by Zimmerli et al[51], acute infections by Staphylococcus aureus associated to orthopedic implants were treated with debridement, irrigation, cleaning and implant retention, combined with ciprofloxacin (750 mg/12 h) and rifampicin (450 mg/12 h) compared against ciprofloxacin as monotherapy (750 mg/12 h); finding a cure rate (100% hip and knee replacement 53%) higher when rifampicin is added with P < 0.05 at 35 mo follow-up[51]. When the microorganism is Staphylococcus aureus or coagulase-negative Staphylococcus and the germ is sensible, several studies recommend the combination of rifampin with fluoroquinolones[43,50-53]. Within fluoroquinolones, the one that best interacts with rifampicin is levofloxacin[54]. When talking about a MRSA, available information is very limited, however, studies report good results with the combination with rifampicin[55]. The combination of linezolid plus rifampicin reported cure rates of 60%[56-58]. However, its use is not recommended for more than six weeks due to toxicity and follow-up serum levels are necessary[59].
As for the duration of antibiotic therapy, the current trend is an initial intravenous therapy of 2-6 wk maximum, followed by 3 to 6 mo of oral antibiotics depending if it is a total hip or knee replacement[1,35]. The rapid change of intravenous to oral antibiotics (7-15 d) allows an early discharge for the patient and avoids catheter-associated infections[49]; this reports a success rate of over 70%[46,50,53]. Some authors recommend a treatment with intravenous antibiotics of less than 3 mo with similar success rates of over 70%[50,60]. However, it is an issue that is still under discussion and more information is needed to this[49].
This type of procedure is not common in the United States, it is more common in Europe[1,61]. The indications for this technique are: (1) relatively healthy patients; (2) insignificant bone loss; (3) viable soft tissue; (4) low virulence microorganism (sensitive Streptococcus aureus, Enterococci, not infections by Pseudomonas or gram-negative bacteria); and (5) that the microorganism is susceptible to oral antibiotics with excellent bioavailability[1,61,62]. The advantages of this technique are: (1) lower cost for the patient/hospital/insurance system; (2) avoidance of a second surgery (in comparison with two-stage revision surgery); and (3) lower morbidity rates[63].
The technique consists of removing all of the pro–sthetic components including the cement (polymethyl methacrylate) aggressive debridement of soft and bone tissue (this being the most important factor). The placement of a new prosthesis, using antibiotic-loaded cement. This technique reports a success rate above 80%[63-65].
The medical treatment for single-stage revision surgery consists of administration of specific intravenous antibiotic treatment for 2-6 wk combined with oral rifampicin and changing the treatment to oral antibiotics for 3 mo. The success rate for this regimen is calculated between 80%-100% and two different approaches for treating these patients are described: (1) identification of the pathogen previous to surgery, followed by 4-6 wk of intravenous/oral antibiotic treatment (high bioavailability) followed by replacement of the prosthesis; and (2) in aseptic loosening, the prosthetic infection is confirmed by cultures, followed by intravenous antibiotic treatment combined with rifampicin[62,66,67].
This is the technique of choice in the United States for the treatment of chronic periprosthetic infections[68-71]. The ideal patient and the indications for this technique are: (1) chronic prosthetic infection; (2) insignificant bone loss; (3) patient in adequate conditions for surgery; (4) patient willing to undergo two surgeries; (5) patients with active fistula; and (6) high-virulence microorganisms (MRSA, Candida)[35,68,72]. This technique reports a success rate of 87%[1,73].
This surgical technique consists of aggressive debridement, removal of all prosthetic components including the cement (polymethyl methacrylate). Subsequently, a cement spacer with antibiotics is placed in block or articulated (to keep space and avoid future soft tissue contractures)[74,75]; in the second stage the cement spacer is removed and a new prosthesis is placed only if there is no evidence of infection. In case of infection, debridement, irrigation and cleaning should be performed again.
Regarding the medical treatment and the time of placement of the second prosthesis, reports vary from two to several months[70,74]. The most used strategy is 4-6 wk of intravenous antibiotic treatment (6 wk for Staphylococcus aureus) followed by 2-8 wk with no antibiotic treatment, obtaining good results[76-79]; in this case, rifampicin is not used, since the components with biofilm were removed[1].
This is a useful treatment but has few indications; it involves the arthrodesis of the limb to allow ambulation and avoid amputation. The indications for this treatment are: (1) non-walking patients; (2) significant bone loss; (3) little and poor quality soft tissue; (4) high-virulence infections (low bioavailability antibiotics); (5) poor general condition of the patient; and (6) failure of two-stage revision surgery[1]. Arthrodesis is achieved by an intramedullary rod or an external fixator[80]. An eradication rate of 60%-100% is reported. Medical treatment involves the administration of intravenous or oral antibiotics (high bioavailability) for 4-6 wk[1].
This treatment is reserved for select group of patients and its indications are: (1) necrotizing fasciitis (not responding to debridement); (2) severe bone loss; (3) soft tissue defect that could be closed primarily; (4) failed attempts at resection and arthrodesis; and (5) non-walking patients[1,73,81]. The technique consists of amputation or disarticulation above the affected areas. The medical treatment consists of antibiotic treatment for 24-48 h if clean and non-contaminated edges were achieved during surgery. In case of bacteremia, sepsis or inadequate debridement, intravenous or oral antibiotic treatment should be continued for 4-6 wk[1].
Despite all initiatives and actions against prosthetic infections, the general incidence of infection ranges between 1%-2%[3,4]. Most actions are focused on improving the diagnostic tools and to combat biofilm[4,13].
Regarding the future of diagnostic imaging studies, the positron emission tomography (PET) scan is the imaging study that provides the most information for the diagnosis of prosthetic infections. The problem with PET scan is the variability of results that has been reported. In a meta-analysis by Kwee et al[82] composed of 11 studies, the PET scan reported a sensibility of 82.1% and a specificity of 86.6% for the diagnosis of prosthetic infection, concluding that there was great heterogeneity in the percentages reported by the studies. However, there are more recent studies that report a sensibility of 95% and a specificity of 98%[83]. More studies are needed to find the real value of PET scan for it to be a part of the diagnostic tools for prosthetic infections[13].
The most important biomarkers for the diagnosis of prosthetic infection are CRP and ESR[10,11,13]. However, interleucin-6 (IL-6) has been reported as an excellent marker for prosthetic infection, even above CRP and ESR. The advantage IL-6 offers is a return to normal levels within days, compared with weeks for CRP and months for ESR[84].
Diagnostic arthrocentesis is the method from which samples are taken for evaluating major and minor criteria for prosthetic infection[10,13]. Research shows that a CRP ELISA of synovial fluid is superior compared to serologic CRP, with a sensibility and specificity of 85%-97% (synovial CRP ELISA) vs 76%-93% (serologic CRP)[85]. But nevertheless, the best biomarker obtained from synovial fluid with reports of a sensibility and specificity of 100% is alpha-defensin[86,87]. This marker is a peptide secreted by the cells in response to microbial byproducts. The advantage it offers is that it is not influenced by inflammatory response nor by antibiotics; it is necessary to keep researching this test for it to be recommended generally[86,87].
As to perioperative tools/strategies to lower the periprosthetic infection there is the covering of prosthetic surfaces with silver ions. It has been reported that silver ions have antimicrobial properties when used in cream, gel and impregnated gauzes for the treatment of ulcers and wounds[88,89]. In a study by Gordon et al[90] the team designed a metallic prosthesis impregnated with silver polymers which showed in vitro activity against biofilm. Another strategy is the covering of the prosthesis with antibiofilm agents; biofilm is defined as a protective membrane of polysaccharides, polypeptides and nucleic acids that create an ideal microenvironment for the reproduction of bacteria and makes them resistant to antibiotics and the patient’s immune system[91,92].
Extensive research has been made about therapies directed specifically to combating the physical integrity of the biofilm such as Deoxyribonuclease I (DNase I) and Dispersin B[93]. DNase I degrades extranuclear DNA, which causes the firmness and stability of the biofilm. Dispersin B is directed against the intracellular adhesin produced by the biofilm[94]; its effects have been proved against S. aeureus, S. epidermidis, and E. coli[94,95].
Regarding intraoperative therapies; disposable antibacterial coating (DAC) is used in the bone-prosthesis interface. DAC is an hydrogel made of hyaluronic acid and polylactic acid to which specific antibiotics against the microorganism can be added; a great advantage since a high dose of antibiotics are added to the surgical site. This gel is smeared on the prosthesis (with no cement) prior to placement and it is reported to release antibiotics for up until 96 h[96].
Prosthetic infections continue to be a devastating complication for patients, health systems and the medical teams who handle these cases. Despite the progress made in diagnostic tools and the unification of criteria for creating treatment algorithms, the management of these cases is still a challenge for the orthopedic surgeon. It is expected that in the near future, better diagnostic tools for prosthetic infections will be created.
Clinical suspicion of the orthopedic surgeon is the cornerstone for achieving a quick diagnosis and choosing the ideal treatment; early diagnosis in acute infections is essential to preserve the prosthesis. In chronic infections, two-stage revision surgery is the treatment of choice in the vast majority of cases.
The current tendency is to reduce the intravenous antibiotic treatment when the bacteria involved are susceptible to oral antibiotics with ample bioavailability and to asses the duration of antibiotic treatment according to the patient’s clinical response, with satisfactory results, with the benefit of shorter hospital stays, decreased complications of catheter use and reduced side effects of prolonged intravenous antibiotic therapy.
| 1. | Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1505] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 2. | Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2079] [Cited by in RCA: 3317] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 3. | Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2006;1-209. [PubMed] |
| 4. | George DA, Gant V, Haddad FS. The management of periprosthetic infections in the future: a review of new forms of treatment. Bone Joint J. 2015;97-B:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lidwell OM. Clean air at operation and subsequent sepsis in the joint. Clin Orthop Relat Res. 1986;91-102. [PubMed] |
| 6. | Lucht U. The Danish Hip Arthroplasty Register. Acta Orthop Scand. 2000;71:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85-A Suppl 1:S75-S80. [PubMed] |
| 8. | Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 782] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 10. | Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992-2994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1212] [Cited by in RCA: 1514] [Article Influence: 100.9] [Reference Citation Analysis (1)] |
| 11. | Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD. Diagnosis of periprosthetic joint infection. J Orthop Res. 2014;32 Suppl 1:S98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, Keith M, Turkelson CM. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:760-770. [PubMed] |
| 13. | Parvizi J, Fassihi SC, Enayatollahi MA. Diagnosis of Periprosthetic Joint Infection Following Hip and Knee Arthroplasty. Orthop Clin North Am. 2016;47:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Daines BK, Dennis DA, Amann S. Infection prevention in total knee arthroplasty. J Am Acad Orthop Surg. 2015;23:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247-1254. [PubMed] |
| 16. | Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res. 1984;117-126. [PubMed] |
| 17. | Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodríguez-Pardo D, Horcajada JP. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56:182-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 18. | Vilchez F, Martínez-Pastor JC, García-Ramiro S, Bori G, Tornero E, García E, Mensa J, Soriano A. Efficacy of debridement in hematogenous and early post-surgical prosthetic joint infections. Int J Artif Organs. 2011;34:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Shaw JA, Chung R. Febrile response after knee and hip arthroplasty. Clin Orthop Relat Res. 1999;181-189. [PubMed] |
| 20. | Lima AL, Oliveira PR, Carvalho VC, Saconi ES, Cabrita HB, Rodrigues MB. Periprosthetic joint infections. Interdiscip Perspect Infect Dis. 2013;2013:542796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Tsaras G, Maduka-Ezeh A, Inwards CY, Mabry T, Erwin PJ, Murad MH, Montori VM, West CP, Osmon DR, Berbari EF. Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2012;94:1700-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Sofka CM. Current applications of advanced cross-sectional imaging techniques in evaluating the painful arthroplasty. Skeletal Radiol. 2007;36:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Tigges S, Stiles RG, Roberson JR. Appearance of septic hip prostheses on plain radiographs. AJR Am J Roentgenol. 1994;163:377-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672-683. [PubMed] |
| 25. | Shih LY, Wu JJ, Yang DJ. Erythrocyte sedimentation rate and C-reactive protein values in patients with total hip arthroplasty. Clin Orthop Relat Res. 1987;238-246. [PubMed] |
| 26. | Sastre S, SorianoÀ , Garcia S, Martínez J-A, Suso S, Mensa J. Serum C-reactive protein as predictor of infected arthroplasty. Eur J Orthop Surg Traumatol. 2006;16:17-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Ghanem E, Antoci V, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13:e444-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for musculoskeletal infection society criteria. J Bone Joint Surg Am. 2014;96:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Spangehl MJ, Younger AS, Masri BA, Duncan CP. Diagnosis of infection following total hip arthroplasty. Instr Course Lect. 1998;47:285-295. [PubMed] |
| 30. | Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 31. | Larsen LH, Lange J, Xu Y, Schønheyder HC. Optimizing culture methods for diagnosis of prosthetic joint infections: a summary of modifications and improvements reported since 1995. J Med Microbiol. 2012;61:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Malekzadeh D, Osmon DR, Lahr BD, Hanssen AD, Berbari EF. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin Orthop Relat Res. 2010;468:2039-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Schwotzer N, Wahl P, Fracheboud D, Gautier E, Chuard C. Optimal culture incubation time in orthopedic device-associated infections: a retrospective analysis of prolonged 14-day incubation. J Clin Microbiol. 2014;52:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Ghanem E, Parvizi J, Clohisy J, Burnett S, Sharkey PF, Barrack R. Perioperative antibiotics should not be withheld in proven cases of periprosthetic infection. Clin Orthop Relat Res. 2007;461:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2193] [Cited by in RCA: 2239] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 36. | Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Haasper C, Buttaro M, Hozack W, Aboltins CA, Borens O, Callaghan JJ, Ivo de Carvalho P, Chang Y, Corona P, Da Rin F. Irrigation and debridement. J Orthop Res. 2014;32 Suppl 1:S130-S135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Odum SM, Fehring TK, Lombardi AV, Zmistowski BM, Brown NM, Luna JT, Fehring KA, Hansen EN. Irrigation and debridement for periprosthetic infections: does the organism matter? J Arthroplasty. 2011;26:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Zmistowski B, Fedorka CJ, Sheehan E, Deirmengian G, Austin MS, Parvizi J. Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty. 2011;26:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Westberg M, Grøgaard B, Snorrason F. Early prosthetic joint infections treated with debridement and implant retention: 38 primary hip arthroplasties prospectively recorded and followed for median 4 years. Acta Orthop. 2012;83:227-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Romanò CL, Manzi G, Logoluso N, Romanò D. Value of debridement and irrigation for the treatment of peri-prosthetic infections. A systematic review. Hip Int. 2012;22 Suppl 8:S19-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Aboltins CA, Page MA, Buising KL, Jenney AW, Daffy JR, Choong PF, Stanley PA. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect. 2007;13:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;125-131. [PubMed] |
| 44. | Van Kleunen JP, Knox D, Garino JP, Lee GC. Irrigation and débridement and prosthesis retention for treating acute periprosthetic infections. Clin Orthop Relat Res. 2010;468:2024-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Schwechter EM, Folk D, Varshney AK, Fries BC, Kim SJ, Hirsh DM. Optimal irrigation and debridement of infected joint implants: an in vitro methicillin-resistant Staphylococcus aureus biofilm model. J Arthroplasty. 2011;26:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63:1264-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 47. | Koyonos L, Zmistowski B, Della Valle CJ, Parvizi J. Infection control rate of irrigation and débridement for periprosthetic joint infection. Clin Orthop Relat Res. 2011;469:3043-3048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 48. | Muñoz-Mahamud E, García S, Bori G, Martínez-Pastor JC, Zumbado JA, Riba J, Mensa J, Soriano A. Comparison of a low-pressure and a high-pressure pulsatile lavage during débridement for orthopaedic implant infection. Arch Orthop Trauma Surg. 2011;131:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | O'Toole P, Osmon D, Soriano A, Berdal JE, Bostrum M, Franco-Cendejas R, Huang D, Nelson C, Nishisaka F, Roslund B. Oral antibiotic therapy. J Orthop Res. 2014;32 Suppl 1:S152-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Vilchez F, Martínez-Pastor JC, García-Ramiro S, Bori G, Maculé F, Sierra J, Font L, Mensa J, Soriano A. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Zimmerli W, Frei R, Widmer AF, Rajacic Z. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J Antimicrob Chemother. 1994;33:959-967. [PubMed] |
| 52. | Cobo J, Miguel LG, Euba G, Rodríguez D, García-Lechuz JM, Riera M, Falgueras L, Palomino J, Benito N, del Toro MD. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy. Clin Microbiol Infect. 2011;17:1632-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 53. | Laffer RR, Graber P, Ochsner PE, Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect. 2006;12:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Anderl JN, Zahller J, Roe F, Stewart PS. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2003;47:1251-1256. [PubMed] |
| 55. | Tornero E, García-Oltra E, García-Ramiro S, Martínez-Pastor JC, Bosch J, Climent C, Morata L, Camacho P, Mensa J, Soriano A. Prosthetic joint infections due to Staphylococcus aureus and coagulase-negative staphylococci. Int J Artif Organs. 2012;35:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Bassetti M, Vitale F, Melica G, Righi E, Di Biagio A, Molfetta L, Pipino F, Cruciani M, Bassetti D. Linezolid in the treatment of Gram-positive prosthetic joint infections. J Antimicrob Chemother. 2005;55:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Gómez J, Canovas E, Baños V, Martínez L, García E, Hernández-Torres A, Canteras M, Ruiz J, Medina M, Martínez P. Linezolid plus rifampin as a salvage therapy in prosthetic joint infections treated without removing the implant. Antimicrob Agents Chemother. 2011;55:4308-4310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Soriano A, Gómez J, Gómez L, Azanza JR, Pérez R, Romero F, Pons M, Bella F, Velasco M, Mensa J. Efficacy and tolerability of prolonged linezolid therapy in the treatment of orthopedic implant infections. Eur J Clin Microbiol Infect Dis. 2007;26:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Pea F, Furlanut M, Cojutti P, Cristini F, Zamparini E, Franceschi L, Viale P. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother. 2010;54:4605-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 60. | Achermann Y, Eigenmann K, Ledergerber B, Derksen L, Rafeiner P, Clauss M, Nüesch R, Zellweger C, Vogt M, Zimmerli W. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): a matched case-control study. Infection. 2013;41:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Hanssen AD, Osmon DR. Assessment of patient selection criteria for treatment of the infected hip arthroplasty. Clin Orthop Relat Res. 2000;91-100. [PubMed] |
| 62. | Ure KJ, Amstutz HC, Nasser S, Schmalzried TP. Direct-exchange arthroplasty for the treatment of infection after total hip replacement. An average ten-year follow-up. J Bone Joint Surg Am. 1998;80:961-968. [PubMed] |
| 63. | Wolf CF, Gu NY, Doctor JN, Manner PA, Leopold SS. Comparison of one and two-stage revision of total hip arthroplasty complicated by infection: a Markov expected-utility decision analysis. J Bone Joint Surg Am. 2011;93:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;101-105. [PubMed] |
| 65. | Carlsson AS, Josefsson G, Lindberg L. Revision with gentamicin-impregnated cement for deep infections in total hip arthroplasties. J Bone Joint Surg Am. 1978;60:1059-1064. [PubMed] |
| 66. | Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512-523. [PubMed] |
| 67. | Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Osmon DR. Prosthetic joint infection diagnosed postoperatively by intraoperative culture. Clin Orthop Relat Res. 2005;439:38-42. [PubMed] |
| 68. | Bejon P, Berendt A, Atkins BL, Green N, Parry H, Masters S, McLardy-Smith P, Gundle R, Byren I. Two-stage revision for prosthetic joint infection: predictors of outcome and the role of reimplantation microbiology. J Antimicrob Chemother. 2010;65:569-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 69. | Biring GS, Kostamo T, Garbuz DS, Masri BA, Duncan CP. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10- to 15-year follow-up study. J Bone Joint Surg Br. 2009;91:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 70. | Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 71. | Toulson C, Walcott-Sapp S, Hur J, Salvati E, Bostrom M, Brause B, Westrich GH. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol: update on “our institution’s” experience from 1989 to 2003. J Arthroplasty. 2009;24:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 72. | Müller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. Diagnosis of periprosthetic infection following total hip arthroplasty--evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J Orthop Surg Res. 2008;3:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Sia IG, Berbari EF, Karchmer AW. Prosthetic joint infections. Infect Dis Clin North Am. 2005;19:885-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Mabry TM, Hanssen AD. Articulating antibiotic spacers: a matter of personal preference. Orthopedics. 2007;30:783-785. [PubMed] |
| 75. | Bloomfield MR, Klika AK, Barsoum WK. Antibiotic-coated spacers for total hip arthroplasty infection. Orthopedics. 2010;33:649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Brandt CM, Duffy MC, Berbari EF, Hanssen AD, Steckelberg JM, Osmon DR. Staphylococcus aureus prosthetic joint infection treated with prosthesis removal and delayed reimplantation arthroplasty. Mayo Clin Proc. 1999;74:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Westrich GH, Walcott-Sapp S, Bornstein LJ, Bostrom MP, Windsor RE, Brause BD. Modern treatment of infected total knee arthroplasty with a 2-stage reimplantation protocol. J Arthroplasty. 2010;25:1015-1021, 1021.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434-1445. [PubMed] |
| 79. | Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81:684-689. [PubMed] |
| 80. | Mabry TM, Jacofsky DJ, Haidukewych GJ, Hanssen AD. Comparison of intramedullary nailing and external fixation knee arthrodesis for the infected knee replacement. Clin Orthop Relat Res. 2007;464:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Fedorka CJ, Chen AF, McGarry WM, Parvizi J, Klatt BA. Functional ability after above-the-knee amputation for infected total knee arthroplasty. Clin Orthop Relat Res. 2011;469:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Kwee TC, Kwee RM, Alavi A. FDG-PET for diagnosing prosthetic joint infection: systematic review and metaanalysis. Eur J Nucl Med Mol Imaging. 2008;35:2122-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | Kobayashi N, Inaba Y, Choe H, Ike H, Fujimaki H, Tezuka T, Hirata Y, Tateishi U, Inoue T, Saito T. Use of F-18 fluoride PET to differentiate septic from aseptic loosening in total hip arthroplasty patients. Clin Nucl Med. 2011;36:e156-e161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, Steckelberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92:2102-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 85. | Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry Award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Enayatollahi MA, Parvizi J. Diagnosis of infected total hip arthroplasty. Hip Int. 2015;25:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE, Parvizi J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473:198-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 88. | Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 739] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 89. | Dunn K, Edwards-Jones V. The role of Acticoat with nanocrystalline silver in the management of burns. Burns. 2004;30 Suppl 1:S1-S9. [PubMed] |
| 90. | Gordon O, Vig Slenters T, Brunetto PS, Villaruz AE, Sturdevant DE, Otto M, Landmann R, Fromm KM. Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob Agents Chemother. 2010;54:4208-4218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 91. | Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985;67:264-273. [PubMed] |
| 92. | Prosser BL, Taylor D, Dix BA, Cleeland R. Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrob Agents Chemother. 1987;31:1502-1506. [PubMed] |
| 93. | Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int J Artif Organs. 2009;32:545-554. [PubMed] |
| 94. | Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Efficacy of combination of chlorhexidine and protamine sulphate against device-associated pathogens. J Antimicrob Chemother. 2008;61:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J Antimicrob Chemother. 2009;64:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 96. | Drago L, Boot W, Dimas K, Malizos K, Hänsch GM, Stuyck J, Gawlitta D, Romanò CL. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res. 2014;472:3311-3323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Anand A, Cui Q, Drosos GI S- Editor: Ji FF L- Editor: A E- Editor: Li D