Published online Nov 22, 2023. doi: 10.5495/wjcid.v13.i4.37
Peer-review started: October 3, 2023
First decision: October 24, 2023
Revised: November 2, 2023
Accepted: November 13, 2023
Article in press: November 13, 2023
Published online: November 22, 2023
Processing time: 49 Days and 16 Hours
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to millions of confirmed cases and deaths worldwide. Elderly patients are at high risk of developing and dying from COVID-19 due to advanced age, decreased immune function, intense inflammatory response, and comorbidities. Shanghai has experienced a wave of infection with Omicron, a new variant of SARS-CoV-2, since March 2022. There is a pressing need to identify clinical features and risk factors for disease progre
To investigate clinical characteristic differences and risk factors between elderly patients with severe and nonsevere Omicron SARS-CoV-2 variant infection.
A total of 328 elderly patients with COVID-19 admitted to the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine from April 2022 to June 2022 were enrolled and divided into a severe group (82 patients) and a nonsevere group (246 patients) according to the diagnosis and treatment protocol of COVID-19 (version 7). The clinical data and laboratory results of both groups were collected and compared. A chi-square test, t test, Mann-Whitney U test, hierarchical log-rank test, univariate and multivariate logistic regression, and hierarchical analyses were used to determine significant differences.
The severe group was older (84 vs 74 years, P < 0.001), included more males (57.3% vs 43.9%, P = 0.037), had a lower vaccination rate (P < 0.001), and had a higher proportion of comorbidities, including chronic respiratory disease (P = 0.001), cerebral infarction (P < 0.001), chronic kidney disease (P = 0.002), and neurodegenerative disease (P < 0.001), than the nonsevere group. In addition, severe disease patients had a higher inflammatory index (P < 0.001), greater need for symptomatic treatment (P < 0.001), longer hospital stay (P = 0.011), extended viral shedding time (P = 0.014), and higher mortality than nonsevere disease patients (P < 0.001). No difference was observed in the application of Paxlovid in the severe and nonsevere groups (P = 0.817). Oxygen saturation, cerebral infarction, and D-dimer were predictive factors for developing severe disease in patients with COVID-19, with D-dimer having an excellent role (area under the curve: 90.1%, 95%CI: 86.1-94.0%). In addition, D-dimer was a risk factor for developing severe COVID-19 according to multivariate stratified analysis.
The clinical course of severe COVID-19 is complex, with a higher need for symptomatic treatment. D-dimer is a suitable biomarker for identifying patients at risk for developing severe COVID-19.
Core Tip: Since March 2022, the Omicron wave has affected Shanghai, China. Many elderly patients with severe and nonsevere Omicron severe acute respiratory syndrome coronavirus 2 variant infections have been admitted to our hospital. These patients have a precise diagnosis, complete examination, and clear treatment results. After China adjusts its coronavirus prevention and control policies in 2023, findings such as those in this article will no longer be available.
- Citation: Liu XQ, Lu GZ, Yin DL, Kang YY, Zhou YY, Wang YH, Xu J. Analysis of clinical characteristics and risk factors between elderly patients with severe and nonsevere Omicron variant infection. World J Clin Infect Dis 2023; 13(4): 37-48
- URL: https://www.wjgnet.com/2220-3176/full/v13/i4/37.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v13.i4.37
Currently, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to millions of confirmed cases and deaths around the world. As of 6:32 pm Central European time, September 27, 2023, there were 770875433 confirmed cases of COVID-19 globally, including 6959316 deaths, reported to the WHO[1].
SARS-CoV-2 not only affects the respiratory tract, causing pneumonia, but it can also affect the gastrointestinal tract, nervous system, and cardiovascular system[2,3]. The severity of symptoms in COVID-19 patients varies from asymptomatic to life-threatening[4]. Among all age groups, elderly patients, defined as 60 years of age or older, are at higher risk of developing and dying from COVID-19[5,6]. In a multicentre study in the Netherlands, the in-hospital mortality of older hospitalized patients with COVID-19 was 38%[7]. From the perspective of epidemic transmission, many older people with disabilities and severe cardiovascular and neurological diseases live together in close contact in long-term care centres, which facilitates transmission of the virus and leads to infection as well as progression of severe COVID-19 in the elderly[8,9]. Based on analysis of global COVID-19 data, it was concluded that the causes of severe illness in elderly infected patients are closely related to their advanced age, decreased immune function, intense inflammatory response in the body, and comorbidities. In previous studies, hypertension, atrial fibrillation, type 2 diabetes, chronic respiratory disease, dementia, and depression were associated with hospitalization rates and mortality in elderly patients with COVID-19[10-12].
Previous studies have shown that excessive inflammation, cytokine storms, and coagulopathy are important pathological mechanisms of COVID-19[13,14]. The neutrophil-to-lymphocyte ratio (NLR) reflects the systemic inflammatory response and level of neutrophil-to-lymphocyte activation. The systemic inflammatory response index (SIRI) may also reflect the host’s immune and inflammatory balance[15]. Additionally, white blood cell count, neutrophil percentage, C-reactive protein (CRP), procalcitonin (PCT), D-dimer, and lactate are closely related to the severity and mortality of COVID-19[16-19].
Shanghai has experienced a wave of infection with Omicron, a new variant of SARS-CoV-2, since March 2022. The Omicron variant, which was first identified in Botswana and South Africa in November 2021, accounted for 41% of all strains by August 20, 2022[20]. Omicron has several subvariants, including BA.1, BA.2, BA.3, BA.4, and BA.5, all of which have a high transmission rate and significant antibody avoidance, posing a great threat to the prevention and control of COVID-19[21-23]. This study retrospectively analysed the baseline clinical features and risk factors of older patients with severe and nonsevere Omicron infection to provide solid evidence for clinical policy-makers, public health officials, researchers, and the general public, to help to identify high-risk groups, and to promote appropriate remediation.
Clinical data for 328 elderly patients diagnosed with COVID-19 and admitted to the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine from April 2022 to June 2022 were collected during hospitalization. Confirmed diagnosis of COVID-19 was based on positive results for a nasopharyngeal swab sample tested by real-time reverse transcription polymerase chain reaction using a SARS-CoV-2 ZC-HX-201-2 kit (Biogerm, Shanghai, China). Elderly patients were defined as those diagnosed at age 60 years or older[6]. The discharge criteria for patients were as follows: (1) Body temperature returned to normal for more than 3 d; (2) respiratory symptoms improved obviously; (3) pulmonary imaging showed obvious absorption of inflammation; and (4) nucleic acid tests were negative twice consecutively (sampling interval of at least 24 h)[24].
In this study, 15 people died, comprising 0 nonsevere disease patients and 15 severe disease patients, and the direct cause of death was comorbidity. This study was approved by the Ethics Committee of the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Ethics Approval No: SH9H-2022-T139-1).
Baseline data, vaccination status, onset time, onset symptoms, viral shedding time, comorbidities, laboratory data, therapeutic drugs, length of hospitalization, and survival for the 328 elderly patients with COVID-19 were collected. Laboratory tests included routine blood tests, CRP, PCT, coagulation function, liver function, cytokines, lactic acid, and other indicators. According to the discharge diagnosis and clinical data during hospitalization, the study cohort was divided into mild, general, severe, and critical severe types according to the clinical classification criteria of the novel coronavirus pneumonia diagnosis and treatment protocol (trial version 7)[24]: (1) Mild type: Fever and cough, nasal stuffiness, and other respiratory tract clinical symptoms are mild; no imaging manifestations of pneumonia; (2) general type: with the above clinical manifestations and imaging manifestations of pneumonia; (3) severe: Conformed to any of the following articles, including shortness of breath, respiratory frequency acuity 30 times/min; oxygen saturation 93% or less in the resting state; arterial blood oxygen partial pressure ≤ 300 mmHg or less oxygen concentration (1 mmHg = 0.133 kPa); and progressively worsening clinical symptoms and lung imaging showing lesions that progressed significantly more than 50% within 24-48 h; and (4) critical severe: Cases meeting any of the following criteria: respiratory failure and requiring mechanical ventilation; shock; with other organ failure requiring intensive care unit care.
Among the 328 elderly patients in this study, mild and general types were included in the nonsevere group (246 cases in total), whereas severe and critical severe types were included in the severe group (82 cases in total). The baseline data at admission, differences in mortality risk, and risk factors for developing severe disease among the patients in the severe and nonsevere groups were analysed retrospectively to verify the ability and clinical significance of using laboratory indicators to identify severe infection.
SPSS Software 25.0 (SPSS Inc., Chicago, United States) was used for statistical analysis. Measurement data with skewed distribution are represented by the median (interquartile range), while measurement data with normal distribution or approximate normal distribution are represented by the mean ± SD. The chi-square test or Fisher exact probability test and t test and the Mann-Whitney U test were used for comparisons between groups. Count data are expressed as the number of cases (percentage). A risk accumulation curve was determined using a stratified log-rank test and univariate and multivariate analyses with logistic regression. A receiver operating characteristic curve (ROC) was used to analyse and calculate the area under the curve (AUC). The optimal critical value of D-dimer and the corresponding sensitivity and specificity were calculated. The layered analysis was drawn by GraphPad 8.0 (GraphPad Software, San Diego, CA, United States). All tests were bilateral. A P < 0.05 was considered statistically significant.
Among the 328 patients with COVID-19, 155 were males and 173 females, with a median age of 77 (68, 86) years. The severe infection group was older than the nonsevere infection group (84 vs 74 years, P < 0.001), included more males (57.3% vs 43.9%, P = 0.037), and had lower vaccination rates (P < 0.001). In terms of comorbidities, severe disease patients had higher rates of chronic respiratory disease (P = 0.001), cerebral infarction (P < 0.001), chronic kidney disease (P = 0.002), and neurodegenerative disease (P < 0.001) than nonsevere disease patients, and the difference was statistically significant. In terms of symptoms, the severe group included more patients with fever (P < 0.001), cough (P < 0.001), nasal stuffiness (P = 0.026), and other symptoms (including impaired smell, poor appetite, and nausea) than the nonsevere group (P < 0.001). In terms of disease severity, the inflammatory indicators SIRI, NLR, tumor necrosis factor-α, interleukin (IL)-10, IL-1, PCT, CRP, white blood cell, neutrophil percentage, lactic acid, and D-dimer in severe disease patients were significantly higher than those in nonsevere disease patients (P < 0.001). The glomerular filtration rate in severe disease patients was lower than that in nonsevere disease patients, and the difference was statistically significant (P = 0.039). Severe disease patients had significantly higher demands for respiratory support, glucocorticoids, anticoagulation (low molecular weight heparin or ordinary heparin), and antibiotics than nonsevere disease patients (P < 0.001). Application of Lianhua Qingwen granules in patients with severe COVID-19 was significantly lower than that in patients with nonsevere COVID-19 (P = 0.007). There was no difference in the application of Paxlovid between the severe and nonsevere groups (P = 0.817). The length of hospitalization (P = 0.011) and virus shedding time (P = 0.014) in severe disease patients were higher than those in nonsevere disease patients, and the difference was statistically significant. In terms of clinical outcome, the number of deaths was 15, among which the mortality rate of nonsevere disease patients was 0% and that of severe disease patients was 18.29%. Thus, the mortality rate of severe disease patients was significantly higher than that of nonsevere disease patients (P < 0.001) (Table 1).
| Variables | Total (n = 328) | Non-severe (n = 246) | Severe (n = 82) | P value |
| Age (yr) | 77.0 (68.0, 86.0) | 74.0 (64.0, 84.0) | 84.0 (75.0, 89.0) | < 0.001 |
| Male sex | 155 (47.4) | 108 (43.9) | 47 (57.3) | 0.037 |
| Vaccinations (times) | 0 (0, 0) | 0 (0, 2) | 0 (0, 0) | < 0.001 |
| Comorbidities | ||||
| Chronic respiratory disease | 37 (11.2) | 20 (7.8) | 17 (20.7) | 0.001 |
| Hypertension | 185 (56.4) | 135 (54.8) | 50 (61.0) | 0.332 |
| Diabetes mellitus | 65 (19.9) | 44 (17.8) | 21 (25.6) | 0.129 |
| Coronary heart disease | 56 (17.0) | 40 (16.1) | 16 (19.5) | 0.478 |
| Cerebral infarction | 64 (19.6) | 34 (13.5) | 30 (36.6) | < 0.001 |
| Chronic kidney disease | 16 (4.8) | 7 (2.6) | 9 (11.0) | 0.002 |
| Immune system disease | 6 (1.9) | 5 (2.2) | 1 (1.2) | 0.589 |
| Neoplastic disease | 31 (9.6) | 23 (9.6) | 8 (9.8) | 0.96 |
| Neurodegenerative diseases | 23 (7.1) | 8 (3.0) | 15 (18.3) | < 0.001 |
| Other comorbidities | 99 (30.1) | 69 (27.8) | 30 (36.6) | 0.138 |
| Symptoms | ||||
| Fever | 61 (18.6) | 33 (13.0) | 28 (34.1) | < 0.001 |
| Pharyngodynia | 58 (17.6) | 41 (16.5) | 17 (20.7) | 0.39 |
| Cough | 149 (45.5) | 98 (39.6) | 51 (62.2) | < 0.001 |
| Nasal stuffiness | 31 (9.6) | 18 (7.4) | 13 (15.8) | 0.026 |
| Diarrhea | 3 (1.0) | 1 (0.4) | 2 (2.4) | 0.11 |
| Other symptoms | 21 (6.4) | 9 (3.5) | 12 (14.6) | < 0.001 |
| Laboratory data | ||||
| D-dimers (mg/ml) | 0.66 (0.30, 1.85) | 0.42 (0.21, 0.82) | 3.19 (1.33, 7.32) | < 0.001 |
| eGFR (ml/min/1.73 m2) | 76.63 ± 23.62 | 78.59 ± 20.82 | 71.09 ± 29.47 | 0.039 |
| TNF-α (pg/ml) | 9.78 (7.47, 12.60) | 8.85 (7.12, 11.10) | 12.37 (10.90, 18.80) | < 0.001 |
| IL-10 (pg/ml) | 5.00 (5.00, 5.82) | 5.00 (5.00, 5.00) | 6.47 (5.24, 8.99) | < 0.001 |
| IL-1β (pg/ml) | 5.00 (5.00, 5.55) | 5.00 (5.00, 5.00) | 5.55 (5.00, 7.17) | < 0.001 |
| PCT (ng/ml) | 1.58 ± 6.76 | 0.14 ± 0.57 | 5.66 ± 12.31 | < 0.001 |
| CRP (mg/L) | 23.41 ± 38.25 | 9.83 ± 21.20 | 61.96 ± 48.30 | < 0.001 |
| WBC (109/L) | 6.82 ± 3.29 | 5.92 ± 1.93 | 9.36 ± 4.72 | < 0.001 |
| Neutrophil percentage | 69.80 ± 14.30 | 64.44 ± 11.38 | 85.02 ± 10.22 | < 0.001 |
| Lactic acid (mmol/L) | 1.80 (1.37, 2.30) | 1.64 (1.25, 2.00) | 2.47 (1.81, 3.20) | < 0.001 |
| SIRI | 3.45 ± 5.08 | 1.71 ± 1.93 | 8.36 ± 7.48 | < 0.001 |
| NLR | 5.27 ± 5.58 | 3.19 ± 3.05 | 11.17 ± 6.79 | < 0.001 |
| Oxygen saturation | 96.13 (93.00, 97.13) | 96.60 (95.98, 97.73) | 91.00 (89.00, 92.90) | < 0.001 |
| Treatment | ||||
| Respiratory support | 108 (33.01) | 29 (10.44) | 79 (96.34) | < 0.001 |
| Paxlovid | 196 (59.62) | 148 (60.00) | 48 (58.54) | 0.817 |
| Glucocorticoids | 78 (23.72) | 24 (8.70) | 54 (65.85) | < 0.001 |
| Anticoagulation (low molecular weight heparin or regular heparin) | 98 (29.81) | 42 (16.09) | 56 (68.29) | < 0.001 |
| Lianhua Qingwen Granule | 212 (64.74) | 169 (69.13) | 43 (52.44) | 0.007 |
| Antibiotics | 113 (34.30) | 46 (17.39) | 67 (81.71) | < 0.001 |
| Length of hospital stays (d) | 8 (5, 11) | 7 (5, 10) | 8 (5, 14) | 0.011 |
| Viral shedding time (d) | 9.25 ± 5.84 | 8.65 ± 4.87 | 10.95 ± 7.74 | 0.014 |
| Death | 15 (4.57) | 0 (0.00) | 15 (18.29) | < 0.001 |
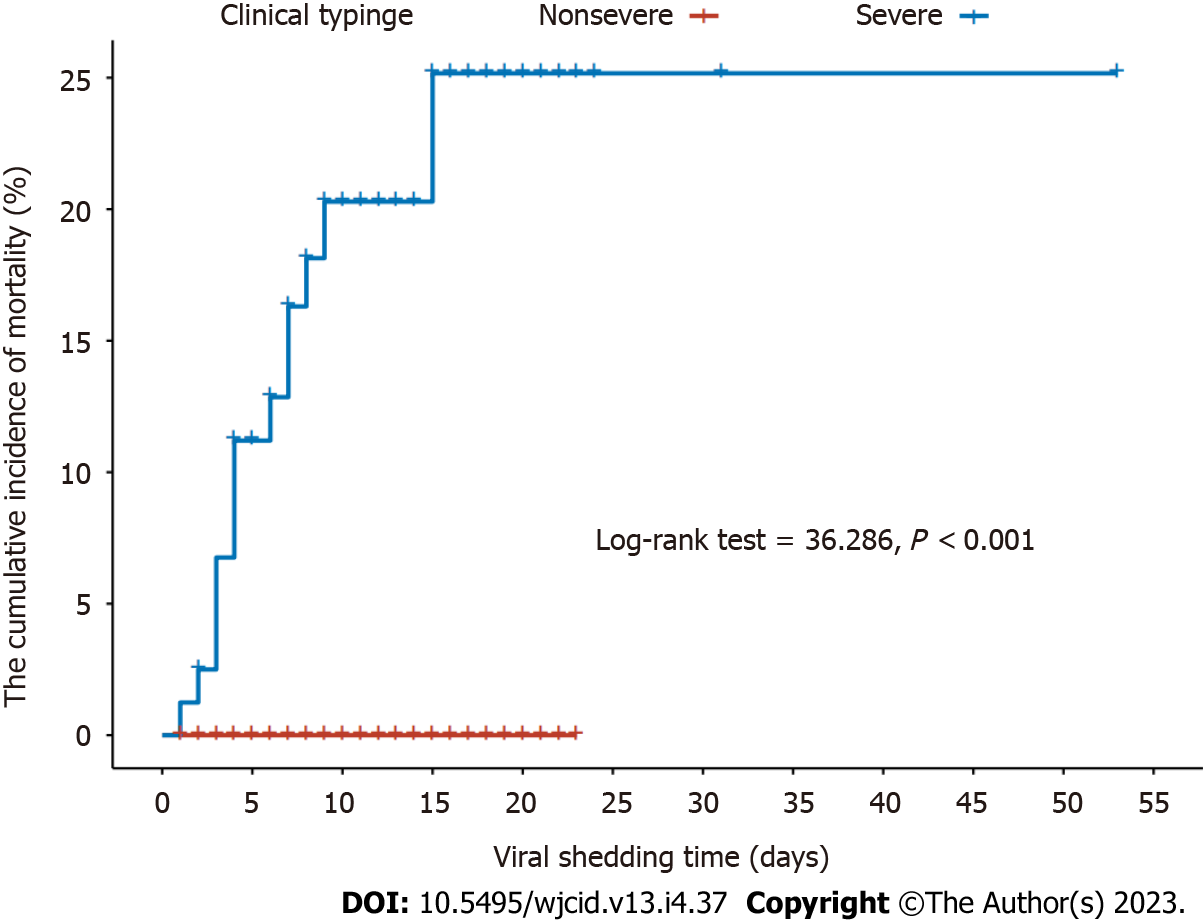
In this study, the viral shedding times of severe and nonsevere COVID-19 patients were 10.95 ± 7.74 and 8.65 ± 4.87 d, respectively. During the viral shedding period, a total of 15 patients died, all of whom had severe COVID-19. The cumulative incidence of death risk during viral shedding was higher in severe disease patients than in nonsevere disease patients (log-rank test = 36.286, P < 0.001) (Figure 1).
Univariate and multivariate logistic regressions were used to analyse risk factors for developing severe infection in COVID-19 patients (Table 2). In univariate regression analysis, only oxygen saturation [Odds ratio (OR) = 0.513, 95%CI: 0.369-0.714; P < 0.001] was a risk factor for developing severe COVID-19. In multivariate logistic regression analysis, oxygen saturation (OR = 0.573, 95%CI: 0.451-0.728; P < 0.001), cerebral infarction (OR = 4.26, 95%CI: 1.012-17.937; P = 0.048), and D-dimer (OR = 1.394, 95%CI: 1.000-1.944; P = 0.05) were predictors of severe infection.
| Variables | Univariate analysis OR (95%CI) | P value | Multivariate analysis OR (95%CI) | P value |
| Age | 1.084 (0.978, 1.202) | 0.126 | - | - |
| Sex | 0.575 (0.1, 3.322) | 0.536 | - | - |
| Vaccinations | 0.636 (0.039, 10.385) | 0.751 | - | - |
| Chronic respiratory disease | 0.559 (0.025, 12.453) | 0.713 | - | - |
| Diabetes mellitus | 7.76 (0.446, 134.92) | 0.16 | - | - |
| Hypertension | 3.267 (0.365, 29.275) | 0.29 | - | - |
| Coronary heart disease | 0.142 (0.01, 1.926) | 0.142 | - | - |
| Cerebral infarction | 7.757 (0.704, 85.443) | 0.094 | 4.26 (1.012, 17.937) | 0.048 |
| Chronic kidney disease | 0.057 (0.001, 4.578) | 0.2 | - | - |
| Neurodegenerative diseases | 19.385 (0.149, 2527.003) | 0.233 | - | - |
| Neoplastic disease | 0.527 (0.022, 12.846) | 0.695 | - | - |
| Immune system disease | 0 (0, 203.169) | 0.213 | - | - |
| WBC | 1.096 (0.669, 1.794) | 0.716 | - | - |
| Neutrophil percentage | 1.125 (0.95, 1.331) | 0.172 | - | - |
| eGFR | 1.044 (0.966, 1.128) | 0.281 | - | - |
| NLR | 1.017 (0.636, 1.626) | 0.943 | - | - |
| SIRI | 1.003 (0.534, 1.883) | 0.993 | - | - |
| CRP | 1.023 (0.984, 1.063) | 0.251 | - | - |
| PCT | 1.552 (0.67, 3.598) | 0.305 | - | - |
| Oxygen saturation | 0.513 (0.369, 0.714) | 0.000 | 0.573 (0.451, 0.728) | 0.000 |
| Lactic acid | 0.768 (0.269, 2.194) | 0.622 | - | - |
| D-dimers | 1.156 (0.754, 1.772) | 0.507 | 1.394 (1, 1.944) | 0.05 |
| Viral shedding time | 1.066 (0.892, 1.274) | 0.484 | - | - |
| Lianhua Qingwen Granule | 0.486 (0.055, 4.302) | 0.517 | - | - |
| Paxlovid | 2.505 (0.19, 33.049) | 0.485 | - | - |
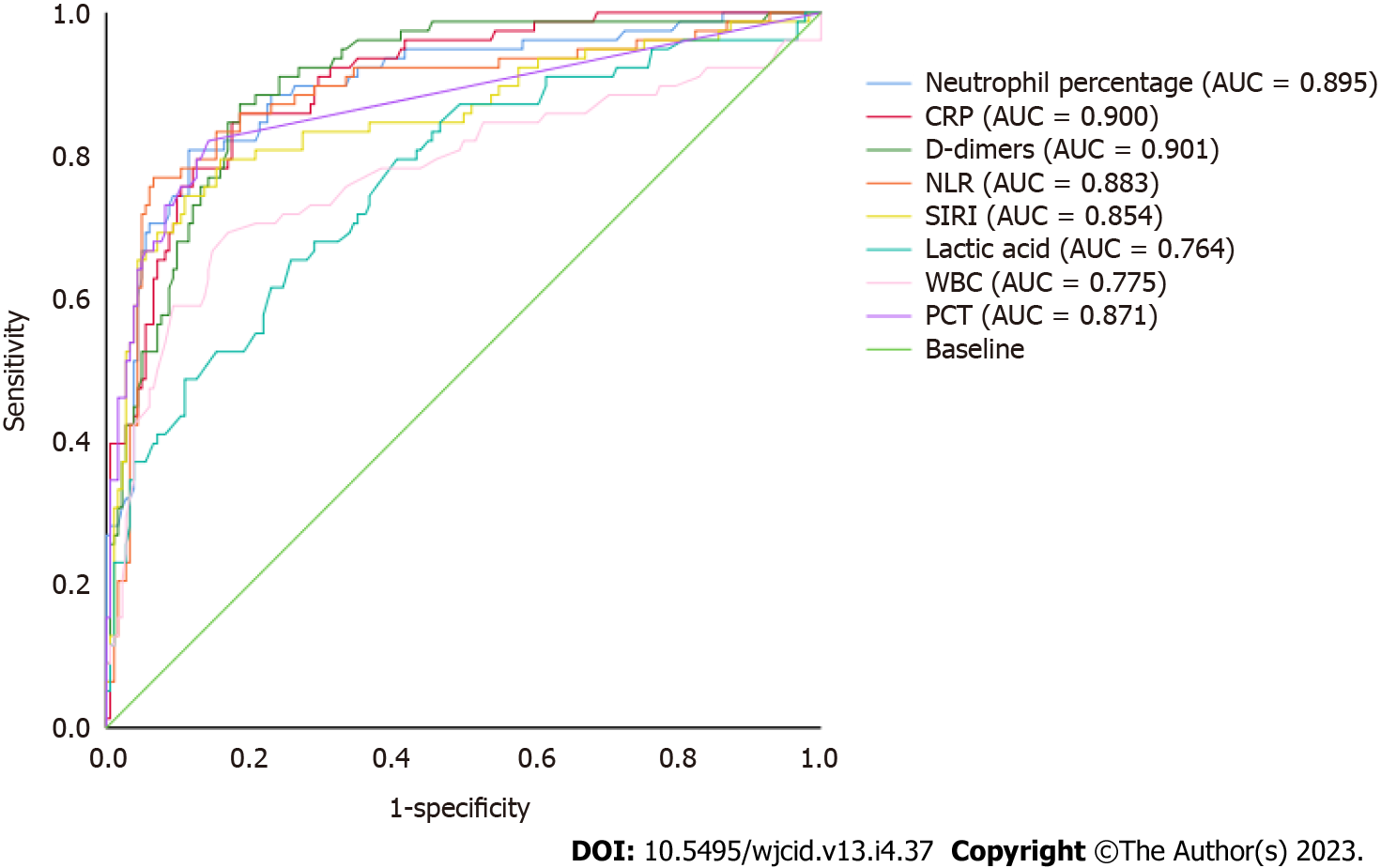
A ROC curve was used to analyse and calculate the AUC of neutrophil percentage, CRP, D-dimer, NLR, SIRI, lactic acid, white blood cell count, and PCT indicators to assess the ability of each indicator to identify severe infection in elderly patients with COVID-19. Among them, the AUC of neutrophil percentage was 0.895, that of CRP 0.900, that of NLR 0.883, that of SIRI 0.854, that of lactic acid 0.764, that of white blood cell count 0.775, and that of PCT 0.871. The AUC of D-dimer was 0.901 (P < 0.001). When the threshold was 1.020 mg/L, the AUC was 90.1% (95%CI: 86.1%-94.0%). The sensitivity and specificity of D-dimer to identify severe disease in elderly patients with COVID-19 were 85.5% and 81.7%, respectively (Figure 2).
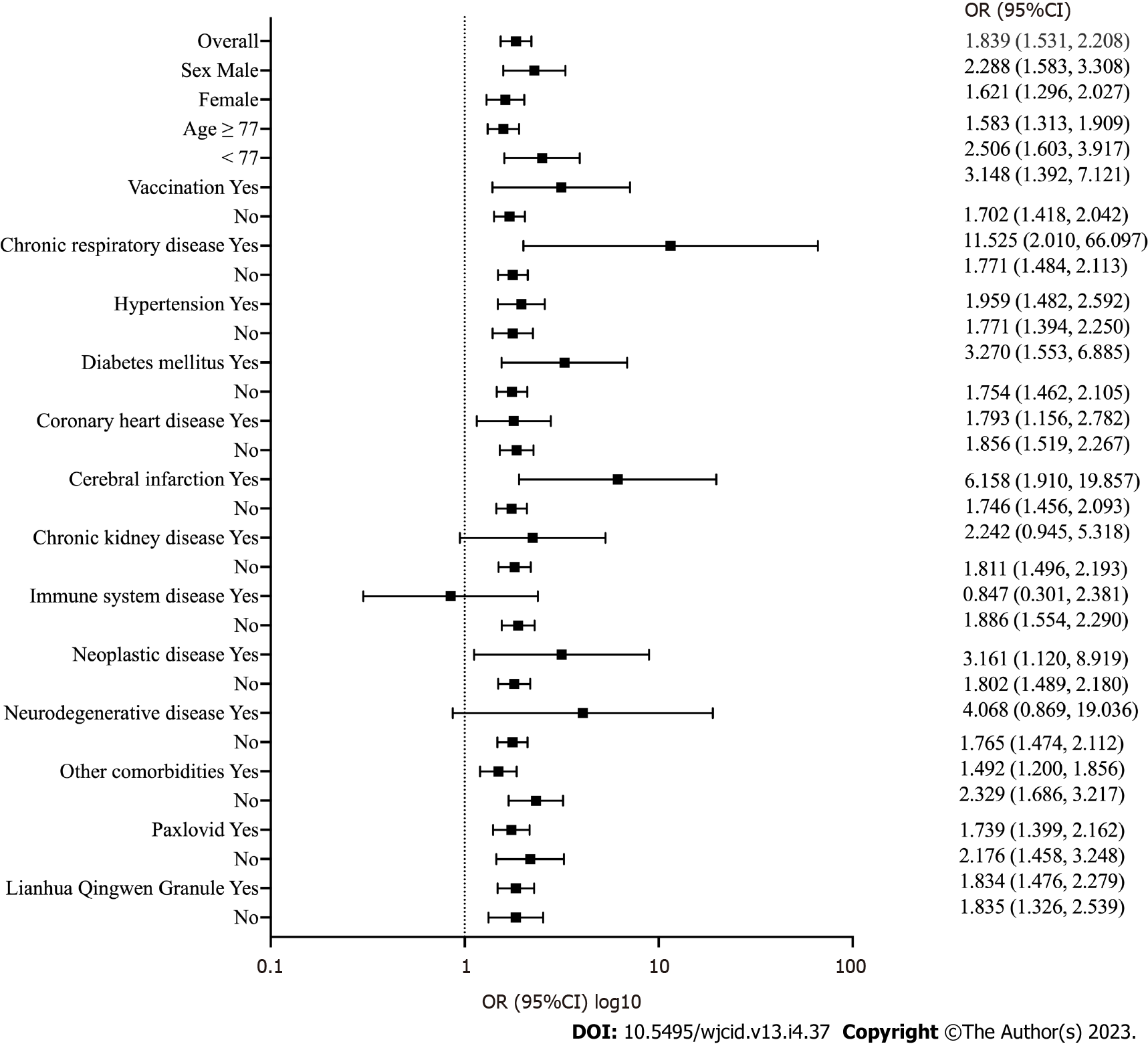
Figure 3 shows multivariate stratified analysis of D-dimer levels in elderly patients with COVID-19. Overall, D-dimer was a risk factor for the development of severe disease in elderly patients with COVID-19 (OR = 1.839, P < 0.001). In further variable stratification analysis, D-dimer remained a risk factor for the development of severe COVID-19, including in female patients (OR = 1.621, P < 0.001), male patients (OR = 2.288, P < 0.001), patients younger than the median age of 77 years (OR = 2.506, P < 0.001), patients older or equal to 77 years old (OR = 1.583, P < 0.001), patients not vaccinated against COVID-19 (OR = 1.702, P < 0.001), patients not vaccinated against COVID-19 (OR = 3.148, P = 0.006), patients without chronic respiratory disease (OR = 1.771, P < 0.001), patients with chronic respiratory disease (OR = 11.525, P = 0.006), patients without hypertension (OR = 1.621, P < 0.001), patients with hypertension (OR = 1.621, P < 0.001), patients without diabetes mellitus (OR = 1.754, P < 0.001), patients with diabetes mellitus (OR = 3.270, P = 0.002), patients without coronary heart disease (OR = 1.856, P < 0.001), patients with coronary heart disease (OR = 1.793, P = 0.009), patients without cerebral infarction (OR = 1.746, P < 0.001), patients with cerebral infarction (OR = 6.158, P = 0.002), patients without chronic kidney disease (OR = 1.811, P < 0.001), patients without immune system disease (OR = 1.886, P < 0.001), patients without neoplastic disease (OR = 1.802, P < 0.001), patients with neoplastic disease (OR = 3.161, P = 0.030), patients without neurodegenerative disease (OR = 1.765, P < 0.001), patients without other comorbidities (OR = 2.329, P < 0.001), patients with other comorbidities (OR = 1.492, P < 0.001), patients without Paxlovid (OR = 2.176, P < 0.001), patients with Paxlovid (OR = 1.739, P < 0.001), patients given Lianhua Qingwen granules (OR = 1.834, P < 0.001), and patients not given Lianhua Qingwen granules (OR = 1.835, P < 0.001).
In addition, in stratified analysis for chronic kidney disease (OR = 1.621, P = 0.067) and neurodegenerative disease (OR = 4.068, P = 0.075), although it did not achieve statistical significance, the OR of D-dimer was still greater than 1.0. In patients with immune system diseases (OR = 0.847, P = 0.753), the OR of D-dimer was less than 1.0, but there was no statistical significance.
Pneumonia is often regarded as a terminal event that complicates long-term diseases, such as dementia, cardiovascular disease, and cancer, in the elderly[25], SARS-CoV-2 mainly causes pulmonary interstitial pneumonia changes, typical bilateral patchy ground glass shadows, and peripheral consolidation. Compared with other age groups, the elderly seem to be more susceptible to COVID-19, and severe disease is an important reason for the high mortality rate and intensive care unit hospitalization rate of elderly patients with COVID-19[26,27]. In previous reports, the case fatality rate of elderly patients with COVID-19 ranged from 8.0% to 37.5%, increasing with age[26,28,29]. In addition, the population characteristics include a higher male proportion, intense inflammatory response in the body, prolonged viral shedding time, and prolonged hospital stay[26,30].
This study found that elderly patients with severe COVID-19 were older and comprised a higher proportion of males than nonsevere COVID-19 patients. The inflammatory reaction in severe disease patients was more intense than that in nonsevere disease patients. In addition, levels of lactic acid and D-dimer in severe disease patients were significantly higher than those in nonsevere disease patients, and the estimated glomerular filtration rate was lower. The length of hospitalization and viral shedding time of severe disease patients were longer than those of nonsevere disease patients. In this study, the severe infection group had lower vaccination rates than the nonsevere infection group; however, the vaccination status was not significant in univariate and multivariate analyses of the development of severe disease in elderly patients with COVID-19. This suggests that vaccination status is associated with a significantly lower risk of hospitalization for COVID-19 but is not associated with the development of severe COVID-19 in elderly patients, which was similar to a previous observational study[31]. Regarding the management and treatment of COVID-19 in this study, no difference was observed in the application of Paxlovid in the severe and nonsevere groups, suggesting that Paxlovid did not benefit patients in terms of avoiding the development of severe COVID-19 in this study. On the other hand, the need for respiratory support, glucocorticoids, anticoagulation (low molecular weight heparin or ordinary heparin), and antibiotic therapy was significantly higher in severe disease patients than in nonsevere disease patients. This is consistent with current research showing that COVID-19, similar to other community-acquired pneumonia, is considered to be a late-stage event that complicates long-term disease[26]. To personalize clinical management of COVID-19, researchers are also reflecting on better therapeutic strategies, including early adoption of non-steroidal anti-inflammatory drugs[32], application of broad-spectrum antimicrobials[33], and a personalized risk-benefit ratio for glucocorticoid use[34]. In terms of clinical outcome, 15 people died in this study; the mortality rate of nonsevere disease patients was 0%, and that of severe disease patients was 18.29%. Hence, the mortality rate of severe disease patients was significantly higher than that of nonsevere disease patients, which was also consistent with previous literature reports[26,30].
In addition to age, the presence and quantity of comorbidities are considered to be key factors in predicting the death of elderly patients. However, the significance of specific comorbidities, such as hypertension, coronary heart disease, and respiratory diseases, in the development of severe COVID-19 in elderly patients varied in previous research[35-38]. The results of this study also showed that the proportions of chronic respiratory diseases, cerebral infarction, chronic renal diseases, and neurodegenerative diseases were higher in severe disease patients than in nonsevere disease patients. Further analysis of the predictive factors of severe disease in elderly patients showed that among all comorbidities, cerebral infarction was the only risk factor for the development of severe disease in elderly patients with COVID-19 in this study.
In addition, studies have found that elderly patients from long-term care centres seem to have a higher rate of severe illness and fatality on admission than elderly patients from family care situations[26]. Indeed, staying in a long-term care centre is a strong risk factor for COVID-19 diagnosis and all-cause mortality[39]. Studies have suggested that this may be related to the fact that elderly COVID-19 patients living in long-term care centres usually have more comorbidities, are physically weaker, and are more susceptible to infection when in a closed environment[26]. In this study, cerebral infarction was a risk factor for severe COVID-19 in elderly patients. This can be explained by the fact that elderly people with cerebral infarction may need to stay in bed for a longer period and need increased daily nursing care. As a result, early identification of COVID-19 tends to be missed in this group of people, and they tend to receive insufficient nursing care after developing COVID-19, leading to severe infection in these patients. Therefore, the results of this study showed that elderly COVID-19 patients with cerebral infarction may be the most vulnerable group of elderly COVID-19 patients during the current wave of Omicron infection in Shanghai.
In previous studies, plasma D-dimer levels were directly related to the development of pulmonary embolism and vascular thrombosis complications during COVID-19 and correlated highly with adverse outcomes[40,41]. In this study, D-dimer was also a risk factor for the development of severe COVID-19 in elderly patients, which is consistent with previous literature reports[42]. In previous studies, NLR, CRP, and neutrophil percentage were demonstrated to be predictors of severe diseases, showing good recognition ability for severe COVID-19[17,37,43]. In this study, it was found that compared with white blood cell count, neutrophil percentage, CRP, PCT, NLR, SIRI, and lactic acid, the ROC curve of D-dimer yielded the largest AUC, with good sensitivity and specificity and an outstanding ability to identify severe COVID-19. In multivariate stratified analysis, D-dimer was a risk factor for the development of severe COVID-19 in elderly patients both at the overall level and stratified by sex, age, vaccination, chronic respiratory disease, hypertension, diabetes mellitus, coronary heart disease, cerebral infarction, chronic kidney disease, immune system disease, neoplastic disease, neurodegenerative disease, other comorbidities, use of Paxlovid, and use of Lianhua Qingwen granules. This confirms the important role of D-dimer in the course and outcome of COVID-19 in elderly patients.
This study has at least two limitations. First, the sample size was small, especially regarding the number of patients in the severe disease group. This may be related to the relatively reduced pathogenicity of the Omicron subtype in the current wave of COVID-19 and the protective effect of the vaccine in reducing the risk of hospitalization for COVID-19, which is a result of active participation in receiving the vaccine in Shanghai. Second, this study was a single-center study, and the patients were limited to those diagnosed with COVID-19 and admitted to the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine from April 2022 to June 2022. Because the outbreak is evolving rapidly around the world, follow-up studies with more patients are needed to improve the statistical power of these findings.
In conclusion, the results of this study suggest that COVID-19 complicates long-term illness in elderly patients. There are considerable differences in disease severity and adverse clinical outcomes between severe and nonsevere cases in older patients with COVID-19. Elderly people are vulnerable to severe illness and death due to their age and comorbidities, especially elderly patients with preexisting cerebral infarction. D-dimer is a risk factor for severe COVID-19 in elderly patients and has a good recognition function for severe disease. Therefore, a comprehensive assessment of the comorbidities of older patients with COVID-19 may help to establish risk stratification for admission of COVID-19 patients, and dynamic monitoring of D-dimer levels can provide valuable information for planning appropriate interventions at the health assistance level.
Elderly patients are at higher risk of contracting and dying from coronavirus disease 2019 (COVID-19) due to advanced age, decreased immune function, intense inflammatory response, and comorbidities. Omicron, a new variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has a high transmission rate and significant antibody avoidance, posing a great threat to the prevention and control of COVID-19.
Previous studies have evaluated risk factors for severity or death among elderly people with COVID-19, though analyses of Omicron infection risk and protective factors among elderly people are relatively few.
To identify clinical features and risk factors for disease progression among elderly patients with Omicron infection to provide solid evidence for clinical policy-makers, public health officials, researchers, and the general public.
A chi-square test, t test, Mann-Whitney U test, hierarchical log-rank test, univariate and multivariate logistic regression analyses, and hierarchical analyses were used to determine significant differences between elderly patients with severe and nonsevere Omicron SARS-CoV-2 variant infection.
The clinical course of severe disease patients is more complex, as both the need for symptomatic treatment and the risk of death are higher than those of nonsevere disease patients. Oxygen saturation, cerebral infarction, and D-dimer are risk factors for developing severe COVID-19. D-dimer also showed a suitable role in identifying severe infection.
Elderly people are vulnerable to severe illness and death due to their age and comorbidities, especially elderly patients with preexisting cerebral infarction. D-dimer is a risk factor for severe COVID-19 in elderly patients and has a good recognition function for severe disease.
A comprehensive assessment of the comorbidities of older patients with COVID-19 may help to establish risk stratification for admission of COVID-19 patients, and dynamic monitoring of D-dimer levels can provide valuable information for planning appropriate interventions at the health assistance level.
We would like to thank Professor Liang Huang, Ph.D. Associate Chief, Director Chengdu Public Health Clinical Center, Chengdu City, 610000, Sichuan Province, China. Email: 15201920@qq.com for reviewing the statistical methods of this research work.
| 1. | WHO Coronavirus (COVID-19) Dashboard. World Health Organization. Available from: https://covid19.who.int/. |
| 2. | Peters LL, Raymer DS, Pal JD, Ambardekar AV. Association of COVID-19 Vaccination With Risk of COVID-19 Infection, Hospitalization, and Death in Heart Transplant Recipients. JAMA Cardiol. 2022;7:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Wei P, Lyu W, Wan T, Zheng Q, Tang W, Li J, Yang JJ. COVID-19: a novel risk factor for perioperative neurocognitive disorders. Br J Anaesth. 2021;127:e113-e115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin Microbiol Rev. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 5. | Lithander FE, Neumann S, Tenison E, Lloyd K, Welsh TJ, Rodrigues JCL, Higgins JPT, Scourfield L, Christensen H, Haunton VJ, Henderson EJ. COVID-19 in older people: a rapid clinical review. Age Ageing. 2020;49:501-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 6. | Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: A review of clinical data in China. Mech Ageing Dev. 2020;188:111255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (16)] |
| 7. | Blomaard LC, van der Linden CMJ, van der Bol JM, Jansen SWM, Polinder-Bos HA, Willems HC, Festen J, Barten DG, Borgers AJ, Bos JC, van den Bos F, de Brouwer EJM, van Deudekom FJA, van Dijk SC, Emmelot-Vonk MH, Geels RES, van de Glind EMM, de Groot B, Hempenius L, Kamper AM, Kampschreur LM, de Koning MMM, Labots G, Looman R, Lucke JA, Maas HAAM, Mattace-Raso FUS, El Moussaoui R, van Munster BC, van Nieuwkoop C, Oosterwijk LBLE, Regtuijt MEM, Robben SHM, Ruiter R, Salarbaks AM, Schouten HJ, Smit OM, Smits RAL, Spies PE, Vreeswijk R, de Vries OJ, Wijngaarden MA, Wyers CE, Mooijaart SP. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing. 2021;50:631-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Srifuengfung M, Thana-Udom K, Ratta-Apha W, Chulakadabba S, Sanguanpanich N, Viravan N. Impact of the COVID-19 pandemic on older adults living in long-term care centers in Thailand, and risk factors for post-traumatic stress, depression, and anxiety. J Affect Disord. 2021;295:353-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Konetzka RT, White EM, Pralea A, Grabowski DC, Mor V. A systematic review of long-term care facility characteristics associated with COVID-19 outcomes. J Am Geriatr Soc. 2021;69:2766-2777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Zhang YJ, Sun XF, Xie B, Feng WJ, Han SL. Exploration of severe Covid-19 associated risk factor in China: Meta-analysis of current evidence. Int J Clin Pract. 2021;75:e14900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Bianchetti A, Rozzini R, Bianchetti L, Coccia F, Guerini F, Trabucchi M. Dementia Clinical Care in Relation to COVID-19. Curr Treat Options Neurol. 2022;24:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, Melzer D. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2224-2230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 332] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 13. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4076] [Article Influence: 679.3] [Reference Citation Analysis (0)] |
| 14. | Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 563] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 15. | Citu C, Gorun F, Motoc A, Sas I, Gorun OM, Burlea B, Tuta-Sas I, Tomescu L, Neamtu R, Malita D, Citu IM. The Predictive Role of NLR, d-NLR, MLR, and SIRI in COVID-19 Mortality. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 16. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1184] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 17. | Chen Y, Zhi H, Zhang K, Zhu G, Liu L, Yan X, Cai Z, Zhao C, Hu Z. Combined predictive performance of age and neutrophilic percentage on admission for severe novel coronavirus disease 2019. Int J Clin Pract. 2021;75:e14257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Milenkovic M, Hadzibegovic A, Kovac M, Jovanovic B, Stanisavljevic J, Djikic M, Sijan D, Ladjevic N, Palibrk I, Djukanovic M, Velickovic J, Ratkovic S, Brajkovic M, Popadic V, Klasnja S, Toskovic B, Zdravkovic D, Crnokrak B, Markovic O, Bjekic-Macut J, Aleksic A, Petricevic S, Memon L, Milojevic A, Zdravkovic M. D-dimer, CRP, PCT, and IL-6 Levels at Admission to ICU Can Predict In-Hospital Mortality in Patients with COVID-19 Pneumonia. Oxid Med Cell Longev. 2022;2022:8997709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Shi D, Yan R, Lv L, Jiang H, Lu Y, Sheng J, Xie J, Wu W, Xia J, Xu K, Gu S, Chen Y, Huang C, Guo J, Du Y, Li L. The serum metabolome of COVID-19 patients is distinctive and predictive. Metabolism. 2021;118:154739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 20. | Omicron Variant Report. Outbreak.info. Available from: https://outbreak.info/situation-reports/omicron. |
| 21. | Ai J, Wang X, He X, Zhao X, Zhang Y, Jiang Y, Li M, Cui Y, Chen Y, Qiao R, Li L, Yang L, Li Y, Hu Z, Zhang W, Wang P. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe. 2022;30:1077-1083.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 22. | Cui Z, Liu P, Wang N, Wang L, Fan K, Zhu Q, Wang K, Chen R, Feng R, Jia Z, Yang M, Xu G, Zhu B, Fu W, Chu T, Feng L, Wang Y, Pei X, Yang P, Xie XS, Cao L, Cao Y, Wang X. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell. 2022;185:860-871.e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 331] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 23. | Iketani S, Liu L, Guo Y, Chan JF, Huang Y, Wang M, Luo Y, Yu J, Chu H, Chik KK, Yuen TT, Yin MT, Sobieszczyk ME, Yuen KY, Wang HH, Sheng Z, Ho DD. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 652] [Cited by in RCA: 644] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 24. | Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin Med J (Engl). 2020;133:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 534] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 25. | Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 26. | D'ascanio M, Innammorato M, Pasquariello L, Pizzirusso D, Guerrieri G, Castelli S, Pezzuto A, De Vitis C, Anibaldi P, Marcolongo A, Mancini R, Ricci A, Sciacchitano S. Age is not the only risk factor in COVID-19: the role of comorbidities and of long staying in residential care homes. BMC Geriatr. 2021;21:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Xue QL. Frailty as an integrative marker of physiological vulnerability in the era of COVID-19. BMC Med. 2020;18:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11609] [Article Influence: 1934.8] [Reference Citation Analysis (2)] |
| 29. | Niu S, Tian S, Lou J, Kang X, Zhang L, Lian H, Zhang J. Clinical characteristics of older patients infected with COVID-19: A descriptive study. Arch Gerontol Geriatr. 2020;89:104058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 30. | Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, Yee NTS, Liu C, Nerurkar SN, Kai JCY, Teng MLP, Li X, Zeng H, Borghi JA, Henry L, Cheung R, Nguyen MH. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 419] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 31. | Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, Cattaneo S, Cereda D, Colombo S, Coluccello A, Crescini G, Forastieri Molinari A, Foti G, Fumagalli R, Iotti GA, Langer T, Latronico N, Lorini FL, Mojoli F, Natalini G, Pessina CM, Ranieri VM, Rech R, Scudeller L, Rosano A, Storti E, Thompson BT, Tirani M, Villani PG, Pesenti A, Cecconi M; COVID-19 Lombardy ICU Network. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1183] [Cited by in RCA: 1074] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 32. | Kelleni MT. NSAIDs and Kelleni's protocol as potential early COVID-19 treatment game changer: could it be the final countdown? Inflammopharmacology. 2022;30:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Kelleni MT. The African Kelleni's roadmap using nitazoxanide and broad-spectrum antimicrobials to abort returning to COVID-19 square one. Inflammopharmacology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Kelleni MT. Tocilizumab, Remdesivir, Favipiravir, and Dexamethasone Repurposed for COVID-19: a Comprehensive Clinical and Pharmacovigilant Reassessment. SN Compr Clin Med. 2021;3:919-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Becerra-Muñoz VM, Núñez-Gil IJ, Eid CM, García Aguado M, Romero R, Huang J, Mulet A, Ugo F, Rametta F, Liebetrau C, Aparisi A, Fernández-Rozas I, Viana-Llamas MC, Feltes G, Pepe M, Moreno-Rondón LA, Cerrato E, Raposeiras-Roubín S, Alfonso E, Carrero-Fernández A, Buzón-Martín L, Abumayyaleh M, Gonzalez A, Fernández Ortiz A, Macaya C, Estrada V, Fernández-Pérez C, Gómez-Doblas JJ. Clinical profile and predictors of in-hospital mortality among older patients hospitalised for COVID-19. Age Ageing. 2021;50:326-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 36. | Covino M, De Matteis G, Polla DAD, Santoro M, Burzo ML, Torelli E, Simeoni B, Russo A, Sandroni C, Gasbarrini A, Franceschi F. Predictors of in-hospital mortality AND death RISK STRATIFICATION among COVID-19 PATIENTS aged ≥ 80 YEARs OLD. Arch Gerontol Geriatr. 2021;95:104383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, Li Y. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. 2020;92:2188-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 38. | Lu G, Zhang Y, Zhang H, Ai J, He L, Yuan X, Bao S, Chen X, Wang H, Cai J, Wang S, Zhang W, Xu J. Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai Omicron wave. Emerg Microbes Infect. 2022;11:2045-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36:287-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 40. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18392] [Article Influence: 3065.3] [Reference Citation Analysis (13)] |
| 41. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3447] [Article Influence: 574.5] [Reference Citation Analysis (0)] |
| 42. | Li Y, Zhao K, Wei H, Chen W, Wang W, Jia L, Liu Q, Zhang J, Shan T, Peng Z, Liu Y, Yan X. Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol. 2020;190:e24-e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 43. | Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6-e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 571] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kelleni MT, Egypt S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY