Published online May 29, 2023. doi: 10.5495/wjcid.v13.i2.11
Peer-review started: September 28, 2022
First decision: November 22, 2022
Revised: November 28, 2022
Accepted: April 27, 2023
Article in press: April 27, 2023
Published online: May 29, 2023
Processing time: 234 Days and 4 Hours
Leishmaniasis is a vector-borne parasitic disease affecting millions of people worldwide. However, in the last decade, the number of cases has been reduced from well-documented endemic parts, but sporadic cases have been reported widely from various non-endemic areas, especially from the southern Himalayan zone. This raises concerns about the emergence of new ecological niches. This warrants a critical evaluation of key factors causing this rapid spread and possibly indigenous transmission. This mini-review article is aimed to briefly address the parasite, the vector, and the environmental aspects in the transmission of leishmaniasis in these new foci against a background of worldwide endemic leishmaniasis with a special focus on the southern Himalayan zone. As the lack of knowledge about the causative parasites, vectors, reservoir hosts, atypical presentations, and their management make the problem serious and may lead to the emergence of public health issues. The present works also reviewed the existing information regarding clinical variations, diagnostic methods, treatment, its outcome, and ignite for further research in these aspects of the disease.
Core Tip: This mini-review article is aimed to briefly address the parasite, the vector, and the environmental aspects in the transmission of leishmaniasis in these new foci against a background of worldwide endemic leishmaniasis with a special focus on the southern Himalayan zone.
- Citation: Sharma A, Kumar S, Panda PK, Yadav S, Kalita D. Emerging leishmaniasis in southern Himalayas: A mini-review. World J Clin Infect Dis 2023; 13(2): 11-23
- URL: https://www.wjgnet.com/2220-3176/full/v13/i2/11.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v13.i2.11
Leishmaniasis is a vector-borne parasitic disease that exists either as zoonosis (in most endemic parts of the world) or anthroponosis (endemic part of the Indian sub-continent) and is transmitted by S and fly. The latter entity is on the verge of elimination, efficiently with the help of assigned memorandum of understanding by the five most endemic countries: India, Nepal, Bangladesh, Bhutan, and Thailand[1]. But at present, a major challenge is the increasing emergence of new ecological niches having indigenous transmission. Recently World Health Organization (WHO) declares it as a category I disease (emerging and uncontrolled), and the World Health Assembly recognizes it as a major public health concern[2]. Leishmaniasis is a disease of low altitude. It does not occur at an altitude of more than 2000ft (600m)[3]. The Southern Himalayan regions (of countries like Pakistan, India, Nepal, and Bhutan) are considered as non-endemic regions probably because of the non-conducive environment for the growth of its vector, i.e., Sand flies. But as several cases of leishmaniasis have been reported from the sea areas, the above observational facts are being indistinct. Most of these cases were found along with the upstream of Himalayas river belts (like Indus, Ganga, Yamuna, and the Brahmaputra) especially in the western part (Islamabad, Jammu & Kashmir, and Himachal Pradesh), the middle part (Uttarakhand), and the eastern part (Nepal and Bhutan) of Himalayas[3-33].
Here, a mini-/narrative review is done considering the available case reports/case series/observational studies from new emerging areas, regarding leishmaniasis disease profile (epidemiology, microbiology, patho-physiology, clinical variations, diagnostic methods, treatment, and outcome, including the entomological assessment of S and fly). This article also intends to focus on the difference between the disease profile of leishmaniasis in the southern Himalayan belt vs the world’s endemic areas in a systematic manner.
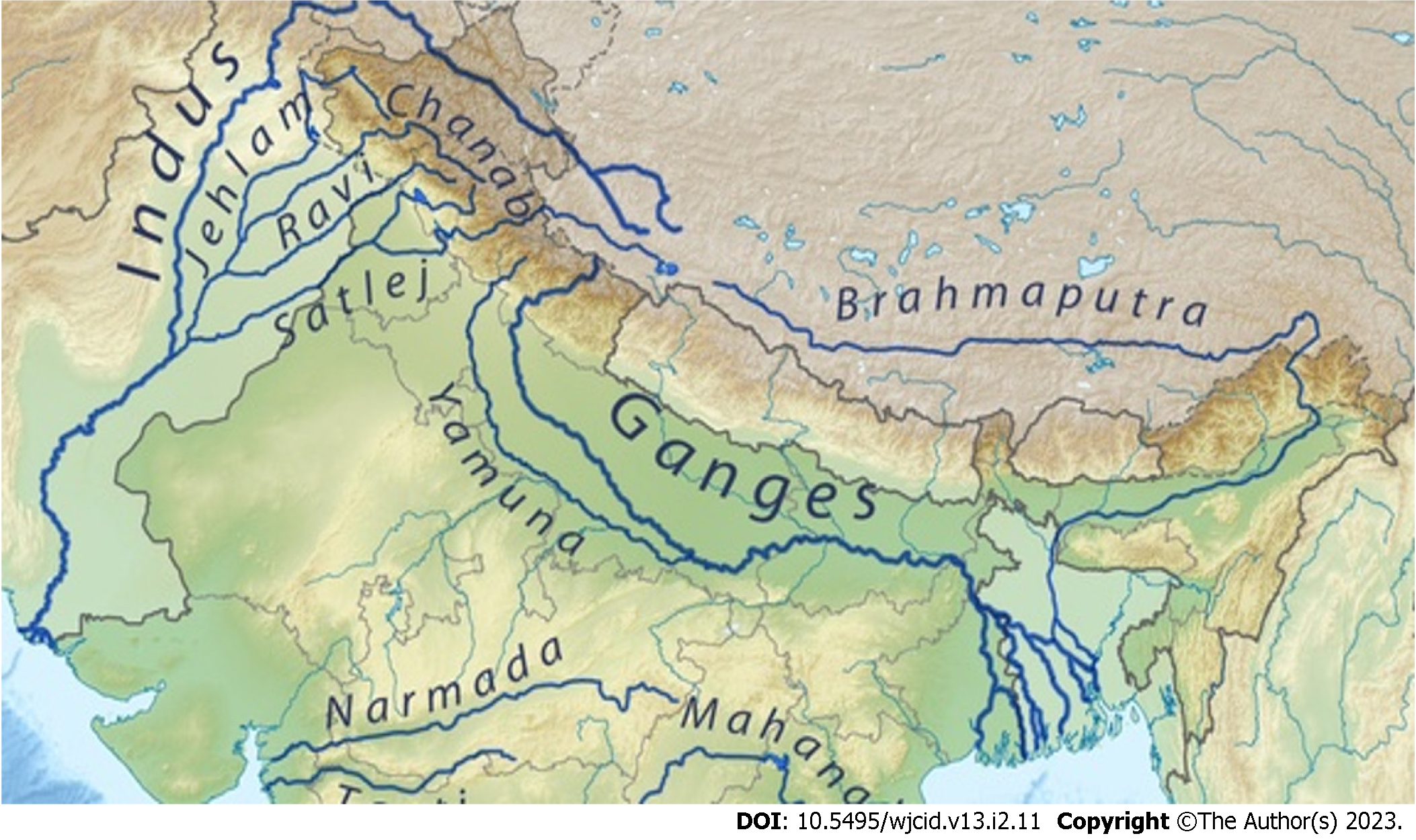
A mini-review of all published (PubMed/Medline, Embase, Cochrane database, Google Scholar) leishmania cases from the Himalayas regions of Pakistan, India, Nepal, and Bhutan were reviewed and analyzed with prime focus on the disease profiles of the cases reported in the lower Himalayan belt (Figure 1). For distinctive comparison and obtaining good inference, the Indian Himalayan belt is further divided into Jammu & Kashmir, Himachal Pradesh (Shimla, Chamba & Kinnaur), and Uttarakhand (Garhwal & Kumaon) regions. Leishmaniasis which was initially considered a disease of plain lower altitude areas along the banks of major rivers is now prevailing in higher altitudes. This ecological shift provides us with an excellent opportunity to study the epidemiological triad and also warranting a need to implement appropriate control measures. Hence, this review is done with the objective to identify the newly reported endemic areas on these hilly terrains related to the disease and multiple factors associated with it, especially in relation to river belts.
Selection: (1) Leishmania disease: Only records that concern the leishmania/Kala-azar in the Indian sub-continent or related topics are included in the selection; (2) Original records: We excluded letters, editorials and comments; and (3) English language: We excluded articles written in other language.
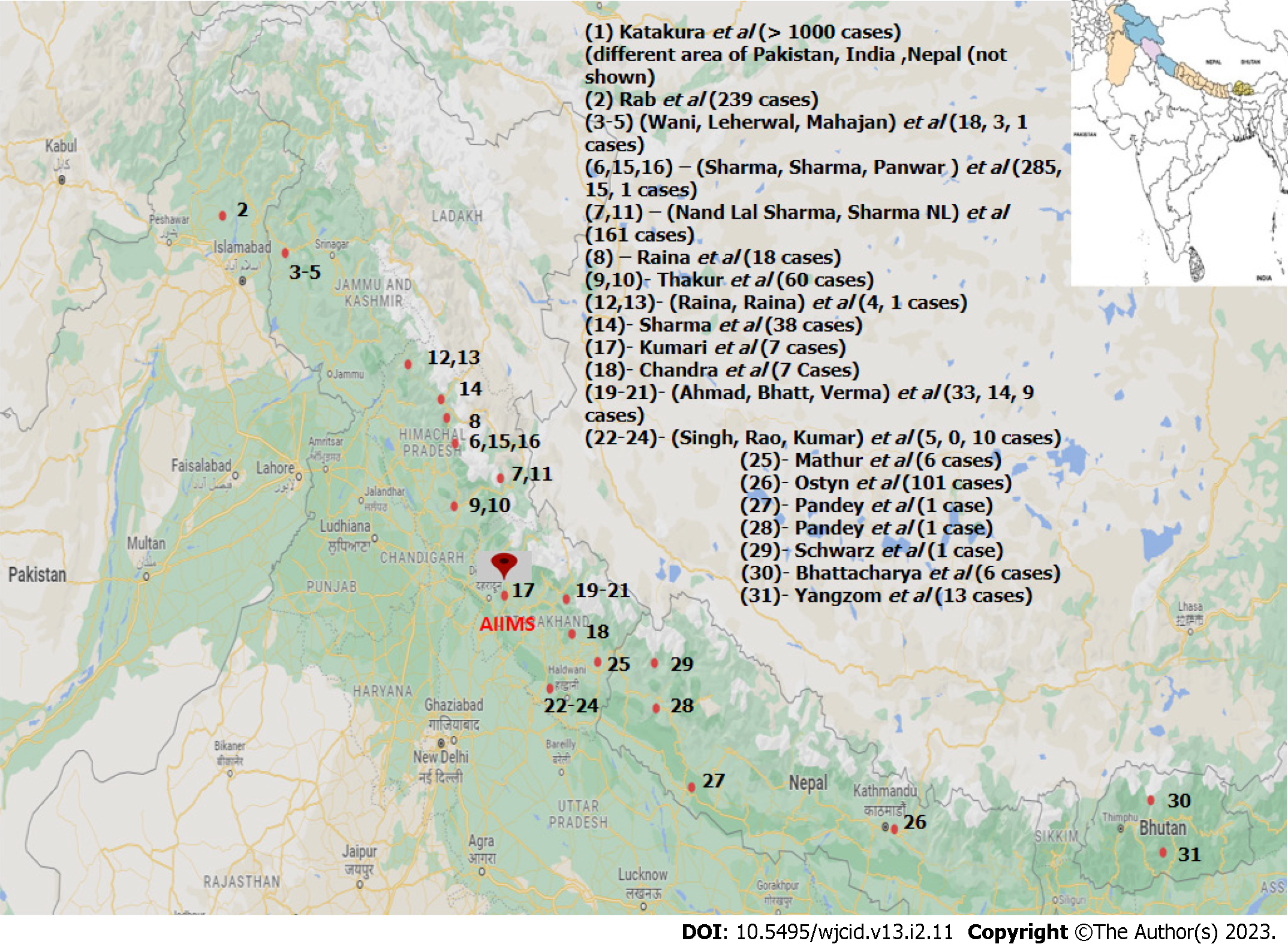
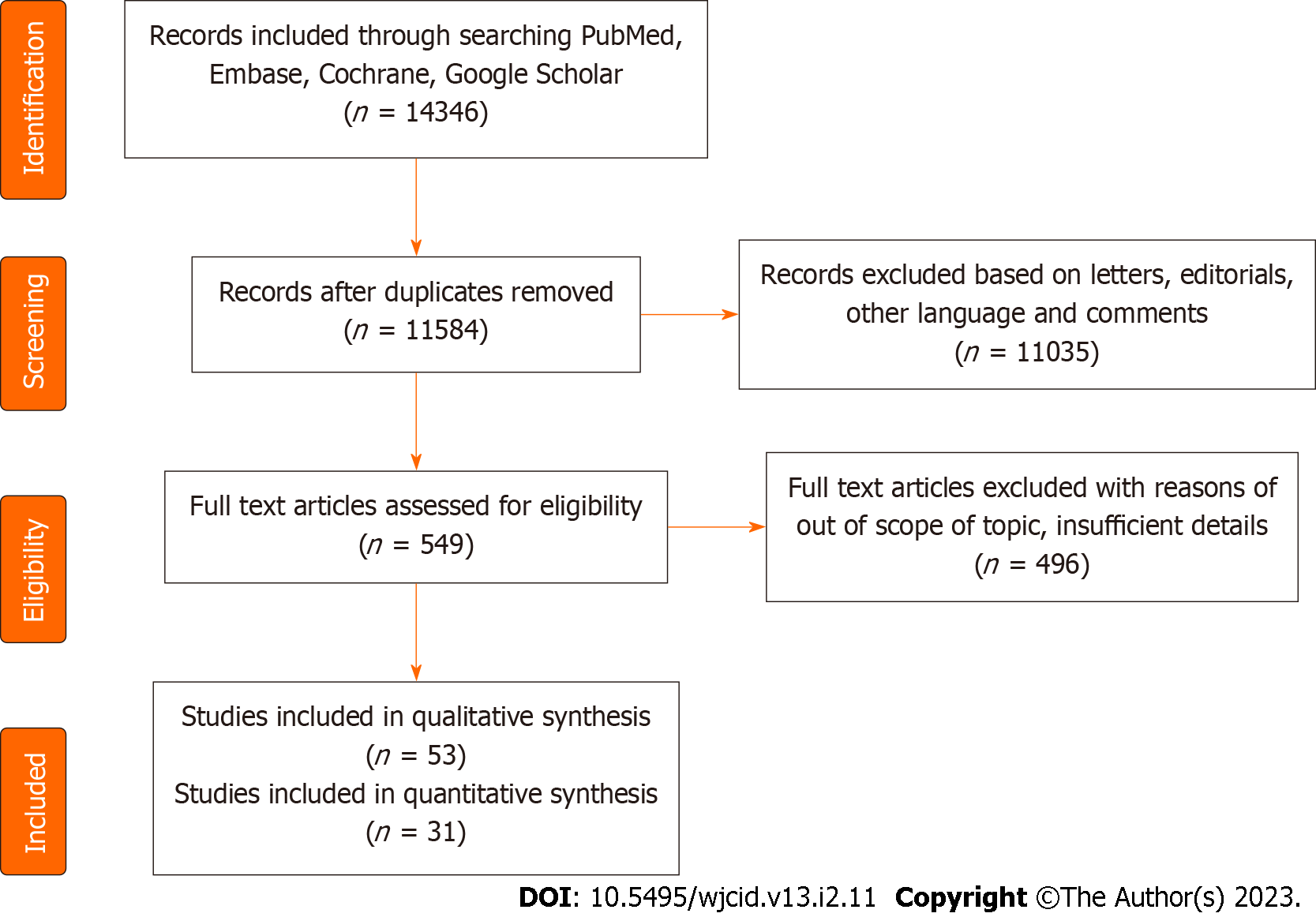
Across all literature and records available, 31 references were found which were relevant to our study (Table 1) among 51 qualitative syntheses (Figure 2). The sample size in these studies varied from a single case report to a study containing more than thousands of cases[3-33].
| Ref. | Sample size | Location (if available district, state, country) | Agent factors | Host factors | Vector identified | Environmental factors | River body associated | Authors conclusion |
| Katakura et al[4] | > 1000 | Different areas of Pakistan, India, and Nepal | In Pakistan Himalayas, Leishmania tropica followed by Leishmania major | CP: Cutaneous leishmaniasis (CL) cases only; No descriptions | In all Himalayas, P. sergenti followed by P. argentipes and papatasi | Altitude is not documented | Indus, Ganges | Microsatellite analysis of the parasites will be a powerful tool for population genetic and epidemiological studies of Leishmania species |
| Rx: Not known | ||||||||
| In India, L. donovani followed by L.tropica | Outcome: Not known | |||||||
| In Nepal, L. major | ||||||||
| Rab et al[5] | 239 (1984-1992) and more cases in the past (before 1984) | Different areas of Northern areas of Pakistan (Bagh, Abbottabad, Chilas, and Baltistan) | Leishmania | Clinical presentation (CP): | Not documented | Altitude is not documented | Indus | The clinical pattern of VL in north Pakistan is akin to that in north-western China, with a marked predilection for young children, and a male preponderance. The infantile VL has risen 10-fold in the last decade from 0.2 to almost 2 per 100 000 population |
| Visceral leishmaniasis (VL – all cases); Not described | ||||||||
| infantum | Rx: Not known | |||||||
| Outcome: Not known | ||||||||
| Wani et al[6] | 18 | Different areas of Uri &Karnah belt, Jammu & Kashmir, India | Leishmania, species not identified | CP: Cutaneous leishmaniasis (CL); mostly nodulo-ulcerative, mostly on the face and single lesion | Not documented | Altitude is not documented. The hot and arid climate of these areas(Uri belt) is quite conducive to the growth and development of leishmania and the sand fly | Not documented | Any patient with nodular/nodulo-ulcerative lesion on exposed parts must be suspected for CL, especially if belonging to the Uri and Karnah region of the Kashmir Valley. The public health authorities should make every effort to contain this new infection in this Valley |
| Rx: Intravenous sodium | ||||||||
| Stibogluconate including two received intra-lesional | ||||||||
| Outcome: Survival for all cases | ||||||||
| Leherwal et al[7] | Three | Uri belt, Jammu & Kashmir, India | Leishmania, species not identified | CP: Cutaneous leishmaniasis (CL); solitary erythematous nodule on the face | Not documented | Altitude is not documented | -do- | Focuses on the diagnostic part. FNAC may be the method of choice for suspected CL in cases of solitary nodular lesions |
| Rx: Not documented | ||||||||
| Outcome: Not documented | ||||||||
| Mahajan et al[8] | One | Uri in South West Kashmir, Jammu & Kashmir, India | Leishmania, species not identified | CP: Visceral leishmaniasis (VL); 2months fever, weight loss, ascites, anemia, Hepato-splenomegaly, | Not documented | Altitude is not documented | -do- | This advice for further research into the epidemiology, geographic distribution, and inter-species interactions of the parasite |
| Rx: Intravenous sodium | ||||||||
| Stibogluconate | ||||||||
| Outcome: Survived | ||||||||
| Sharma et al[9] | 285 | Nirmand village,Shimla & Kullu Districts of Himachal Pradesh, India | Among 14 cases, Leishmania tropica (3) and Leishmania donovani (11) | CP: CL; mostly nodulo-ulcerative, mostly on extremities | Among 41 cases, P. longiductus (29), P. major(8), P. kandelaki (2), and 2 remained unidentified | Altitude is not documented | Satluj river | Different leishmania species and vectors compared to other parts of India are found in these Himalayas |
| Tissue smear positivity for amastigotes was 43% | Rx: Intra-leisonal sodium | The climate of the affected areas varies from temperate to subtropical | ||||||
| Stibogluconate | ||||||||
| Outcome: Survival for all cases | ||||||||
| Sharma et al[10] | 161 new localized cases of LCL from May 2001 and December 2003 | sub-alpine valley in the mountainous region of the Kinnaur District,Himachal Pradesh, India | L. donovani in eight cases and L. tropica in two cases | Histopathology showed non-caseating epitheloid cell granuloma in 77% of the cases. Lesions involved mainly the face | Phlebotomus longiductus is a possible vector | Altitude, 700-2,900 m above sea level | Satluj River | Intralesional sodium stibogluconate was effective in all patients |
| Raina et al[11] | 18 | Shimla, Kinnaur & Kullu Districts of Himachal Pradesh, India | Leishmania, species not identified | CP: VL - prolonged fever, weight loss, ascites, pancytopenia, hepato-splenomegaly, lymphadenopathy, diarrhea, and epistaxis | Not documented | Altitude, 924 - 2960 m above sea level | Satluj and Beas river | Initial failure to suspect VL in this area might cause a diagnostic delay |
| Rx: Intravenous sodium | There is a favorable therapeutic response without recurrence of symptoms during 6 months of follow-up | |||||||
| Stibogluconate | The patients had never visited any of the endemic areas | |||||||
| Outcome: 14 Survives and 4 deaths | ||||||||
| Thakur et al[46] | Cases of CL During 2014–2018 in the study area | case reports came from Districts of Kinnaur, Shimla, and Kullu and the previously nonendemic districts of Mandi and Solan,Himachal Pradesh, India | L. donovani variants distinct from the viscerotropic L. donovani strain from northeast India | Coexistence of VL and CL | Not documented | Not documented | Not documented | The scenario appears somewhat similar to Sri Lanka and Kerala, where L. donovani parasites cause cutaneous disease, albeit with differences in the region-specific L. donovani variants |
| Thakur et al[47] | Sixty CL patients over the period from 2014 to 2018 | Satluj river belt in Himachal Pradesh, Khaneri/rampur (location of medical college),Himachal Pradesh, India | Presence of L. seymouri co-infection in the unusual CL cases in Himachal Pradesh (HP) caused by L. donovani variants | Coexistence of VL and CL | Not documented | Not documented | Satluj river | Found the presence of Leptomonas seymouri in 38.5% (22/57) of the patients along with L. donovani detected in all the samples. L. seymouri is a monoxenous insect trypanosoma, generally incapable of infecting humans |
| Sharma et al[49] | None | Shimla, Kinnaur, &Kullu Districts of Himachal Pradesh, India | Not applicable | Not applicable | Among 62 cases, Phlebotomus longiductus (46), P. major (8), P. kandelaki (8) | Our patients reported having been out of the state or district during the three years the preceding onset of symptoms | Satluj river | Phlebotomus longiductus may be the primary vector for human leishmaniases in this endemic focus, however, it needs another study to prove the vector species corresponding to the type of leishmania species |
The studies reviewed were specially chosen from the southern Himalayan region to emphasize the growing concern of leishmania in newly endemic areas. Among all reviewed studies, one was conducted in north Pakistan, twenty-three in north India, four in Nepal, and two in Bhutan. Among Indian studies, three were in Kashmir, eleven in Himachal Pradesh (Shimla, Chamba & Kinnaur), and nine in Uttarakhand (Garhwal & Kumaon). One study was multi-centric, covering vast geographical areas falling in Pakistan, India, and Bhutan[4].
Considering the pivotal role of the environment in the natural history of disease meticulous scrutiny of various articles was done. The majority of studies included in this review have been conducted along the banks of major river-belts of the terrain (Figure 3). In northern Pakistan, the major river associated was Indus and its tributaries. In northern India, the Uri Belt of Jammu & Kashmir, the river belt of Satluj and Ravi in Himachal Pradesh, and the bank of the river Ganges in Uttarakhand were the major site of focus. In Nepal, a total of four studies have been reported which were conducted along the banks of river Budhi Ganga and Kailash. One study from the mid-west region of Nepal has not documented an associated river, but further search for location indicates the site belongs to the banks of river Karnali. Similarly, studies from eastern Bhutan have not specified associated rivers but the described areas are mainly located between the three major rivers-Drangme Chhu, Kuru Chhu, and Mangde Chhu, all are tributaries of the Brahmaputra river. A multi-national study from South and South-east Asia also reported Indus and the Ganges to be the major associated river[4]. Among all these studies none of them established a direct association between the presence of any major water bodies & ecological niche conducive for the vector species.
Although the majority of the reviewed studies did not identify the vector species, Phlebotomus argentipes was the pre dominant vector species among all the reported cases[5-7]. Few studies have also found some different species as a possible vector such as P. longiductus> P.major> P. kandelaki as a leading species of the vector in studies of Shimla & Kullu districts of Himachal Pradesh, India[8,9]. Similarly, one study from Bhutan has also reported four different phlebotomine species[10].
The existence of L. donovani was ubiquitous however the quest to identify the predominant causative leishmania species remains unresolved as the majority of the studies did not identify any. Among the studies included in our review, five studies have reported L. donovani[10-15], while two studies reported L. infantum[16], as the predominant leishmanias pecies. Few studies indicated the presence of dual-species like both L. tropica and L. donovani[4,8,9], were documented in three studies and both L. infantum and L. Donovani[17] were documented in a single study. It Is also recorded that L. donovani variants found in Himachal Pradesh, India were different from the viscera tropic leishmania strain predominant in north east India[11].
The majority of the studies reported cases of visceral leishmaniasis (VL) with high-grade prolonged fever, malaise, abdominal discomfort[3,7,10,13,14,16,18-27]. Cutaneous leishmaniasis (CL) was reported in a few studies with clinical presentation of nodulo-ulcerative lesions or solitary erythematous nodule[4,8,9,12,28,29]. Three studies reported cases with both types (VL and CL) of leishmaniasis[11,17,30]. Another three studies did not identify the type of leishmaniasis however they described a clinical picture of hepatomegaly and weight loss as a common feature in their studies[15,31,32].
Methods of laboratory diagnosis were not documented in any of the reviewed literature, however, smear-positive by Giemsa or Leishman technique for Leishmania donovani (LD) bodies are reported in most cases. LD bodies were demonstrated in the bone marrow in the case of VL and from the skin in the case of CL[13,18,28]. Some studies also found LD bodies in splenic aspirate, lymph node aspirate, duodenal and colonic mucosal biopsy in patients presenting with diarrhea[3,6,9,22]. Only in a few reference studies, there were records of other methods (mostly rK39 ICT) as an additional test. One case report of a pregnant lady was found rK16 test positive, rather than commonly used rK39 antigen[21]. Secondary hemophagocytosis lymphocytic syndrome (HLH) in VL cases was diagnosed either by 4 out of 6 criteria of HLH diagnosis or by bone marrow aspirate examination for hemophagocytosis[20,21,23]. Rarely polymerase chain reaction (PCR) for the leishmania kinetoplast mini circle gene was tested and found to be positive in a case of L. donovani infection which was confirmed on subsequent sequencing of the PCR – amplification method[27]. An age-old aldehyde test was found positive for five out of six cases of kala-azar, however, they confirm edit either by rK39 testing or by bone marrow aspiration examination for the L D bodies[33].
Pharmacological therapies with sodium stibogluconate, amphotericin-B or miltefosine, either single or in various combinations had been reported in 21 reviewed studies. Studies were done in northern Pakistan and the Uri belt of Kashmir did not document the pharmacotherapy used and hence the subsequent outcomes[4,16,29]. In the case of VL, studies had reported intravenous sodium stibogluconate alone is sufficient for upto 84% of cases (19 survivals and 5 deaths, out of 24 cases)[8,18,25]. However, some studies were not clear about the route of stibogluconate therapy (intravenous or intralesional). Plain amphotericin-B showed > 90% recovery rate and liposomal showed upto 100% cure rate[7,13,20,21,22,31,32]. Various studies have a different outcome for the combinations of drugs, like, in one study, a combination of sodium stibogluconate and plain amphotericin-B resulted in 2 deaths out of 4 cases (50%cure rate), while three drugs combination (sodium stibogluconate + plain amphotericin-B+ miltefosine) for 33 cases resulted in all cure with one relapse which later treated with liposomal amphotericin-B (100% curerate)[23].
For CL diagnosed cases use of intra-lesional sodium stibogluconate alone showed recovery of all 285 cases (100% cure rate)[8]. Inspiring results were also seen in cases where the combination of intravenous and intra-lesional stibogluconate resulted in the survival of all 18 cases[28].
In case of relapse or failure, liposomal or plain amphotericin-B was most commonly used, this showed diverse efficacy in different studies. Like in one, out of 10 cases, 6 survived, 3 Lost to follow up and 1 resulted in death after the use of plain amphotericin-B[26]. While in another study, plain amphotericin-B was sufficient for the relapsed case after initial sodium stibogluconate (intralesional or intravenous not explained)[10]. A similar instance was reported in a study where plain amphotericin-B was given after failed miltefosine therapy and the case survived[10].
The dose of all drugs was not available in studies, however, a single dose (10 mg/kg) of liposomal amphotericin-B was used with a 100% cure rate including one relapse case after use of plain amphotericin-B[13].
The thirty-one studies of southern Himalayas show emerging leishmaniasis in high-altitude areas. The disease profile is distinctive from typical endemic areas. This can be discussed under various aspects of disease profile.
Leishmaniasis is prevalent mainly in the poor and marginalized communities of the world, predominantly of the Indian subcontinent like Bangladesh, India, and Nepal. However, recent studies are suggestive of the emergence of new endemic foci in various parts of the world as well. In 2017, 94% of new VL cases were reported in seven countries: Brazil, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan while the majority of CL cases reported from Afghanistan, Algeria, Brazil, Colombia, the Islamic Republic of Iran, Pakistan, Peru, Saudi Arabia, and the Syrian Arab Republic[34]. Some latest sporadic cases have also been reported from Bhutan and Thailand[32]. All these countries share a similar topography, ecological and environmental factors (high humidity, adequate rainfall, and surface dampness) which are favorable for the proliferation of Phlebotomes. Results of recent studies demonstrate that now leishmaniasis is not confined to a specified topography, rainfall, temperature, or vegetation, it has now continuously expanded its geographical distribution which can be explained by factors such as rapid growing globalization, global warming, deforestation, and urbanization. These facts can’t be confirmed as very few epidemiological studies are available on this issue. Furthermore, reviewing the literature, it was observed that the majority of the cases have been reported along with the major river belts in these new areas. This observation is highly suggestive of possible up stream migration of vectors along the rivers. In the past 15 years of reporting, good numbers of cases were found in newly endemic areas of Bhutan, Nepal, India (Uttarakhand, Himachal Pradesh, Jammu and Kashmir),and Northern parts of Pakistan.
Sandfly, vector of VL and CL, includes many species of the genus Phlebotomus (in the Old World) and Lutzomyia longipalpis (in the New World)[35,36]. Although the majority of the reviewed studies have not mentioned the associated vectors, Phlebotomus argentipes was found to be the predominant vector among the reported cases except in Himachal Pradesh (India) where P. longiductus and P. major were identified in co-existence. Interestingly P. argentipes remain closely associated with the exclusive cases of VL while P. longiductus (most common) and P. major were associated with areas where both CL & VL forms were found (Table 2). Therefore, the associated area needs an entomological study to know the basic characteristics of the vector and associated factors.
| Sr No. | Geographical area | Causative agent | Vector | Clinical picture |
| 1 | Northern areas of Pakistan[2] | LeishmaniaInfantum | Not identified | Visceral leishmaniasis |
| 2 | Indian states of Jammu & Kashmir[3-5] | Not identified | Not identified | Cutaneous leishmaniasis most common with a single case study of visceral Leishmaniasis |
| 3 | Himachal Pradesh[6-16] | L. donovani & L. tropica | P. longiductus (most common) & P. major | Both cutaneous & visceral forms ofLeishmaniasis |
| 4 | Uttarakhand (Garhwal)[17-21] | L. donovani | P. argentipes | Visceral leishmaniasis |
| 5 | Uttarakhand (Kumaon)[22-25] | Not identified | P. argentipes | Visceral leishmaniasis |
| 6 | Nepal[26-29] | L. donovani | P. argentipes | Visceral leishmaniasis |
| 7 | Bhutan[30,31] | L. donovani | P. argentipes | Visceral leishmaniasis |
L. donovani transmission in East Africa consists of both anthroponotic and zoonotic components[37]. In Sudan, rodents and dogs were found to be reservoirs; however, observation in the majority of outbreaks reflects anthroponotic predominant transmission[38,39]. While in SEAR countries, the human being is the only reported reservoir. In this review also, we found a similar finding of the human being as the sole reservoir for VL.
Major species of parasites of VL are reported as L. donovani in South Asia and L. infantum in the Mediterranean region along with some sporadic cases in Central Asia, China, Mexico and Central Brazil[35,40]. The central western area of Brazil which is considered an area of recent transmission for VL and is on the risk for CL, L. longipalpis was the widespread species discovered[40]. In the new world, the most common etiological agent is L. infantum. The current review also documents similar findings of L. donovani in the majority of studies but one study from the Himalayan areas of Pakistan reported L. infantum in the majority[16]. A study in Brazil documented to have detected for the first time the presence of either L. infantum or L. braziliensis circulating in the domestic host[41]. In India, VL is caused by L. donovani in the north eastern region, and CL is caused by L. tropica in the western Thar Desert region[42]. Himachal Pradesh is a more recently leishmaniasis endemic state in north-west India where VL and CL coexist. The incidence of CL is higher than that of VL and most cases are attributable to L. donovani[33,43]. One of the studies conducted in the same region reported an interesting presence of Leptomonas seymouri co-infection in CL with L. donovani[30]. Undoubtedly there may be some missing links and associations that are still unknown and undiscovered since no other areas around Himachal Pradesh of the southern Himalayan region reported any remarkable epidemiological studies. Therefore, this review may act as a catalyst to perpetuate epidemiological search in this region to establish various niches.
CL in the New World is generally caused by L. mexicana, while CL of the Old World is caused by five species of Leishmania: L. infantum (more common), L. tropica, L. major, L. aethiopica, and L. donovani. However, a study in the Indian sub-continent documents L. tropica in Pakistan, L. donovani and L. major in Nepal are the most common organism causing CL[4]. PKDL is caused primarily by L. donovani both in India and Sudan with only a few cases by L. infantum or L. chagasi[28].
This shows the existence of different types of species for both VL and CL in different parts of the South Asian countries including the southern Himalayas of the Indian Sub-continent. The rationale behind this diversity and associated epidemiological factors needs to be studied further.
VL has different clinical features in the endemic, epidemic, or sporadic situation. It tends to be relatively chronic and mostly affects children in endemic areas. Both the VL and CL are endemic in Pakistan and India while only VL is endemic in Nepal and Bhutan (WHO updates). Study analysis revealed that the characteristics of the disease vary with the environment. Here we see the preponderance of VL in Bhutan, Nepal, and Uttarakhand (India) with the coexistence of CL and VL in the Indian states of Himachal Pradesh, Jammu and Kashmir and Pakistan (Table 1).
Most cases are asymptomatic, but some eventually develop VL on follow-up, more commonly in males[35]. Risk factors for progression to VL include malnutrition, genetic factor and other co-infections, mainly HIV. The major classical presentation is prolonged fever, fatigue, loss of appetite and weight, and left hypochondrium discomfort. There may be non-tender splenomegaly with or without hepatomegaly, pallor, and lymphadenopathy (especially in Sudan, commonly by Viannia subgenus species). The darkening of the skin is typical for the Indian variant (Hindi name, kala-azar). Clinically CL usually exhibits painless, multiple, round-to-oval crater-form dry nodular lesions, mostly at the site of inoculation. Usually, these cutaneous lesions heal spontaneously in 1year, often with disfiguring scars. PKDL is extremely rare, confined mainly in two regions endemic to kala-azar the Indian sub-continent and Sudan plus adjoining areas (up to 50% and 10% of patients with kala-azar respectively)[44-46].
Among all the studies reviewed none of them documented an asymptomatic period. The majority documented similar classical VL and CL symptoms except a few, which documented some atypical presentations like ascites, diarrhea, epistaxis, HLH syndrome, and hypergammaglobulinaemia (Table 1). Few cases of PKDL were reported from the hilly area of Uttarakhand too. The occurrence of PKDL after VL treatment in Nepal is also low as compared to neighboring countries[47].
For diagnosis of Leishmaniasis many tests like dual path platform, a rapid immune-chromatographic test, and enzyme linked immune-sorbent assay (ELISA) are recommended by the Brazilian Ministry of Health[41]. Govt. of India recommends various tests for the detection of leishmania, including serology, aldehyde test, complement fixation test, indirect hem-agglutination test, ELISA, direct agglutination tests (DAT), spleen or bone marrow aspirates, and rK39[43]. The diagnostic policy for leishmaniasis is variable depending on the level of health systems. In first-line centers or rural hospitals of the highly endemic zone, the rK39 test is mostly used. Parasitological diagnosis is necessary for relapse identification. In low- endemic areas, more specific tests like PCR or parasitic demonstration are found necessary, as PCR is more sensitive than microscopic examination, therefore, can detect more asymptomatic infections. However, it is not available in most centers, and evaluation of its diagnostic accuracy and proper standardization is needed. For relapse, serological tests such as DAT, ELISA, and rK39 rapid test are usually positive and frequently used in majority areas but are of limited value, as a positive result may be due to antibodies persisting after a past episode of VL, so better to show parasitological evidence for confirmation. A study in Brazil documented use of nested PCR (LnPCR) and PCR-restriction fragment length polymorphism for identification of Leishmania species[41]. A careful perusal of studies in this review showed a comprehensive use of various diagnostic procedures with no conclusive evidence towards any particular method. Future studies in these regions are need of the hour to formulate a diagnostic policy suitable for primary to tertiary health care levels.
Depending upon the sensitivity of drugs and the economic status, the treatment regime varies in different parts of the world. Liposomal amphotericin- B monotherapy (total dose of 20 to 21 mg/kg) is the preferred treatment in Europe, North America, and South America[48,49]. In East Africa, first-line therapy consists of a combination treatment of sodium stibogluconate and paromomycin for 17 d; the efficacy of liposomal amphotericin-B, miltefosine and paromomycin monotherapy are unacceptably low[50]. WHO Expert Committee and the Regional Technical Advisory Group of SEAR recommends liposomal amphotericin-B in a single dose of 10 mg/kg body weight as the first line treatment regimen for the Indian subcontinent within the current elimination strategy, given its high antimicrobial efficacy, safety, ease of use and assured compliance[1].
The majority of the studies in this review comply with the above standards and none of them documented parallel or supplemental pharmacotherapy other than the recommended regimen. However, agreeable documentation about the efficacy of the above drugs cannot be established in these emerging foci, as different studies had different outcomes. On summarizing the treatment outcomes liposomal amphotericin-B has emerged as the most effective therapy against the disease (with 100% cure rate achieved with single-dose). Furthermore, it is also found to be effective in VL-associated HLH and the explanations were that it inhibits macrophage function, reduces cytokine expression, and antigen-induced proliferation of T and B cells in vitro, causing a dual effect on both HLH and VL[13]. At last, the treatment regimen must follow national or regional guidelines, if applicable. Species identification usually is not critical to treatment decisions for VL (incontrast with CL)[51]. Multiple trial studies regarding drugs and doses should be done for the best suitable management protocol in these new niches.
As said before, the availability of only a few studies related to the Himalayan regions is the major limitation of this review. Limited studies have covered the factors determining the transmission of VL in these new foci. The paucity of data limits the freedom to give any conclusive remarks on this new possible niche of leishmaniasis. A detailed analysis of these factors and the molecular characterization of vector species and leishmaniasis strain are still lacking. However, this mini-review aspires to highlight the surge of new cases in non-endemic areas as a matter of public health importance and research.
Despite substantial progress towards VL elimination in most endemic parts of the world, recently reported the emergence of new endemic foci in Southern Himalayas, forecast a great challenge for public health. Upstream river belts are a possible path of Sandfly spread towards these non-endemic areas, need a better environmental study to prove. In these areas, P. argentipes is found to be a predominant vector, L. donovani as a major parasite cause of VL, and L. tropica, L. donovani, and L. major as a major cause of CL in Pakistan, India, and Nepal respectively. Isolated VL is seen in Bhutan, Nepal, and the Uttarakhand state of India, while both VL and CL are seen in other Himalayan areas. Moreover, patients of these areas have a typical clinical presentations (ascites, diarrhea, epistaxis, HLH syndrome, and hypergammaglobulinaemia) so they need a high index of clinical suspicion, prompt diagnosis, and management. Single-dose liposomal amphotericin-B holds a 100% cure rate. As the a typical disease is recognized as a major threat to ongoing leishmaniasis elimination, so continuous monitoring of the disease type and associated parasitic variants and vector species should be implemented as part of the ongoing leishmaniasis elimination and maintenance programs. Studies on vector species and alternate reservoirs are also required for a better understanding of region-specific disease transmission and epidemiology.
| 1. | World Health Organization. Kala-Azar elimination program: report of a WHO consultation of partners, Geneva, Switzerland, February 10-11, 2015. Available from: https://apps.who.int/iris/bitstream/handle/10665/185042/9789241509497_eng.pdf?sequence=1. |
| 2. | Hirve S, Kroeger A, Matlashewski G, Mondal D, Banjara MR, Das P, Be-Nazir A, Arana B, Olliaro P. Towards elimination of visceral leishmaniasis in the Indian subcontinent-Translating research to practice to public health. PLoS Negl Trop Dis. 2017;11:e0005889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Raina S, Mahesh DM, Kaul R, Satindera KS, Gupta D, Sharma A, Thakur S. A new focus of visceral leishmaniasis in the Himalayas, India. J Vector Borne Dis. 2009;46: 303-306. [PubMed] |
| 4. | Katakura K. Molecular epidemiology of leishmaniasis in Asia (focus on cutaneous infections). Curr Opin Infect Dis. 2009;22:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Verma SK, Ahmad S, Shirazi N, Kusum A, Kaushik RM, Barthwal SP. Sodium stibogluconate-sensitive visceral leishmaniasis in the non-endemic hilly region of Uttarakhand, India. Trans R Soc Trop Med Hyg. 2007;101:730-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Rao JS, Sharma SK, Bhattacharya D, Saxena NB. Sandfly survey in Nainital and Almora districts of Uttaranchal with particular reference to Phlebotomus argentipes, vector of kala-azar. J Commun Dis. 2001;33:7-11. [PubMed] |
| 7. | Bhattacharya SK, Rinzin N, Chusak P, Dash AP, Chowdhury R, Tobgay T, Narain JP. Occurrence & significance of kala-azar in Bhutan. Indian J Med Res. 2010;132:337-338. [PubMed] |
| 8. | Sharma NL, Mahajan VK, Negi AK. Epidemiology of a new focus of localized cutaneous leishmaniasis in Himachal Pradesh. J Commun Dis. 2005;37:275-279. [PubMed] |
| 9. | Sharma NL, Mahajan VK, Kanga A, Sood A, Katoch VM, Mauricio I, Singh CD, Parwan UC, Sharma VK, Sharma RC. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: preliminary findings of the study of 161 new cases from a new endemic focus in himachal pradesh, India. Am J Trop Med Hyg. 2005;72:819-824. [PubMed] |
| 10. | Yangzom T, Cruz I, Bern C, Argaw D, den Boer M, Vélez ID, Bhattacharya SK, Molina R, Alvar J. Endemic transmission of visceral leishmaniasis in Bhutan. Am J Trop Med Hyg. 2012;87:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Thakur L, Singh KK, Kushwaha HR, Sharma SK, Shankar V, Negi A, Verma G, Kumari S, Jain A, Jain M. Leishmania donovani Infection with Atypical Cutaneous Manifestations, Himachal Pradesh, India, 2014-2018. Emerg Infect Dis. 2020;26:1864-1869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Sharma RC, Mahajan VK, Sharma NL, Sharma A. A new focus of cutaneous leishmaniasis in Himachal Pradesh (India). Indian J Dermatol Venereol Leprol. 2003;69:170-172. [PubMed] |
| 13. | Kumari S, Dhawan P, Panda PK, Bairwa M, Pai VS. Rising visceral leishmaniasis in Holy Himalayas (Uttarakhand, India) - A cross-sectional hospital-based study. J Family Med Prim Care. 2020;9:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ostyn B, Uranw S, Bhattarai NR, Das ML, Rai K, Tersago K, Pokhrel Y, Durnez L, Marasini B, Van der Auwera G, Dujardin JC, Coosemans M, Argaw D, Boelaert M, Rijal S. Transmission of Leishmania donovani in the Hills of Eastern Nepal, an Outbreak Investigation in Okhaldhunga and Bhojpur Districts. PLoS Negl Trop Dis. 2015;9:e0003966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Pandey BD, Pun SB, Kaneko O, Pandey K, Hirayama K. Case report: Expansion of visceral leishmaniasis to the western hilly part of Nepal. Am J Trop Med Hyg. 2011;84:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Rab MA, Evans DA. Leishmania infantum in the Himalayas. Trans R Soc Trop Med Hyg. 1995;89:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Sharma NL, Mahajan VK, Negi AK, Verma GK. The rK39immunochromatic dipstick testing: a study for K39 seroprevalence in dogs and human leishmaniasis patients for possible animal reservoir of cutaneous and visceral leishmaniasis in endemic focus of Satluj river valley of Himachal Pradesh (India). Indian J Dermatol Venereol Leprol. 2009;75:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Mahajan D, Bhat ML, SinghJB, Hans D. Visceral LeishmaniasisInA Native Kashmiri Boy. JK Sci. 2009;11:152-153. |
| 19. | Raina S, Raina RK, Sharma R, Rana BS, Bodh A, Sharma M. Expansion of visceral leishmaniasis to northwest sub-Himalayan region of India: A case series. J Vector Borne Dis. 2016;53:188-191. [PubMed] |
| 20. | Raina RK, Raina S, Sharma M. Visceral leishmaniasis-associated hemophagocytosis: A tale of two unexpected diagnoses from a nonendemic region. Trop Parasitol. 2017;7:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Pawar S, Ragesh R, Nischal N, Sharma S, Panda PK, Sharma SK. Unique Triad of 'Pregnancy, Kala Azar and Hemophagocytic Lymphohistiocytic Syndrome from a Non-Endemic Region'. J Assoc Physicians India. 2015;63:65-68. [PubMed] |
| 22. | Chandra H, Chandra S, Kaushik R, Bhat N, Shrivastava V. Hemophagocytosis on bone marrow aspirate cytology: single center experience in north himalayan region of India. Ann Med Health Sci Res. 2014;4:692-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Ahmad S, Chandra H, Bhat NK, Dhar M, Shirazi N, Verma SK. North Indian state of Uttarakhand: a new hothouse of visceral leishmaniasis. Trop Doct. 2016;46:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Kumar Bhat N, Ahuja V, Dhar M, Ahmad S, Pandita N, Gupta V, Chandra S. Changing Epidemiology: A New Focus of Kala-azar at High-Altitude Garhwal Region of North India. J Trop Pediatr. 2017;63:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Singh S, Biswas A, Wig N, Aggarwal P, Sood R, Wali JP. A new focus of visceral leishmaniasis in sub-Himalayan (Kumaon) region of northern India. J Commun Dis. 1999;31:73-77. [PubMed] |
| 26. | Kumar A, Rawat V, Thapliyal N, Saxena SR. Kala-azar-A case series from the nonendemic area, Uttarakhand. Ann Trop Med Public Health. 2013;6:355-357. [DOI] [Full Text] |
| 27. | Mathur SB, Arya AK. Nonmigrant children with visceral leishmaniasis from the nonendemic area of Uttarakhand. J Trop Pediatr. 2014;60:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Wani GM, Ahmad SM, Khursheed B. Clinical study of cutaneous leishmaniasis in the Kashmir Valley. Indian Dermatol Online J. 2015;6:387-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Leherwal MA, Yasin SB, Ahmed SB. Diagnosis of cutaneous leishmaniasis by FNAC-Report of three cases. J Cytol. 2004;21:103-105. |
| 30. | Thakur L, Kushwaha HR, Negi A, Jain A, Jain M. Leptomonas seymouri Co-infection in Cutaneous Leishmaniasis Cases Caused by Leishmania donovani From Himachal Pradesh, India. Front Cell Infect Microbiol. 2020;10:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Pandey BD, Pandey K, Kaneko O, Yanagi T, Hirayama K. Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am J Trop Med Hyg. 2009;80:580-582. [PubMed] |
| 32. | Schwarz D, Andrews J, Gauchan B. Visceral leishmaniasis in far western Nepal: another case and concerns about a new area of endemicity. Am J Trop Med Hyg. 2011;84:508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Sharma NL, Sood A, Arora S, Kanga A, Mahajan V, Negi AK, Sharma AK. Characteristics of Leishmania spp. isolated from a mixed focus of cutaneous and visceral leishmaniasis in Himachal Pradesh (India). Int J Third World Med. 2009;7. [DOI] [Full Text] |
| 34. | World Health Organization. Visceral leishmaniasis-WHO publishes validation documents as countries approach elimination. Available from: https://www.who.int/neglected_diseases/news/Visceral_leishmaniasis_WHO_publishes_validation_document/en/. |
| 35. | World Health Organization. Control of the Leishmaniasis: Report of the WHO Expert Committee Meeting, Geneva. March 22-26, 2010. Available from: https://apps.who.int/iris/handle/10665/44412. |
| 36. | Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990;4:1-24. [PubMed] [DOI] [Full Text] |
| 37. | Alvar J, Bashaye S, Argaw D, Cruz I, Aparicio P, Kassa A, Orfanos G, Parreño F, Babaniyi O, Gudeta N, Cañavate C, Bern C. Kala-azar outbreak in Libo Kemkem, Ethiopia: epidemiologic and parasitologic assessment. Am J Trop Med Hyg. 2007;77:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Bucheton B, Kheir MM, El-Safi SH, Hammad A, Mergani A, Mary C, Abel L, Dessein A. The interplay between environmental and host factors during an outbreak of visceral leishmaniasis in eastern Sudan. Microbes Infect. 2002;4:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Dereure J, El-Safi SH, Bucheton B, Boni M, Kheir MM, Davoust B, Pratlong F, Feugier E, Lambert M, Dessein A, Dedet JP. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008;2:e313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 41. | Menezes JA, Ferreira Ede C, Andrade-Filho JD, de Sousa AM, Morais MH, Rocha AM, Machado-Coelho GL, Lima FP, Madureira AP, Garcia TC, Freitas CR, Soares RP, Margonari C. An Integrated Approach Using Spatial Analysis to Study the Risk Factors for Leishmaniasis in Area of Recent Transmission. Biomed Res Int. 2015;2015:621854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Thakur L, Singh KK, Shanker V, Negi A, Jain A, Matlashewski G, Jain M. Atypical leishmaniasis: A global perspective with emphasis on the Indian subcontinent. PLoS Negl Trop Dis. 2018;12:e0006659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | National Center for Vector Borne Diseases Control (NCVBDC). Guidelines-Diagnosis and treatment of Kala-azar. [Accessed on 10 October 2021] Available from: http://nvbdcp.gov.in/Doc/Guidelines-Diagnosis-Treatment-KA.pdf. |
| 44. | Baghestani S, Handjani F, Sodeifi M, Kumar PV. Post-kala-azar dermal leishmaniasis. Eur J Dermatol. 1998;8:277-279. [PubMed] |
| 45. | Croft SL. PKDL--a drug related phenomenon? Indian J Med Res. 2008;128:10-11. [PubMed] |
| 46. | Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 385] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 47. | Uranw S, Ostyn B, Rijal A, Devkota S, Khanal B, Menten J, Boelaert M, Rijal S. Post-kala-azar dermal leishmaniasis in Nepal: a retrospective cohort study (2000-2010). PLoS Negl Trop Dis. 2011;5:e1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, Carvalho E, Ephros M, Jeronimo S, Magill A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2017;96:24-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 49. | Alvar J, Croft S, Olliaro P. Chemotherapy in the treatment and control of leishmaniasis. Adv Parasitol. 2006;61:223-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, Edwards T, Rashid J, Mbui J, Musa B, Abuzaid AA, Ahmed O, Fadlalla A, El-Hassan A, Mueller M, Mucee G, Njoroge S, Manduku V, Mutuma G, Apadet L, Lodenyo H, Mutea D, Kirigi G, Yifru S, Mengistu G, Hurissa Z, Hailu W, Weldegebreal T, Tafes H, Mekonnen Y, Makonnen E, Ndegwa S, Sagaki P, Kimutai R, Kesusu J, Owiti R, Ellis S, Wasunna M. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis. 2012;6:e1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 51. | Sundar S, Singh A. Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther Adv Infect Dis. 2016;3:98-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mukhopadhyay S, United States; Rychtář J, United States S-Editor: Gong ZM L-Editor: A P-Editor: Ju JL