Published online Jan 15, 2021. doi: 10.5495/wjcid.v11.i1.27
Peer-review started: September 10, 2020
First decision: October 21, 2020
Revised: November 7, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 15, 2021
Processing time: 125 Days and 1.4 Hours
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). This disease was declared a worldwide health problem with the characteristics of a pandemic. Most patients have mild symptoms and a good prognosis. Information on the evolution and prognosis of COVID-19 in solid organ recipients is scarce.
We describe two patients who underwent liver transplantation with a positive test result for detection of the viral sequence for COVID-19, using reverse-transcription polymerase chain reaction (RT-PCR), immediately before transplantation. The patients showed good evolution in the postoperative period, without signs of graft dysfunction. The immunosuppressive therapy was not modified. Both patients were discharged for subsequent outpatient follow-up.
In conclusion, it is expected that the experience at this center can be used as an example, aimed at the continuation of transplantations by other services and, thus, the morbidity and mortality of patients with liver disease on the transplantation waiting list can be reduced. Transplant centers must be able to readjust daily to the evolution of the COVID-19 pandemic.
Core Tip: Coronavirus disease 2019 (COVID-19), caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its consequences have resulted in high rates of morbidity and mortality worldwide in the first half of this year. This infection shows worse outcomes in certain at-risk populations, including those with cirrhosis of any etiology. Most patients with decompensated cirrhosis have poor quality of life and a high chance of progressing to death if they have high prognostic scores, such as the Model for End-Stage Liver Disease score. The definitive treatment for these patients is liver transplantation. Data related to the evolution and outcome of these patients when infected with SARS-CoV-2, including those undergoing transplantation, are scarce and contributions to the literature on this topic can help the adequate management of these patients, supporting the development of additional research and even guidelines. Thus, the publication of this report on two cirrhotic patients with COVID-19 who underwent liver transplantation is justified.
- Citation: Bastos Limeira CB, Veras CM, Lima Paiva JHHG, e Neves MSS, Teles de Carvalho TM, de Assunção Ferreira NS, Mont`Alverne Pierre AM, Brasil IRC. Liver transplantation in patients with SARS-CoV-2: Two case reports. World J Clin Infect Dis 2021; 11(1): 27-34
- URL: https://www.wjgnet.com/2220-3176/full/v11/i1/27.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v11.i1.27
In December 2019, the first cases of viral pneumonia of unknown origin were documented in Wuhan, the capital of China's Hubei province. The virus was identified as a new coronavirus, called “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)”. The infection was documented in hospital and community settings. Soon the virus spread throughout the Chinese territory and, subsequently, increasing numbers of cases were also observed in several continents[1]. Considering the severity of the situation, the World Health Organization (WHO) declared Coronavirus Disease 2019 (COVID-19) a public health emergency of international interest[2]. Liver transplantation programs were affected worldwide.
Most patients with COVID-19 have mild symptoms and a good prognosis. It is worth mentioning that asymptomatic cases have been described. However, patients with risk factors can develop severe SARS-CoV-2 disease, secondary to severe pneumonia, pulmonary edema, severe acute respiratory syndrome, acute kidney injury, coagulopathy or multiple-organ failure[3].
Based on data from other viruses, including SARS-CoV-2, immunosuppressed patients with COVID-19 were expected to have more severe clinical manifestations and a longer period of viral dispersal. However, the effects of immunosuppression on COVID-19 are not well established. Due to this fact, it remains controversial whether organ transplantation should be performed during the COVID-19 pandemic. Some recent guidelines suggested that transplantation can be performed as long as careful measures are taken[4].
A timely and accurate diagnosis, especially in cases with the potential to develop into the severe form of the disease, is extremely important to provide adequate clinical support to patients, and to limit the spread of the virus. Currently, detection of the viral sequence by reverse-transcription polymerase chain reaction (RT-PCR) is the test routinely used to confirm the diagnosis of SARS-CoV-2 infection[5].
This study aims to describe two patients with confirmed SARS-CoV-2 infection who successfully underwent liver transplantation.
Case 1: A 55-year-old male patient was admitted on March 9, 2020 to Hospital Geral de Fortaleza, state of Ceará, Brazil with a clinical picture of hepatic encephalopathy, abdominal pain, fever and upper gastrointestinal bleeding.
Case 2: A 40-year-old male patient, followed at the Liver Transplantation Service of Hospital Geral de Fortaleza was admitted on June 22, 2020 to undergo a liver transplantation with a Model for End-Stage Liver Disease-Sodium (MELD-Na) score of 24. The patient had no clinical complaints and was hemodynamically stable.
Case 1: The patient had a diagnosis of alcoholic-induced liver cirrhosis and a one-year withdrawal period.
Case 2: The patient was followed at the Liver Transplantation Service of Hospital Geral de Fortaleza due to liver cirrhosis caused by hepatitis B virus infection.
Case 1: The patient had no comorbidities, such as hypertension or diabetes. Moreover, he had no recent travel history.
Case 2: Previous complications in this patient included portal vein thrombosis. He had a history of peripheral vascular disease, with a healing venous ulcer in the left lower limb, with no signs of active infection. He had no other comorbidities and denied a travel history in recent months. He had a recent hospitalization history (20 d before) for intravenous antibiotic therapy due to erysipelas.
Cases 1 and 2: No relevant family history.
Case 1: Physical examination revealed the presence of massive ascites. The other systems showed no changes. On admission, vital signs showed a respiratory rate of 22 breaths/min (brpm), heart rate of 97 beats/min (bpm), 96% oxygen saturation in ambient air and blood pressure of 140/80 mmHg.
Case 2: On clinical examination, only mild jaundice and an ulcer in the left lower limb without signs of infection were observed. The other systems showed no changes. On hospital admission, vital signs showed a respiratory rate of 18 brpm, heart rate of 89 bpm, oxygen saturation of 97% in ambient air and blood pressure of 110/70 mmHg.
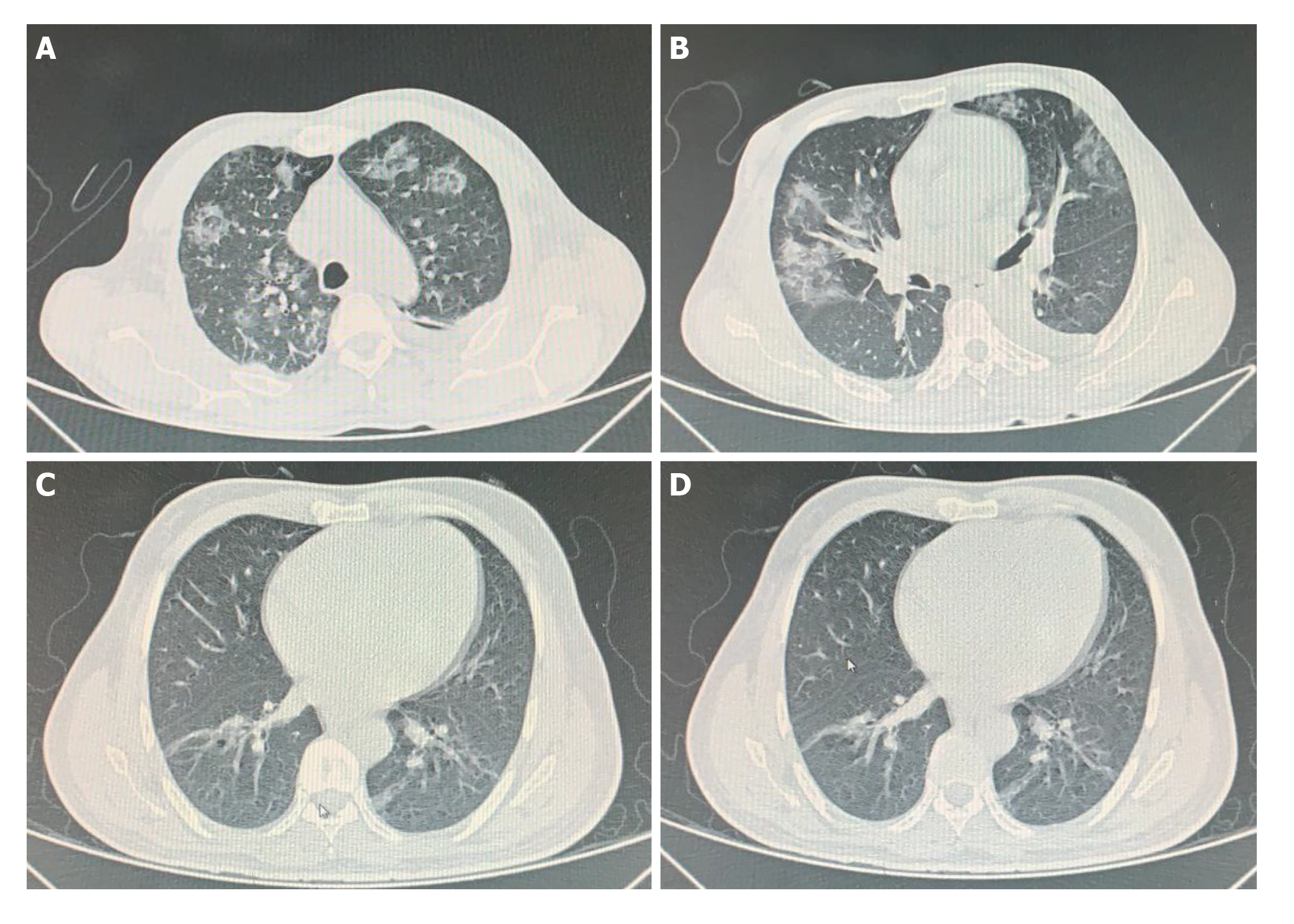
Case 1: A computed tomography scan of the chest was performed, which showed lungs with reduced volume, left pleural effusion, atelectasis of the adjacent parenchyma and multiple diffuse ground-glass opacities (Figure 1A and B).
Case 2: A computed tomography scan of the chest showed evidence of discrete foci of ground glass attenuation affecting the bases of the lungs and discrete bilateral parenchymal bands (Figure 1C and D).
Case 1: The patient’s evolution required dialysis for acute kidney injury and his ascites were refractory to clinical measures, and required several relief paracenteses. Piperacillin/tazobactam therapy was started, due to bacterial peritonitis. An upper gastrointestinal endoscopy was performed, which did not show the presence of gastroesophageal varices, but demonstrated the presence of severe candidiasis and thus, antifungal therapy with fluconazole was started, which was later replaced by caspofungin. However, due to the lack of improvement in the patient’s clinical status and laboratory tests, he was listed for liver transplantation according to the MELD-Na score of 35 and the Child-Pugh score of C. Despite the absence of respiratory symptoms, screening for SARS-CoV-2 infection was performed, with the collection of a nasopharyngeal swab for viral sequence detection by RT-PCR, but the result, which was positive, was only released after the transplant had been performed.
Liver transplantation was carried out on March 25, 2020, according to the standard surgical technique. During the procedure, the recipient developed cardiorespiratory arrest in asystole during the graft reperfusion period, which was effectively reversed with a cardiac massage cycle. The time of cold and hot ischemia was 6 h and 28 min and 32 min, respectively. The patient was extubated in the immediate postoperative period in the Intensive Care Unit and an O2 saturation of 96% was maintained in ambient air, with an oxygenation index of 400.
The donor was a 56-year-old female patient with a previous history of diabetes mellitus. The patient developed a history of hypertensive peak and a decrease in the level of consciousness due to an intraparenchymal brain hematoma, with significant midline deviations. There was no screening for COVID-19, as the transplant occurred at the beginning of the pandemic and there was no defined protocol on the screening of donors at that time.
Case 2: According to the protocol of our service, screening for SARS-CoV-2 infection was performed using a nasopharyngeal swab (RT-PCR), as well as chest radiography. There were no alterations in the chest X-ray, and it was not possible to obtain the nasopharyngeal swab result before transplantation. Considering the patient’s clinical condition of chronic liver failure at risk of worsening, the transplant team chose to proceed with the surgery.
The donor was a 42-year-old male patient who suffered a traumatic brain injury. He was receiving piperacillin-tazobactam due to a bacterial infection. He screened negative for COVID-19 following RT-PCR.
During the intraoperative period, the patient developed massive bleeding during the anastomoses, requiring vigorous volume replacement (3500 mL), 4 units of fresh frozen plasma and 2 units of packed red blood cells, in addition to blood recovery by cell-salvage. Moreover, an intraoperative thrombectomy was performed for portal vein thrombosis.
During the postoperative period, the patient was transferred to the Intensive Care Unit for patients with Coronavirus (ICU-COVID), and required invasive mechanical ventilation and vasopressors. Laboratory test results are shown in Table 1. The nasopharyngeal swab collected prior to surgery for viral sequence detection by RT-PCR was positive for SARS-CoV-2.
| Case 1 | Case 2 | |||||
| At hospital admission | Preoperative | At hospital discharge | At hospital admission | Preoperative | At hospital discharge | |
| Fibrinogen | 83 | NA | NA | 178 | NA | NA |
| aPTT | 1.97 | 1.6 | 1.2 | 1.4 | 0.96 | 0.88 |
| Hemoglobin | 8.7 | 6.0 | 11.1 | 11.8 | 8.9 | 6.4 |
| Hematocrit | 24.8 | 19.5 | 32.9 | 35.5 | 25.9 | 18 |
| Leukocytes | 22900 | 15100 | 9300 | 3100 | 16700 | 4300 |
| Lymphocytes | 711 | 615 | 2615 | 1092 | 754 | 492 |
| Platelets | 109000 | 100000 | 376000 | 42000 | 53000 | 20000 |
| INR | 2.72 | 2.78 | 1.02 | 1.84 | 1.28 | 1.32 |
| Total bilirubin | 7.44 | 23.25 | 0.74 | 2.87 | 2.07 | 1.46 |
| Albumin | 2.5 | NA | NA | 3.2 | NA | NA |
| Urea | 136 | 113 | 55 | 16 | 53 | 69 |
| Creatinine | 2.1 | 5.4 | 1.0 | 1.3 | 1.2 | 1.02 |
| AST | 105 | NA | 28 | 68 | 3005 | 1924 |
| ALT | 59 | NA | 39 | 35 | 1807 | 950 |
| Sodium | 125 | 127 | NA | 129 | 131 | NA |
SARS-CoV-2 infection and alcoholic-induced liver cirrhosis.
Following the release of the RT-PCR test result with a detectable SARS-CoV-2 viral load, the patient was transferred to the ICU-COVID; he was initially asymptomatic and therapy was started with azithromycin 500 mg/d for 5 d, ivermectin 12 mg/d for 2 d and oseltamivir 150 mg/d for 5 d. Additionally, an immunosuppression protocol was prescribed, with methylprednisolone 250 mg/d with dose tapering on subsequent days, associated with tacrolimus 1 mg/kg/d with a goal serum level of 4-7 ng/mL and everolimus 2 mg/d.
Prophylactic intravenous fluconazole for candidemia (risk due to several transfusions) and immunosuppression protocol with methylprednisolone 250 mg/d with dose tapering on subsequent days, associated with tacrolimus 1 mg/kg/d with a goal serum level of 4-7 ng/mL were initiated, plus everolimus 1 mg/d. Additionally, due to the history of hepatitis B, hyperimmune immunoglobulin and entecavir were prescribed. The patient developed a pulmonary infection, and piperacillin-tazobactam, azithromycin 500 mg/d for 5 d and ivermectin 12 mg/d for 2 d were started.
On the third postoperative day, he had a fever peak of 38.5ºC associated with desaturation (91% oxygen saturation), and antibiotics were replaced by polymyxin B and meropenem, as the blood culture showed the growth of Escherichia coli sensitive to such drugs. Oxygen was supplied through a nasal catheter, with a flow rate of 3 L/min, with the patient remaining comfortable and with an oxygen saturation > 94%. Table 1 shows the laboratory test results. The patient continued to receive acetylsalicylic acid and sulfamethoxazole with prophylactic trimethoprim, according to the liver transplantation protocol of the service. Over the next few days, the patient showed an improvement curve and no new clinical complications. He was discharged to outpatient follow-up after 38 d of hospitalization.
The patient’s evolution showed clinical improvement and he was extubated on the second postoperative day, with an initial need for 5 L/min of oxygen through a nasal catheter to maintain adequate oxygen saturation and an oxygenation index of 420. Over the next few days, complete weaning from oxygen support was attained.
He showed clinical and laboratory improvement and was discharged from the ICU after 1 wk, and discharged from the hospital after 12 d. He was then referred to outpatient follow-up. During the follow-up period, a new nasopharyngeal swab was collected to screen for SARS-CoV-2 infection, 16 days after the first test and a detectable result has remained to date.
The liver is the second most commonly transplanted solid organ worldwide, second only to the kidney. The transplanted population is exposed to several emerging diseases and may even develop symptomatic and, sometimes, severe infections[6,7]. The SARS-CoV-2 pandemic presents a challenging scenario to the reality of transplantations, considering that due to immunosuppression, newly transplanted patients are subject to a high risk of developing complications from infections[8].
The most frequently reported symptoms of SARS-CoV-2 infection in the general population comprise fever, dry cough, myalgia and headache. A Swiss study described the results of a series of 21 patients submitted to solid-organ transplantations who contracted COVID-19, in whom the clinical presentation did not significantly differ from the symptoms described in the general population[9]. In the reported cases, the patients did not have flu-like symptoms during hospitalization.
However, case 1 had a fever peak and showed oxygen desaturation on the third postoperative day, which can be attributed to symptoms of infection by SARS-CoV-2 or by another bacterial infectious process. The evolution of case 2 showed a slower weaning from oxygen support in the postoperative period. A North American study reported a worse prognosis in solid organ recipients with COVID-19[10]. Preliminary data indicate that late transplant recipients have more severe disease than recent transplant recipients, suggesting that immunosuppression itself is not a criterion for severity, and a metabolic component, such as arterial hypertension, diabetes and obesity, which are typically present in late recipients is responsible for the worse prognosis in this population[11].
A study reported on four transplant recipients who were diagnosed with SARS-CoV-2 between 7 and 10 d after the transplant. Three had a good evolution and one died due to a cause unrelated to COVID-19[12]. In our center, only these two patients were transplanted with SARS CoV-2 infection detected by RT-PCR during surgery and both showed a good evolution. To date, there has been no description in the world literature of other recipients with SARS-CoV-2 infection detected by RT-PCR immediately before transplantation. Despite our small sample, our data confirmed the recent literature indicating that immunosuppression alone is not a factor of poor prognosis in the presence of COVID-19. As recommended by the transplant societies, our patients were screened for COVID-19 prior to the procedure, in order to predict possible adverse developments in the postoperative period and allow more adequate multidisciplinary patient care. However, the difficulty in obtaining the results and the fact that the patients did not have respiratory symptoms were essential in the decision by the medical team to proceed with the transplant, even without the COVID-19 test results. Another important fact was the patients’ disease severity, as both patients had an important risk of worsening liver disease, given their high MELD score.
There are reports in the literature of several pathogens that can be transmitted through grafting. In the case of heart and lung transplantation, the International Society of Heart and Lung Transplantation recommends considering the exclusion of suspected or confirmed donors with SARS-CoV-2 infection, as the microorganism is predominantly found in respiratory secretions[13]. With regard to liver transplantation and COVID-19 infection, recent recommendations suggest that the procedure can be performed during the pandemic[14]. However, transmission via the liver graft cannot be excluded, since the virus has been found in blood in up to 15% of cases. A study described the autopsy results of 27 patients and showed that SARS-CoV-2 can be detected in multiple organs, including the lungs, pharynx, heart, liver, brain and kidneys[15]. It is noteworthy that liver damage may be caused by direct liver injury due to COVID-19, medication-induced hepatotoxicity and immune-mediated inflammation.
In our center, we chose to continue to perform transplants during the pandemic, limiting the procedure to candidates with greater need for transplantation, as in the described cases. The use of personal protective equipment to reduce the transmission chain as much as possible is mandatory among health professionals, and any professional who is symptomatic or has positive results for COVID-19 is removed from the procedure.
The current proposal in our center is to screen all possible donors for SARS-CoV-2 infection using RT-PCR. If the donor is positive, they are immediately excluded.
With regard to the recipient, the current proposal of this transplantation center is that during the outbreak of certain diseases, as in the case of COVID-19, an initial screening is carried out by telephone, to determine flu-like symptoms and contact with suspected or confirmed cases of SARS-CoV-2 infection. It is also advised that the patient should remain in social isolation for at least 14 d before the transplant, to avoid possible infectious contamination.
A clinical history of flu-like symptoms is again performed upon hospital admission. Additionally, chest X-rays and nasopharyngeal swab screenings are performed to minimize the risk of transmission. Computed tomography of the chest is reserved for patients with significant alterations shown on chest X-rays. If the patient is suspected of having COVID-19, the transplant is postponed and should be performed in a timely manner. However, it is important to emphasize that the clinical condition is taken into account, in order to define whether the patient has the possibility of an adverse evolution if the transplant is postponed, especially in patients without respiratory complaints.
As relevant data are scarce, it is important to identify a population of recipients that can safely undergo solid organ transplant even with RT-PCR detected SARS-CoV-2 infection, in whom the risk of not undergoing the transplantation is higher than that of the infection.
To date, there is no proven therapy for the treatment of symptomatic coronavirus cases. Recent studies have shown clinical improvement after the use of corticosteroid therapy in cases of severe acute respiratory syndrome associated with COVID-19[16].
Hydroxychloroquine, lopinavir/ritonavir and remdesivir were not used in the present study and immunosuppressive therapy after liver transplantation was not altered, as the patients showed progressive clinical improvement and they were easily weaned from mechanical ventilation in the postoperative period.
Two cases of successful liver transplant are described in patients with a positive test for COVID-19 immediately after transplantation, with minimal symptoms and no graft dysfunction after the procedure. In this new post-COVID-19 era, the experience in this center can be used as an example, in order that other services can continue to perform transplants and, thus, reduce the morbidity and mortality of this population on the waiting list. Transplant centers must be able to readjust daily to evolution of the COVID-19 pandemic and care during the pandemic must be intensified, requiring a donor and recipient screening process to detect COVID-19. If the disease is detected, transplantation should be carefully considered. It is worth mentioning that the care and use of personal protective equipment by the multidisciplinary team is crucially important to prevent the viral propagation cycle.
| 1. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19017] [Article Influence: 3169.5] [Reference Citation Analysis (9)] |
| 2. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13056] [Article Influence: 2176.0] [Reference Citation Analysis (4)] |
| 3. | Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020;382:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1819] [Cited by in RCA: 1871] [Article Influence: 311.8] [Reference Citation Analysis (0)] |
| 4. | Kumar D, Manuel O, Natori Y, Egawa H, Grossi P, Han SH, Fernández-Ruiz M, Humar A. COVID-19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Liu H, He X, Wang Y, Zhou S, Zhang D, Zhu J, He Q, Zhu Z, Li G, Sun L, Wang J, Cheng G, Liu Z, Lau G. Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int. 2020;14:432-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, Zambrano-Achig P, del Campo R, Ciapponi A, Sued O, Martinez-Garcia L, Rutjes A, Low N, Bossuyt PM, Perez-Molina JA, Zamora J. FALSE-NEGATIVE RESULTS OF INITIAL RT-PCR ASSAYS FOR COVID-19: A SYSTEMATIC REVIEW. MedRxIV. 2020.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | El Kassas M, Alboraie M, Al Balakosy A, Abdeen N, Afify S, Abdalgaber M, Sherief AF, Madkour A, Abdellah Ahmed M, Eltabbakh M, Salaheldin M, Wifi MN. Liver transplantation in the era of COVID-19. Arab J Gastroenterol. 2020;21:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 8. | Muller X, Tilmans G, Chenevas-Paule Q, Lebossé F, Antonini T, Poinsot D, Rode A, Guichon C, Schmitt Z, Ducerf C, Mohkam K, Lesurtel M, Mabrut JY. Strategies for liver transplantation during the SARS-CoV-2 outbreak: Preliminary experience from a single center in France. Am J Transplant. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Tschopp J, L'Huillier AG, Mombelli M, Mueller NJ, Khanna N, Garzoni C, Meloni D, Papadimitriou-Olivgeris M, Neofytos D, Hirsch HH, Schuurmans MM, Müller T, Berney T, Steiger J, Pascual M, Manuel O, van Delden C; Swiss Transplant Cohort Study (STCS). First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20:2876-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 11. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 12. | Kolonko A, Dudzicz S, Wiecek A, Król R. COVID-19 infection in solid organ transplant recipients: A single-center experience with patients immediately after transplantation. Transpl Infect Dis. 2020;e13381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic. [cited July 1, 2020]. Available from: https://ishlt.org/ishlt/media/documents/SARS-CoV-2_-Guidance-for-Cardiothoracic-Transplant-and-VAD-centers.pdf. |
| 14. | American Association for the Study of Liver Diseases. Clinical Insights for Hepatology and Liver Transplant providers during the COVID-19 Pandemic. [cited April 13, 2020]. Available from: https://www.aasld.org/sites/default/files/2020-04/ AASLD-COVID19-ClinicalInsights-4.07.2020-Final.pdf. |
| 15. | Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1254] [Cited by in RCA: 1445] [Article Influence: 240.8] [Reference Citation Analysis (1)] |
| 16. | Randomised Evaluation of COVID-19 Therapy. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. 2020. [cited June 27, 2020]. Available from: https://www.recoverytrial.net/news/Low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cesaretti M, Wang W S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wu YXJ