INTRODUCTION
Hypertension is a major public health problem associated with high cardiovascular morbidity and mortality. Generally the prevalence of high blood pressure appears to be around 30%-45% in the whole population, which shows a higher prevalence with ageing. In case of adults, hypertension is defined as a systolic blood pressure of at least 140 mmHg and a diastolic of 90 mmHg according to the various guidelines (e.g., the new ESH/ESC guideline). However, there are some subgroups of patients in whom the goal blood pressure is different. For instance, the elderly can benefit from lowering systolic blood pressure only to between 140 and 150 mmHg. In diabetic patients, however, the target blood pressure is lower than in the general population. In these patients the diastolic blood pressure should be less than 85 mmHg. According to the concept of J-curve hypothesis, it can be harmful to reduce both systolic and diastolic blood pressure to markedly low values.
Hypertension is an important risk factor of cardiovascular diseases, stroke, renal disease and peripherial artery disease[1]. According to epidemiological data, hypertensive heart disease (HHD) is one of the most important hypertensive organ damage. The most common consequences of HHD are heart failure, ischemic heart disease and arrhythmias. The Framingham Heart Study showed that 20 mmHg elevation of systolic blood pressure is associated with 50% increased risk of heart failure[2]. Hypertension is of course not the sole factor contributing to the development of heart failure but multi-variate analysis using time-dependent modelling revealed that myocardial infarction conferred the greatest risk of developing heart failure. As a consequence of its high prevalence, hypertension carried the greatest population-attributable risk[3]. Thus blood pressure lowering (antihypertensive therapy) markedly reduces the incidence of major cardiovascular (CV) events like HHD and heart failure[4].
Registries proved that nearly half of the patients with heart failure have a preserved ejection fraction (HfpEF). HFpEF is most common among the elderly, women and patients with left ventricular hypertrophy[5].
DEVELOPMENT OF HHD
Hypertensive heart disease encompasses a wide spectrum including asymptomatic cardiac hypertrophy and clinical heart failure (with either preserved or reduced ejection fraction). Elevated blood pressure changes the structure and function of blood vessels and left ventricle. These alterations are also known as remodeling, which is an adaptive mechanism in response to long-term changes in hemodynamic conditions, but it may also subsequently contribute to the pathophysiology of circulatory disorders[6,7].
Alterations in left ventricle, for instance hypertrophy and ischemia, predispose to heart failure in hypertensive patients. Cardiac hypertrophy is an adaptive response, a compensatory mechanism to pressure or volume overload directing to the attenuation of wall tension and the maintenance of cardiac output. The left ventricle mass can increase either as a result of wall thickening or ventricular dilation. The relative wall thickness (the ratio of the left ventricular wall thickness to diastolic diameter) determines the type of hypertrophy (eccentric or concentric). It is influenced by the type of overload (pressure or volume), by the neurohormonal activation (plasma renin level), extracellular matrix changes, concomitant diseases (coronary artery disease, diabetes mellitus, obesity), demographic and genetic factors (e.g., ACE gene polymorphism)[7,8].
Sustained hypertrophy is often the initial step towards the progression of congestive heart failure[7].
It is now well known that symptomatic heart failure can occur either in the setting of reduced (HFrEF) or preserved ejection fraction (HFpEF)[9]. The classic course of HHD progression is a so-called “burned-out” of left ventricle in which hypertension leads to concentric hypertrophy followed by diastolic and finally systolic insufficiency[10].
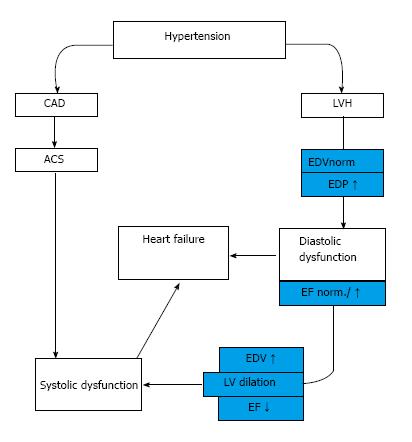
In an other group of hypertensive patients the development of myocardial infarction causes directly systolic heart failure (HFrEF) independently from hypertrophy[8] (Figure 1).
Figure 1 Development of hypertensive heart disease.
HT: Hypertension; LVH: Left ventricle hypertrophy; EDV: End-diastolic volume; EDP: End-diastolic pressure; EF: Ejection fraction; CAD: Coronary artery disease; ACS: Acut coronary syndrome.
HISTOLOGY
High blood pressure caused alterations in cardiac structure and function, eventually resulting in impaired myocardial performance, coronary haemodynamics and apoptosis.
It has been well established that pathogenesis of HHD involves all components of the heart, including myocytes and non-myocytic cells, such as fibroblasts and endothelial cells, extracellular matrix proteins, fibrillar collagen, and coronary vessels[11].
Structural remodeling of HHD is characterized by enlarged cardiac myocytes with altered energy metabolism, fibroblast proliferation and activation, fibroblast-myofibroblast transformation and excessive collagen deposition, which all lead to a more rigid myocardium[12,13]. Coronary resistance vessels are also affected, perivascular fibrosis of intramyocardial coronary arteries and arterioles produce intimal-medial thickening[14].
NEUROHUMORAL MECHANISMS
The remodeling and growth regulation of the heart involve several mechanisms including neurogenic, humoral, autocrine and paracrine factors.
The activation of renin-angiotensin-aldosterone system (RAAS) is one of the most important processes, which contribute to the development of hypertension including vasoconstriction, generation of reactive oxygen species (ROS), vascular inflammation, vascular and cardiac remodeling (hypertrophy and fibrosis). Therefore the RAAS system plays a prominent part in accelerating hypertensive organ damages[15,16]. Moreover angiotensin converting enzyme (ACE) is responsible for the production of angiotensin II (Ang II), which correlates to left ventricle hypertrophy. Individuals have different plasma ACE concentrations due to the insertion/deletion polymorphism of ACE gene, which also shows a close relationship to ventricular hypertrophy[12].
Mineralocorticoids have a physiological role in volume regulation, but they also activate the sympathetic nervous system (SNS), which results in baroreceptor dysfunction, impaired arterial compliance and marked myocardial and vascular fibrosis[17].
The sympathetic hyperactivity rises blood pressure directly (even without RAAS activation), possesses metabolic effects (e.g., insulin resistance) and facilitates the development of LVH.
It has been well established that pathogenesis of cardiac remodeling is also associated with insulin resistance, increased activity of insulin-like growth factor-1 and myocardial pro-fibrotic matricellular protein osteopontin, thyroid hormons and the elevated level of brain and atrial natriuretic peptides[12].
STRESS-INDUCED SIGNALING PATHWAYS
It is well known that hypertension induced oxidative stress plays an important role in the development of cardiac injury. Potential sources of ROS are the NADPH oxidases, nitric oxide synthase, lipoxygenases, cyclo-oxygenases, xanthine oxidase, cytochrome P450 enzymes, and the mitochondrial respiratory chain[18]. ROS mediated damages are implicated in endothelial dysfunction, inflammation, hypertrophy, apoptosis, cell migration, fibrosis and angiogenesis[19]. ROS impair the function of ion-channels and decrease the amount of high energy phosphates. These changes can result in alterations of myocyte and smooth muscle cell calcium homeostasis leading to increased cell proliferation[20]. Oxidative stress can lead to single stranded DNA breaks and changes in signaling pathways evolving alterations in LV structural and mechanical properties[21].
The single stranded DNA breaks provoke the activation of nuclear poly(ADP-ribose) polymerase-1 (PARP) enzyme, which can decrease the cellular NAD+ and ATP pools leading to energy depletion with inadequate glycolysis and mitochondrial respiration, promoting apoptotic or necrotic cell death[21-25].
The activation of PARP-enzyme has a central role in the pathophysiology of several cardiovascular diseases including the development of HHD, transition of HHD to HF by influencing collagen production via modulation of different kinase cascades[21,22]. Cellular adaptations of the heart are typically initiated by stress responsive signaling pathways, which serve as central transducers of cardiac hypertrophic growth and/or ventricular dilation.
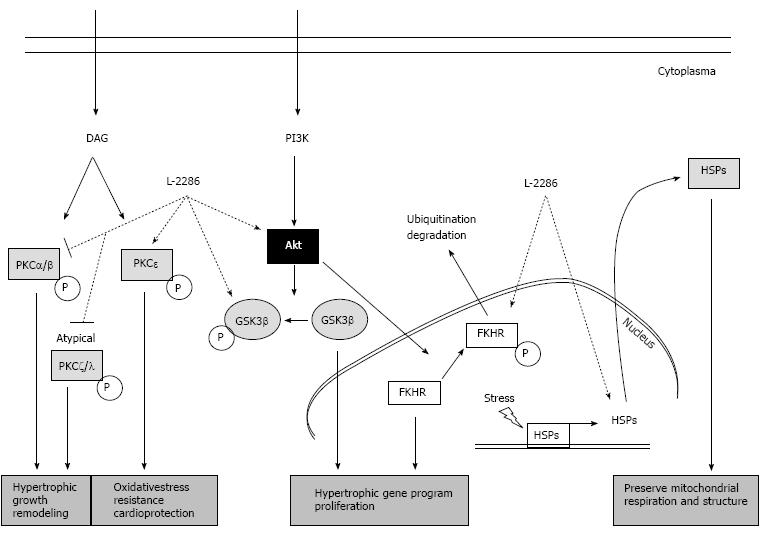
These signaling pathways include extracellular signal-regulated protein kinases (ERK), p38 mitogen-activated protein kinases (p38-MAPK), c-Jun NH2-terminal kinases (JNK), several protein kinase C (e.g., PKC delta and epsilon) isoforms and Akt-1/glycogen synthase kinase-3b (GSK-3β) signaling cascade. These cascades have also been implicated in affecting the decision of myocytes to either survive (Akt-1/GSK-3β, ERK, PKCepsilon, JAK) or undergo programmed cell death (p38 MAPK, PKC delta, JNK)[20-22] (Figure 2).
Figure 2 Summary of protein kinase C and Akt-1/GSK-3β signal pathway and the alterations due to poly(ADP-ribose) polymerase-1 inhibition (22 with permission of Deres L and the authors).
DAG: Diacylglycerol; FKHR: Forkhead transcription factor; GSK-3β: Glycogen synthase kinase-3 β; HSP: Heat shock protein; PARP: Poly(ADP-ribose) polymerase-1; PI3K: Phosphatidylinositide 3-kinase; PKC: Protein kinase C.
It has been observed that RhoA/ROCK pathway is also involved in hypertension and in the development of consequent cardiac hypertrophy. It has a close relationship to Ang II, which can increase ROCK activity and contributes to the maintenance of hypertension, to the increased medial thickness and perivascular fibrosis in coronary arteries[26]. This mechanism also affects stretch-induced ERK activation and vascular smooth muscle cell growth[27].
TREATMENT STRATEGIES IN HYPERTENSION
The main goal of antihypertensive therapy is the prevention of organ damages thus the prevention of life-threatening consequences such as stroke, myocardial infarction HHD or heart failure[1]. Although previous clinical trials focused mainly on improving mortality in HF, nowadays it is recognized that preventing heart failure is better for the patients and financially it is cost-effective for the health care system. It is well-known that effective antihypertensive therapy reduces the incidence of heart failure by more than fifty percent[28].
Based on current guidelines, the cornerstones of antihypertensive pharmacological therapy are diuretics, beta-blockers, angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB) and calcium antagonists (CA)[29].
Blocking sympathetic hyperactivity is thought to be an essential tool in the treatment of CV diseases. Besides the blood pressure lowering effect of beta-blockers, they are able to reduce sympathetic overactivation. Moreover, they reverse left ventricular remodeling and can decrease the incidence of heart failure. Among diuretics, the thiazides mean the first line of choice because of efficacy and price. They are recommended in left ventricular hypertrophy, and can reduce cardiovascular morbidity and mortality.
ARBs and especially ACE-inhibitors significantly decrease all cause mortality in patients with hypertension. ACE-I can both prevent developing HF and decrease LV and vascular wall remodeling. A large body of evidence suggests that all of these are induced by the downregulation of enzymatic pathways involved in the interstitial collagen formation. CA effectively reduce blood pressure by dilating arteries with decreasing Ca(2+) influx into smooth muscle cells of the arterial wall. They can be used in combination therapy with most of the antihypertensive drugs. According to the statement of ESC and ESH, all above mentioned drugs are suitable for the initiation and maintenance of antihypertensive treatment because the main benefits of these drugs are due to the lowering of BP per se and are largely independent of the drugs employed[28].
NEW THERAPEUTIC POSSIBILITIES IN HYPERTENSION
Although there is an increasing number of effective antihypertensive drugs that can be used in the clinical practice, there are many patients who can not reach the goal blood pressure. In the United States, there are approximately 70 million hypertensive patients and about 40 million of them do not have their blood pressure under proper control. The main factors in the background of this phenomenon are side effects, drug intolerance or interactions and therefore poor adherence of patients to the prescribed medication[29]. Therefore in the last several years experimental researches tried to focus on treatments that alleviate end-organ damage itself without lowering blood pressure.
This approach is supported firstly by large trials with statin therapy. The main role of statins was the prevention of coronary artery disease, myocardial infarction and other adverse cardiovascular events. Statins possess both lipid-dependent and lipid independent effects. They are able to lessen inflammation, improve endothelial function and decrease thrombogenicity[30].
In the background of the favorable pleiotropic effects of statins, we need to mention the modulation of intracellular pathways, involved in cell growth regulation/apoptosis and gene expression (Ras, Rac, Rab and Rho)[30,31]. It has already been demonstrated primarily in experimental but also in human studies that high dose atorvastatin inhibits the synthesis of isoprenoids, which are functionally important in the Rho/Rho-associated coiled-coil containing kinase (ROCK) pathway[30]. Moreover, the inhibition of Rho/ROCK pathway by statins may cause improvement in endothelial function and decrease vascular inflammation and atherosclerosis. The localization of these proteins has been shown in vascular smooth muscle cells but their role needs to be determined in the context of atherosclerosis. These findings open an option for specific ROCK1 or ROCK2 inhibitors, which could have greater therapeutic effect with less toxicity[30]. Furthermore, statins decrease the number of angiotensin-1 receptors through RhoA, Ras, Rac1 and the Rho/kinase system, which regulates the ROS formation through NADPH oxidase[32].
The ASCOT-LLA study revealed the role of statins in the prevention of CV events among hypertensive patients[33,34]. Large clinical trials demonstrated that statin therapy may provide clinical benefits to patients with heart failure. Analysis of the Daunia Heart Failure Registry in 2013 elucidated that treatments with atorvastatin are associated with fewer cardiac deaths and better left ventricular performance[35,36].
Mitochondrial dysfunction also seems to be an important factor in the development of HHD[29,37,38]. Another therapeutic strategy can be the stimulation of mitochondrial biogenesis through the AMPK or the eNOS/Nitric Oxide/Cyclic Guanosine Monophosphate pathway[37-43]. Resveratrol, which has a well-known positive effect in the prevention of cardiovascular diseases, is a potent stimulator of the mitochondrial biogenesis[44-49]. An other way is to augment the mitochondrium against oxidative stress. ACE-I and ATII receptor blockers, which are originally antihypertensive drugs, bear antioxidant properties beside blood pressure lowering effect. However, it is not clear whether they target mitochondrial reactive oxygen species (ROS) formation directly or indirectly[50,51]. Thirdly, regulating mitochondrial iron homeostasis and reducing mitochondrial iron content may also yield to cardioprotection because of inhibition of hydroxyl radical formation and mitigation of oxidative stress[36].
There is an expanding number of evidence that the previously mentioned resveratrol significantly attenuates the development of cardiac dysfunction[52]. This ability is already proved in spontaneously hypertensive rats (SHR), transverse aortic constricted rats (TAC), models of hypertension and pressure overload-induced heart failure. Although resveratrol alone does not have any systolic or diastolic blood pressure lowering effect, in TAC rats resveratrol markedly increased glutathione, sodium oxide dismutase 2 levels and decreased 4-hydroxy-2-nonenal - a marker of lipid peroxidation - and LV macrophage and mast cell infiltration. Furthermore, a combination of resveratrol with hydralazine treatment significantly reduced blood pressure, improved systolic and diastolic function, decreased fibrosis and improved vascular geometry. The low-dose resveratrol itself was unable to reach these favourable actions. However, resveratrol alone alleviated cardiac fibrosis and some of the functional abnormalities in SHRs[53].
The cardiomyocyte function enhancer ranolazine reduces myoplasmic free Ca(2+) during diastole at high-stimulus rates. Therefore ranolazine showed to be effective in reducing diastolic dysfunction with inhibition of the increased late sodium current in the SHR leading to reduced Ca(2+) overload[54].
Hu et al[55] found that HGF expression is attenuated in hypertrophic and fibrotic myocardium of spontaneolusly hypertensive rats (SHR) and injected recombinant adenovirus hepatocyte growth factor gene (Ad-HGF gene) in the left ventricular free wall. The upregulation of myocardial HGF expression in SHR animals significantly suppressed myocardial fibrosis, collagen I content, LVMI, LVEDP, and increased -dP/dtmax value[55].
In the last decade PARP inhibitors received growing attention. Although they do not have any antihypertensive effect, our workgroup demonstrated that an isoquinoline derivative PARP-inhibitor, i.e., L-2286 has beneficial effects against oxidative cell damage, ischemia-reperfusion injury and the development of postinfarction, or long-term high blood pressure-induced heart failure in hypertensive animals (SHR)[21,22,25,56,57]. The PARP-inhibitor treatment significantly decreased the collagen deposition in the myocardium thus with echocardiography less prominent septal and posterior wall thickness could be measured. Moreover, in old SHR animals the transition of already developed HHD into manifest heart failure was also blocked by pharmacological PARP-inhibition. In an other long-term experiment, PARP-inhibitors decreased also the hypertensive remodeling of the great vessels in spontaneously hypertensive rats. Our experimental data also proved that the influence on the Akt-1/GSK-3β, MAPKs, MKP-1 and PKC pathways could be the underlying mechanism behind the PARP-inhibition[21,22,25,56,57].
The concept that it is possible to prevent organ damages without blood pressure lowering effect in hypertension is very promising since the goal blood pressure can not be reached in a high number of patients. This is why PARP-inhibitor co-administration could give us a potential new therapeutical approach beside the antihypertensive therapy to prevent hypertension induced organ damages.
CONCLUSION
Although several effective novel and modern antihypertensive therapies were introduced in the last decade, hypertension caused organ damages, especially HHD and heart failure, remain a leading cause of morbidity and mortality in hypertensive patients. That is the reason for the growing number of researches trying to focus on treatments that alleviate end-organ damage itself even without lowering blood pressure. Several drugs, like statins or PARP-inhibitors exert beneficial effect on intracellular signaling, and could be an important part of the treatment of hypertensive patients in the future.
P- Reviewer: Satoh S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/